Comment on Seldin et al, page 3945
In this issue, Seldin and colleagues report that the benefits of high-dose melphalan chemotherapy and autologous peripheral blood stem cell transplantation (SCT) can be safely extended to selected patients 65 years or older with immunoglobulin light-chain (AL) amyloidosis. This finding from the Boston Group, who pioneered this treatment in AL amyloidosis, indicates that older patients should not be excluded from consideration for SCT.
The data reported by Seldin and colleagues show that age plays an important role in both a patient's eligibility for autologous peripheral blood stem cell transplantation (SCT) and in melphalan dose adjustments. Among the older patients, 34.3% were eligible for SCT, compared with 68.4% of younger patients (P < .001), and only 19% of older patients were eligible for full-dose melphalan (200 mg/m2) compared with 65% of younger patients (P < .001). The response rate in older patients, who received a lower dose of melphalan, was less than in younger patients (32% versus 43.5%, respectively), although the difference was not significant. This is consistent with data from large series of immunoglobulin light-chain (AL) amyloidosis patients treated with SCT showing that lower melphalan doses are associated with significant reductions of response rate.1,2 Despite increasing experience with SCT in AL amyloidosis and appropriate melphalan dosage adjustment,2 treatment-related mortality (TRM) remains substantial—it is consistently higher in multicenter trials (usually more than 20%) than in experienced single-center studies (approximately 10%).
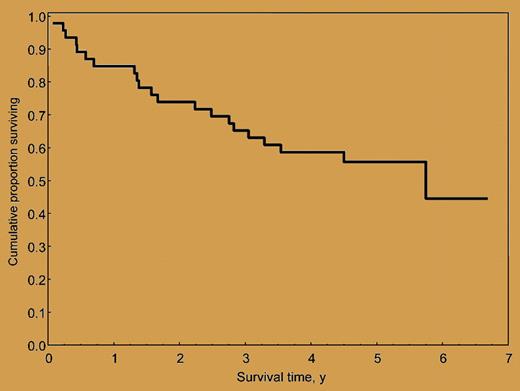
Overall survival of 46 AL amyloidosis patients treated with M-Dex. The median survival is 5.1 years, and the median follow-up of living patients is 5 years (range, 3.5-6.7 years).
Overall survival of 46 AL amyloidosis patients treated with M-Dex. The median survival is 5.1 years, and the median follow-up of living patients is 5 years (range, 3.5-6.7 years).
Two large studies from the Boston Group1 and the Mayo Clinic2 showed that melphalan 200 mg/m2 followed by SCT produced an unsurpassed high rate of hematologic responses (76%2 with 40% complete responses 1 year after SCT1 ) that translated into extended survival (approaching 8 years).1 However, the outcome of SCT using lower doses of melphalan conditioning (100-140 mg/m2) with regard to hematologic response (53%2 with 33% complete responses 1 year after SCT1 ) and survival (2.9 years)1 appears close to that achievable with less intensive and less toxic chemotherapy regimens. For instance, the association of oral melphalan and dexamethasone (M-Dex) in 46 AL amyloidosis patients ineligible for SCT produced hematologic responses in 67% (33% complete responses) and a median overall survival of 5.1 years (see figure) without significant toxicity.3 The efficacy and tolerability of M-Dex was confirmed by a French randomized multicenter trial comparing M-Dex and SCT. Sixty-five percent of patients treated with M-Dex achieved a hematologic response (32% complete responses), with TRM of 2%.4 No significant difference in hematologic response rate and survival was observed between the 2 treatments. However, in this multicenter study, the TRM of SCT was 24%, and only 29 patients undergoing transplantation were evaluable for response. Collectively, these findings indicate that M-Dex may be a viable alternative to SCT, particularly for patients requiring a reduction of the dose of melphalan conditioning. A phase 3 study comparing these 2 treatments in larger numbers of patients is warranted.
The therapy of AL amyloidosis is improving, but the quest for the best treatment continues. The effectiveness and tolerability of other regimens, such as the combination of cyclophosphamide, thalidomide, and dexamethasone,5 and of novel agents, such as lenalidomide6,7 and bortezomib, should also be tested in controlled, large trials. International collaboration is thus required in order to define the optimal therapy for AL amyloidosis.
The author declares no competing financial interests. Dr Merlini is also affiliated with the Fondazione Instituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal