Abstract
In utero hematopoietic cell transplantation (IUHCT) is a nonmyeloablative approach that takes advantage of normal immunologic development to achieve donor specific tolerance. Despite the many potential advantages of the fetal recipient, IUHCT across MHC barriers has been limited by low levels of engraftment and the inability to consistently achieve allochimerism.
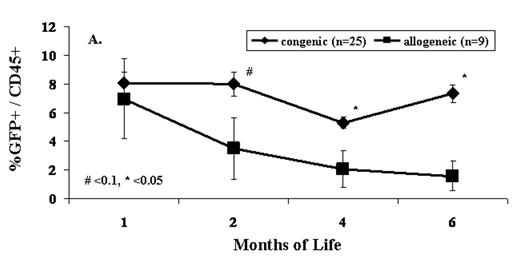
Although the immature immune system of the developing fetus has long been appreciated as a principal advantage of IUHCT, the competence of the fetal immune system to act as a barrier to IUHCT has been a source of debate. Until now, comparisons of allogeneic and congenic engraftment have been inconclusive due to methodologic limitations resulting in minimal and inefficient engraftment. In this study, a new intravascular technique that allows definitive administration of much higher doses of donor cells was employed to directly compare the incidence and levels of engraftment following in utero transplantation of either congenic or allogeneic bone marrow (BM) or enriched hematopoietic stem cells (HSCs). 20E+06 B6 GFP BM cells (H2Kb+, GFP+) or 1E+05 cKit+Sca-1+Lin- B6 GFP cells (H2Kb+, GFP+) were intravenously injected via the vitelline vein into gestational day 14 Balb/c (H2Kd+, allogeneic) or C57Bl/6 (H2Kb+, congenic) fetal mice. The peripheral blood (PB) of recipients was serially analyzed by flow cytometry for GFP+ donor cells at 1, 2, 4 and 6 months of age. A separate group of animals was harvested at 1 week of age (2 weeks after injection) to assess donor chimerism in PB and BM. Our results demonstrate that 100% of surviving recipients of whole BM demonstrate engraftment at 1 week of age, but that 70% of allogeneic recipients lose engraftment by 1 month of age, and 80% ultimately fail to sustain long-term chimerism. In contrast, all congenic recipients maintain engraftment at 6 months of age (Table 1). Chimerism levels in allogeneic recipients drop significantly after 1 month of life while those in congenic recipients remain stable. This results in a significant difference in engraftment levels in allogeneic and congenic recipients beyond 1 month of life (Fig 1). Similar results were seen when enriched HSCs were the donor cell source. 100% (2/2) of congenic recipients of enriched HSCs demonstrated stable low level PB engraftment up to 6 months of life (0.14–0.55% GFP+ donor cells). In contrast, no allogeneic recipients (0/9) of enriched HSCs were chimeric from 1 to 6 months of life. In combination, these results demonstrating a 100% efficiency of long-term engraftment in congenic recipients and loss of engraftment by 1 month of age in the majority of allogeneic recipients strongly implicate an adaptive immune barrier to allogeneic engraftment after IUHCT. Better understanding of the immune mechanisms limiting allogeneic engraftment after IUHCT is required to allow the development of successful strategies for IUHCT.
Efficiency of Engraftment after IUHCT in Congenic and Allogeneic Recipients
| . | 1 week of age (BM) . | 1 week of age (PB) . | 1 month of age (PB) . | 6 months of age (PB) . |
|---|---|---|---|---|
| congenic | 100% (8/8) | 100% (8/8) | 100% (25/25) | 100% (25/25) |
| allogeneic | 100% (8/8) | 100% (8/8) | 29% (9/31) | 19% (6/31) |
| . | 1 week of age (BM) . | 1 week of age (PB) . | 1 month of age (PB) . | 6 months of age (PB) . |
|---|---|---|---|---|
| congenic | 100% (8/8) | 100% (8/8) | 100% (25/25) | 100% (25/25) |
| allogeneic | 100% (8/8) | 100% (8/8) | 29% (9/31) | 19% (6/31) |
Figure
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal