Comment on Pak et al, page 3730
In this issue, Pak and colleagues report that hepcidin production in mice is suppressed after phlebotomy or erythropoietin administration but that the suppression is reversed by inhibitors of erythropoiesis.
Iron is a precious metal for the organism because of its unsurpassed versatility as a biologic catalyst. It is involved in essential biologic functions such as oxygen transport, electron transfer, and DNA synthesis. However, when not appropriately shielded, iron plays a key role in the formation of extremely toxic oxygen radicals that can damage biologic molecules, cells, tissues, and entire organisms. All life forms have thus evolved exquisite regulatory mechanisms that, under normal conditions, delicately control iron metabolism at both cellular and organismal levels.1 In mammals, the amount of total body iron is regulated at the level of absorption in the proximal duodenum but, normally, iron absorption represents approximately only 1/30 of the plasma iron turnover. The major fraction (∼ 80%) of plasma iron turnover is represented by this metal's fluxes into the bone marrow for hemoglobin synthesis in developing red blood cells and the movement of iron from macrophages, which very efficiently recycle hemoglobin iron back to plasma transferrin. Total plasma iron (∼ 3 mg) exchanges 10-fold every day and, until recently, we have been totally ignorant about the mechanisms that govern this tightly controlled pool.FIG1
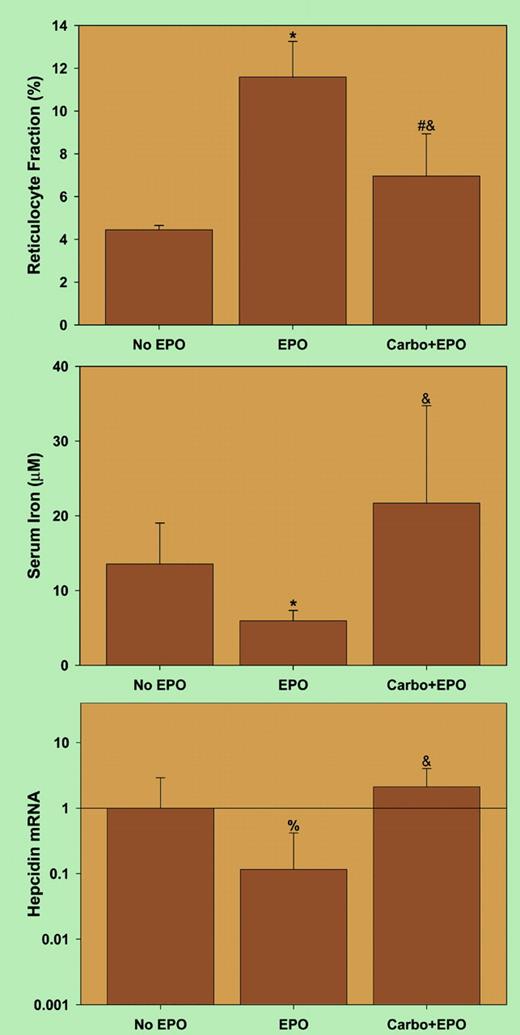
Erythropoietin (EPO) suppresses hepcidin indirectly by inducing erythropoiesis. See the complete figure in the article beginning on page 3730.
Erythropoietin (EPO) suppresses hepcidin indirectly by inducing erythropoiesis. See the complete figure in the article beginning on page 3730.
The discovery of hepcidin2,3 has partially rectified our ignorance of this important regulatory mechanism. Now regarded as the principal iron regulatory hormone, hepcidin synthesis is stimulated by high organismal iron levels. Hepcidin blocks both intestinal absorption and iron release from stores (mainly macrophages) by inducing the internalization and degradation of ferroportin (cellular iron exporter). The opposite scenario develops when iron levels are low and, hence, hepcidin is a negative feedback controller in organismal iron homeostasis. Additionally, hepcidin production and levels negatively correlate with erythropoietic activity, but signals involved in this regulation are unknown.
Pak and colleagues investigated the link between erythropoiesis and hepcidin synthesis. At the core of their report is an experiment in which the authors confirm that anemia4 (following phlebotomy) causes a dramatic decrease in hepcidin mRNA levels. Strikingly, hepcidin mRNA levels did not respond to phlebotomy when inhibitors of erythropoiesis or antierythropoietin antibodies were administered prior to phlebotomy. This experiment provides clear evidence that erythropoietic activity is necessary for anemia-mediated suppression of hepcidin production. In additional control experiments, the authors excluded the possibilities that the inhibitors of erythropoiesis increased hepcidin expression via (1) inflammatory stimulation or (2) interference with tissue hypoxia. Moreover, the authors showed that hepcidin is not directly regulated by erythropoietin. This study considerably enhances our understanding of mechanisms involved in supplying appropriate amounts of iron from stores to plasma for erythropoiesis. Of interest, Nečas' laboratory independently reported that a decrease in hepcidin mRNA levels in hepatocytes from phenylhydrazine-treated mice was abrogated by ablation of bone marrow by irradiation.5
The article by Pak et al contains one important finding that, however, was not commented upon by the authors. As shown in the figure, conditions of erythropoietin administration, combined with erythropoiesis inhibitors, led to an unexpected dyad of elevated plasma iron with increased hepcidin synthesis, suggesting that decreasing hepcidin levels is not physiology's only mode of promoting iron release from stores. Additional evidence telling us that the regulation of iron release from stores is fairly complex comes from “classical” experiments revealing that upon increased demand, hemoglobin-derived iron is much more available for erythropoiesis than storage iron in the reticuloendothelial system.6 This implies that the so-called erythroid regulator, which presumably links iron release from stores to erythropoietic demand, involves more than “just hepcidin.” There is no doubt, based on current experimental evidence, that hepcidin, in response to elevated iron levels or inflammation, inhibits iron release from iron “donor” cells. However, there may be an alternative pathway by which the metal is highly efficiently released from hemoglobin-recycling macrophages when there is an exceptionally high demand for this metal.
The authors declare no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal