Abstract
TNFα has previously been used in anticancer therapy. However, the therapeutic application of TNFα was largely limited due to its general toxicity and the fact that it activates the NF-κB–family transcription factors, which are proinflammatory and antiapoptotic. To overcome this problem in vitro, specific NF-κB inhibitors or transcription or protein synthesis inhibitors such as actinomycin D and cycloheximide are usually used in combination to increase TNFα killing of tumor cells. However, these agents also cause harmful side effects in vivo. We show here that wogonin, derived from the popular Chinese herb Huang-Qin, attenuates NF-κB activity by shifting TNFα-induced free radical ·O2– to a more reduced nonradical product, H2O2, and thereby sensitizes TNFα-resistant leukemia cells to TNFα-induced apoptosis. Importantly, wogonin does not affect the viability of normal peripheral blood T cells. Wogonin also sensitizes TRAIL-induced apoptosis. Our data suggest a potential use of wogonin as a TNFα or TRAIL adjuvant for cancer treatment. Our data also demonstrate how a herbal compound enhances killing of tumor cells with reduced side effects compared with other treatments.
Introduction
Tumor necrosis factor-alpha (TNFα) was isolated in 1985 as the first mammalian protein with cytotoxicity to tumor cells and that induced tumor regression in mice.1 It is now known that TNF signaling may either induce cellular activation, apoptosis, or necrosis.2 TNFα was thought to be a potent anticancer agent due to its cytotoxicity against a number of tumor cell lines. However, the clinical use of TNFα is limited because of its systemic toxicity largely due to activation of the proinflammatory NF-κB–family transcription factors.3-5 NF-κB, apart from its proinflammatory activity, is also a negative regulator that antagonizes TNFα-induced killing.4,6 Clinically, the only success in TNFα therapy has occurred with isolated limb perfusion for a limited subset of susceptible tumors, such as melanoma and sarcoma.7 In general, TNFα is considered a key inducer of proinflammatory genes, and its primary role is to stimulate innate inflammatory responses to fight infections, whereas the function of its proapoptotic capability remains mysterious.8
Apoptosis involves 2 main pathways: the extrinsic pathway, which is initiated by binding of ligands to specific death receptors on the cell surface, and the intrinsic pathway, which is initiated at the mitochondria. TNFα is an inducer of the extrinsic pathway. In mammalians, TNFα signals through 2 distinct receptors, TNF receptor 1 (TNF-R1), the primary receptor for soluble TNFα, and TNF-R2, the main receptor for membrane-associated TNFα.5,9 The TNF-R1 signaling pathway can either trigger activation of NF-κB—crucial for TNFα-mediated immunity, inflammation, and proliferation—or activation of apical caspases as well as the c-Jun–N-terminal kinase (JNK) cascade, which leads to programmed cell death.2 TNF-R1 signaling can be divided into 2 distinct stages that sequentially activate NF-κB and apical caspases. Within a few minutes after binding of TNFα to TNF-R1 (stage 1), a signaling complex containing the receptor itself and the adaptor proteins TRADD, TRAF2, and RIP1 but lacking the FAS/APO-1–associated death domain adaptor protein FADD forms and transduces signals that lead to activation of NF-κB and the JNK cascade.2,10,11 At later time points (stage 2), possibly after receptor internalization, RIP1, TRAF2, and TRADD dissociate from the receptor and recruit FADD and caspase-8 to form the second complex, which signals programmed cell death.2 Because of the long delay in the second complex assembly, NF-κB, activated at stage 1, has sufficient time to activate a variety of antiapoptotic factors, including members of the Bcl-2 family (Bcl-2, Bcl-xL), caspase inhibitors (FLIP, c-IAPs), and inhibitors of JNK activation (XIAP, GADD45β),4,5,12-14 which block the apoptotic pathway. Thus, in most instances, TNF-R1 signaling induces apoptosis only when NF-κB signaling is blocked.4,8,15
In recent years, traditional Chinese herbal remedies have gradually gained considerable attention as a new source of anticancer drugs as well as new chemotherapy adjuvants to enhance the efficacy of chemotherapy and to ameliorate the side effects of cancer chemotherapy. Although the healing mechanisms are still largely unknown, some of the drugs have been used to help cancer patients to fight their disease with reduced side effects compared with other treatments.16 Huang-Qin (Scutellaria baicalensis Georgi) is one of the most popular herbal remedies in China and several other Asian countries. The extracts from Huang-Qin have been widely used for clinical treatment of hyperlipemia, atherosclerosis, hypertension, dysentery, the common cold, and inflammatory diseases such as atopic dermatitis. One of the active components of Huang-Qin is 5,7-dihydroxy-8-methoxyflavone, also called wogonin. Wogonin is a flavonoid and has been shown to exert antioxidant,17 antiviral,18 antithrombotic,19 and anti-inflammatory activities. The antiinflammatory activity of wogonin is at least in part due to its ability to suppress expression of monocyte chemotactic protein-1, a crucial factor for early inflammatory responses.20 Wogonin also suppresses several inflammation-associated genes such as inducible nitric oxide synthase, cyclooxygenases, and lipoxygenases and, consequently, inhibits production of nitric oxide (NO) and prostaglandin E2.21-28 Wogonin has also been shown to exert cytostatic activities in several cancer cell lines, including bladder tumors,29 hepatocellular carcinoma,30 and myelogenous leukemia.31 Importantly, wogonin lacks toxicity for normal peripheral blood mononuclear cells.32
In this study, we show that wogonin can sensitize TNFα-resistant leukemia cells to TNFα-induced apoptosis. Wogonin directly shifts TNFα-induced free radical ·O2– to a more reduced state and thereby down-regulates TNFα-induced NF-κB activity. Besides TNFα, the tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) has been shown to kill various tumor cell lines in vitro and in vivo without being toxic to mice and nonhuman primates.33,34 However, 50% of tumor cell lines were TRAIL resistant.34 To overcome the resistance, combinations of TRAIL and chemotherapeutic drugs have been used to increase antitumor activity. In this study, we also show that wogonin can sensitize tumor cells to TRAIL-induced cell death.
Materials and methods
Cell lines and culture
The malignant T-cell line CEM was cultured in RPMI 1640 medium (GIBCO, Grand Island, NY) supplemented with 10% FCS, 50 μg/mL gentamicin (GIBCO), 6 mM HEPES (GIBCO; 1 M solution), and 2 mM l-glutamine (GIBCO; 200 mM solution) at 37°C and 5% CO2. The Jurkat T-cell clone 16 (J16) and Jurkat T cells bearing an integrated NF-κB–dependent luciferase reporter (J-Luc-κB) (kindly provided by Thomas Wirth, Department of Physiological Chemistry, Ulm University, Germany) were cultivated in DMEM, 10% FCS, and 50 μM β-mercaptoethanol. Primary acute myeloid leukemia (AML) cells were obtained from bone marrow aspirates of AML patients (detailed information about this patient will be provided upon request) by Ficoll gradient and cultured in RPMI medium supplemented with 10% FCS, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Preparation of human T cells from peripheral blood
Human peripheral T cells were prepared as described previously35 and were more than 90% CD3+. For activation, resting T cells (day 0) were cultured at 2 × 106/mL with 1 μg/mL PHA for 16 hours (day 1). Day 1 T cells were then washed 3 times and cultured for an additional 5 days in the presence of 25 U/mL IL-2 (day 6).
Redox measurement
TNFα-(100 ng/mL) or wogonin-(1 to 20 μM) treated cells were stained for 30 minutes with 5 μM of the H2O2-sensitive fluoresent dye dichlorofluorescein diacetate (DCFDA, FL-1) or 5 μM of the ·O2–-sensitive dye dihydroethidium (DHE, FL-2) (Molecular Probes, Eugene, OR) at 37°C in the dark, washed 3 times with PBS, and subsequently assayed by FACScan (BD Biosciences, Heidelberg, Germany).
Determination of apoptosis
Cells were plated in triplicate and treated with wogonin (Wako Pure Chemical Industries, Osaka, Japan) in combination with either TNFα (purchased from Sigma, Taufkirchen, Germany) or LZ-TRAIL and His-TRAIL36 for the indicated periods of time at 37°C. Apoptotic cell death was examined by a forward scatter/side scatter (FSC/SSC) index of apoptotic-like change in cell size and granularity by FACScan37 or by analysis of DNA fragmentation according to the method of Nicoletti.38
Luciferase assay
The Jurkat T cells bearing J-Luc-κB were treated with TNFα in the presence or absence of wogonin. After 8 hours of treatment, cells were collected and lysed in passive lysis buffer (Promega, Heidelberg, Germany). Luciferase activity was determined in 10 μL of cell lysates using the luciferase assay substrate (Promega) with a Duolumat LB9507 luminometer (Berthold, Bad Wildbad, Germany).
Results
Wogonin sensitizes malignant cells to TNFα- and TRAIL-induced apoptosis
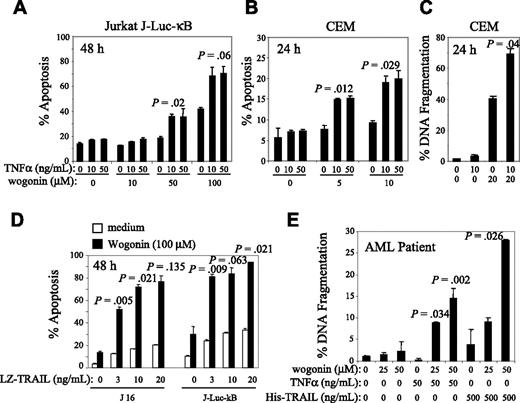
TNFα is toxic for a number of tumors; however, it also induces a profound inflammatory response through activation of NF-κB. Many tumor cells are resistant to TNFα largely due to NF-κB activation.12,14,15 Recently, we have found that wogonin can induce apoptotic cell death through the mitochondrial pathway in different malignant T-cell lines (M.L.-W., unpublished data, May 2006). Therefore, we asked whether wogonin could cooperate with the extrinsic (the receptor-mediated) pathway, such as the TNFα signaling pathway, to enhance killing of tumor cells. To address this question, malignant T-cell lines, the T-cell leukemia cell lines CEM and Jurkat bearing J-Luc-κB, were used as a test system to monitor apoptosis and NF-κB activity induced by TNFα and wogonin. Treatment of CEM and Jurkat cells with wogonin alone resulted in apoptotic cell death in a dose-dependent manner determined by both FSC/SSC and by DNA fragmentation (Figure 1A-C). At the concentration of 10 to 50 ng/mL, CEM and Jurkat T cells are resistant to TNFα-induced cell death (Figure 1A-C). However, treatment of these cells with a combination of TNFα and wogonin resulted in a synergistic (not only additive) increase in TNFα-induced cell death (Figure 1A-C). Thus, wogonin can sensitize TNFα-resistant cells to TNFα-induced apoptotic cell death.
To investigate whether wogonin can also sensitize other TNF-related ligands (eg, TRAIL-mediated cell death), we carried out experiments with a combination of wogonin and TRAIL. Similar to the combination of TNFα and wogonin, wogonin significantly enhanced TRAIL-induced apoptosis in 2 different Jurkat cell lines tested (Figure 1D). Treatment with 100 μM wogonin alone or with 10 to 20 ng/mL TRAIL alone resulted in 10% to 20% apoptotic cell death. However, in the combination of TRAIL and wogonin, 70% to 80% of cells underwent apoptosis. The experiments demonstrate that wogonin can also sensitize TRAIL-mediated apoptosis. To further investigate whether wogonin can enhance receptor-mediated apoptosis in primary tumor cells, AML cells freshly isolated from AML patients were subjected to the combination treatment. The AML cells were relatively resistant to TRAIL (approximately 4% cell death) and even showed less death when treated with TNFα. Treatment of the cells with the combination of wogonin either with TNFα or with TRAIL resulted in 8% to 15% and 9% to 28% death in 24 hours, respectively (Figure 1E). Thus, wogonin can sensitize TNFα- and TRAIL-resistant cells to TNFα- and TRAIL-induced cell death.
Wogonin neutralizes TNFα-induced free radical ·O2–
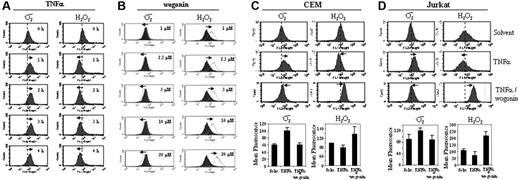
It is well known that reactive oxygen species (ROSs) can activate NF-κB. Several lines of evidence indicate that TNFα mediates its intracellular signaling through ROSs.39 The direct evidence that TNFα can induce ROSs came from more recent studies showing H2O2 accumulation in TNFα-treated fibroblasts.40-42 To investigate whether TNF receptor signaling triggers ROS production in malignant T lymphocytes, we monitored the redox status using the oxidation-sensitive fluorescent dyes, DCFDA (for H2O2) and DHE (for ·O2–) in J-Luc-κB cells treated with TNFα for different times. TNFα treatment resulted in production of ROSs in J-Luc-κB T cells. In contrast to the results previously found for fibroblasts, TNFα does not induce accumulation of H2O2 in Jurkat T cells. Instead, TNFα induces generation of the free radical ·O2– accompanied by reduction in endogenous H2O2 levels (Figure 2A). This observation indicates that TNFα can shift the cellular redox potential to a more oxidative state in Jurkat T cells.
Wogonin sensitizes malignant T cells to TNFα-induced apoptosis. (A) Jurkat cells bearing J-Luc-κB and (B-C) CEM cells were preincubated with different amounts of wogonin for 30 minutes and then treated with different doses of TNFα. Apoptotic cell death was quantified by either the apoptotic changes in cell size and granularity determined by FSC/SSC FACS analysis (A-B) or DNA fragmentation (C) determined by the Nicoletti assay. (D) J16 and J-Luc-κB were treated with 100 μM wogonin in combination with different doses of LZ-TRAIL for 48 hours. Apoptotic cell death was determined by FSC/SSC FACS analysis. (E) AML cells freshly isolated from an AML patient were treated with different combinations of wogonin and TNFα or wogonin and His-TRAIL for 24 hours. Apoptotic cell death was determined by the Nicoletti assay. Data are representative from 1 of 3 different AML patients. Error bars are SD of triplicate assays.
Wogonin sensitizes malignant T cells to TNFα-induced apoptosis. (A) Jurkat cells bearing J-Luc-κB and (B-C) CEM cells were preincubated with different amounts of wogonin for 30 minutes and then treated with different doses of TNFα. Apoptotic cell death was quantified by either the apoptotic changes in cell size and granularity determined by FSC/SSC FACS analysis (A-B) or DNA fragmentation (C) determined by the Nicoletti assay. (D) J16 and J-Luc-κB were treated with 100 μM wogonin in combination with different doses of LZ-TRAIL for 48 hours. Apoptotic cell death was determined by FSC/SSC FACS analysis. (E) AML cells freshly isolated from an AML patient were treated with different combinations of wogonin and TNFα or wogonin and His-TRAIL for 24 hours. Apoptotic cell death was determined by the Nicoletti assay. Data are representative from 1 of 3 different AML patients. Error bars are SD of triplicate assays.
Wogonin has been reported to possess antioxidant activity.17 To investigate whether wogonin can directly scavenge ROSs, we monitored the redox status in J-Luc-κB cells treated with different concentrations of wogonin. In contrast to TNFα, administration of wogonin resulted in a dose-dependent reduction in ·O2– levels accompanied by an increase in H2O2 levels (Figure 2B). Similar to Jurkat T cells, TNFα induces generation of ·O2– and simultaneously reduces the level of H2O2 in CEM T cells (Figure 2C). Thus, wogonin can shift the cellular redox equilibrium to a more reduced state, a reaction that mimics the function of the antiradical enzyme superoxide oxido reductase dismutase (SOD).
TNFα shifts H2O2 to ·O2–, and wogonin converts ·O2– to H2O2 in malignant T cells. (A) Jurkat J-Luc-κB cells were treated with 100 ng/mL TNFα for different times as indicated, and the redox status was monitored by the oxidation-sensitive fluorescent dyes for ·O2– and H2O2. (B) Jurkat J-Luc-κB cells were treated with different doses of wogonin as indicated. After 30 minutes, the redox status was measured as in panel A. Black profiles represent the baselines without wogonin treatment. Shifted ·O2– and H2O2 are indicated by the thin lines and arrows. (C-D) Wogonin neutralizes ·O2– generated by the TNFα treatment. CEM and Jurkat J-Luc-κB cells were treated with 100 ng/mL TNFα in the presence or absence of 50 μM wogonin. The ·O2– and H2O2 levels were measured after 2 hours of treatment by the oxidation-sensitive fluorescent dyes as in panel A. Data from panels C and D are representative of 3 and 4 independent experiments, respectively, and are also presented as bar charts below the profiles (error bars indicate SD of triplicate experiments.)
TNFα shifts H2O2 to ·O2–, and wogonin converts ·O2– to H2O2 in malignant T cells. (A) Jurkat J-Luc-κB cells were treated with 100 ng/mL TNFα for different times as indicated, and the redox status was monitored by the oxidation-sensitive fluorescent dyes for ·O2– and H2O2. (B) Jurkat J-Luc-κB cells were treated with different doses of wogonin as indicated. After 30 minutes, the redox status was measured as in panel A. Black profiles represent the baselines without wogonin treatment. Shifted ·O2– and H2O2 are indicated by the thin lines and arrows. (C-D) Wogonin neutralizes ·O2– generated by the TNFα treatment. CEM and Jurkat J-Luc-κB cells were treated with 100 ng/mL TNFα in the presence or absence of 50 μM wogonin. The ·O2– and H2O2 levels were measured after 2 hours of treatment by the oxidation-sensitive fluorescent dyes as in panel A. Data from panels C and D are representative of 3 and 4 independent experiments, respectively, and are also presented as bar charts below the profiles (error bars indicate SD of triplicate experiments.)
To investigate the effect of wogonin on TNF signaling, CEM and Jurkat J-Luc-κB cells were treated with TNFα in the presence or absence of wogonin. Interestingly, in the presence of wogonin, the TNFα-induced elevation of ·O2– is neutralized to almost normal levels in both CEM and Jurkat T cells (Figure 2C-D). These data demonstrate that wogonin can mimic SOD function to convert TNFα-induced ·O2– to the nonradical H2O2.
Wogonin attenuates TNFα-induced NF-κB activity
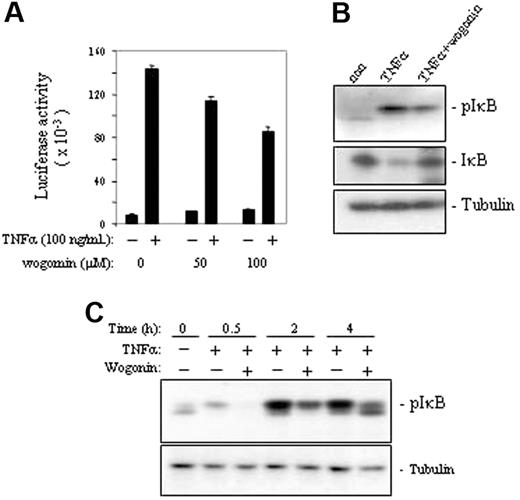
It is known that NF-κB can be activated by ROSs and that NF-κBis a negative regulator of TNFα-induced cell death. To investigate whether blocking TNFα-induced ·O2– by wogonin leads to down-regulation of NF-κB activity, we monitored the NF-κB activity by measuring the luciferase activity of the TNFα-treated J-Luc-κB cells in the absence or presence of wogonin. The experiments showed that wogonin attenuated TNFα-induced NF-κB activity in a dose-dependent manner (Figure 3A). Further investigation of the molecular mechanisms showed that wogonin prevented phosphorylation of IκB by TNFα and thereby blocked TNFα-induced degradation of IκB (Figure 3B-C). These experiments demonstrate that wogonin sensitizes TNFα-induced apoptosis by down-regulation of NF-κB activity.
Wogonin attenuates TNFα-induced NF-κB activity. (A) J-Luc-κB cells were treated with or without TNFα in the presence or absence of different amounts of wogonin as indicated. NF-κB activities were determined by luciferase assays after 8 hours of treatment. Data are representative of 3 independent experiments in triplicate assays. Error bars indicate SD. (B) Western analysis of TNFα-induced NF-κB activation in the presence and absence of 100 μM wogonin. (C) Kinetic analysis of wogonin-mediated suppression of TNFα-induced phosphorylation of IκB. J-Luc-κB cells were preincubated with 100 μM wogonin for 30 minutes and then stimulated with 50 ng/mL TNFα for 0.5 to 4 hours as indicated.
Wogonin attenuates TNFα-induced NF-κB activity. (A) J-Luc-κB cells were treated with or without TNFα in the presence or absence of different amounts of wogonin as indicated. NF-κB activities were determined by luciferase assays after 8 hours of treatment. Data are representative of 3 independent experiments in triplicate assays. Error bars indicate SD. (B) Western analysis of TNFα-induced NF-κB activation in the presence and absence of 100 μM wogonin. (C) Kinetic analysis of wogonin-mediated suppression of TNFα-induced phosphorylation of IκB. J-Luc-κB cells were preincubated with 100 μM wogonin for 30 minutes and then stimulated with 50 ng/mL TNFα for 0.5 to 4 hours as indicated.
ROSs are not involved in wogonin-induced apoptosis
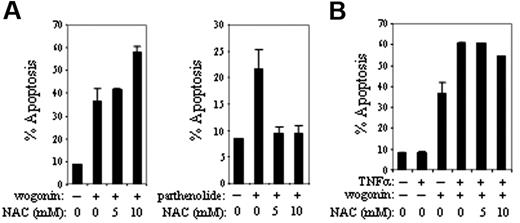
We observed that the levels of H2O2 were elevated in cells treated with wogonin. Normally, H2O2 is mostly degraded to H2O by glutathione (GSH) peroxidase and catalase. However, in the presence of transition metals H2O2 may give rise to a highly reactive hydroxyl radical (·OH) by the Fenton or the Haber-Weiss reaction.43 Therefore, elevation of H2O2 by wogonin might cause generation of ROSs and lead to apoptotic cell death. To investigate whether ROSs are involved in wogonin-induced apoptosis, cells were treated with wogonin in the presence or absence of the antioxidant N-acetyl-cysteine (NAC). Although wogonin can shift the redox status to produce more H2O2, NAC did not show any inhibitory effect on wogonin-induced cell death (Figure 4A, left). As a control, apoptosis induced by parthenolide, a compound that induces apoptosis via generation of ROSs,44 was blocked by NAC (Figure 4A, right). Also, no significant inhibitory effects of NAC were seen on wogonin/TNFα-induced apoptosis (Figure 4B). Thus, wogonin does not induce generation of ROSs to induce apoptosis.
Wogonin does not sensitize normal T cells to TNFα-induced apoptosis
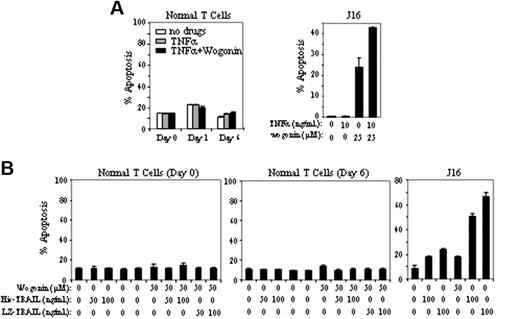
In contrast to malignant T cells, wogonin does not sensitize freshly isolated peripheral blood T cells to TNFα-(Figure 5A) or TRAIL-induced apoptosis (Figure 5B). Freshly isolated (resting) T cells (day 0) are known to be resistant toward activation-induced cell death (AICD), whereas T cells activated for several days in culture become sensitive toward AICD.35 To investigate whether T cells at different activation stages have different susceptibilities to wogonin/TNFα or wogonin/TRAIL treatment, peripheral blood T cells activated by PHA overnight (day 1) or further cultured for 5 days in IL-2–containing medium (day 6) were subjected to wogonin/TNFα or wogonin/TRAIL treatment. In all cases, no toxicity was seen in peripheral blood T cells. As control, wogonin elevated TNFα- and TRAIL-induced apoptosis in Jurkat cells (Figure 5). These results demonstrate that wogonin can selectively enhance TNFα- or TRAIL-induced apoptosis in malignant T cells.
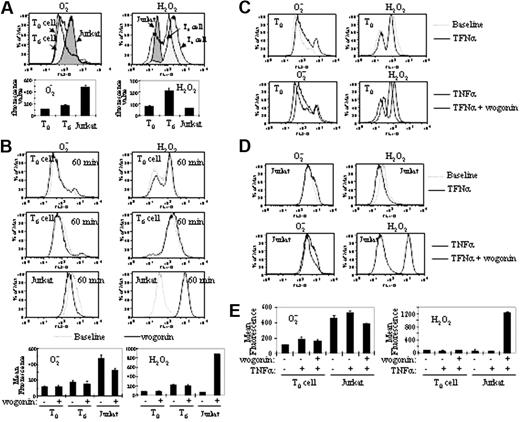
Wogonin has a stronger effect on the redox status of malignant T cells
Increasing evidence shows that tumor cells have altered redox regulation and produce ROSs at elevated rates in vitro and in vivo.45 To further investigate the mechanism by which wogonin selectively enhances malignant but not normal T cells to TNFα-induced apoptosis, we first compared the redox status of malignant with that of normal T cells. In agreement with other studies,45 malignant Jurkat T cells produce significantly higher levels of the free radical ·O2– accompanied by much lower levels of H2O2 than that of normal T cells (Figure 6A). Wogonin also reduces the ·O2– levels and shifts the cellular redox equilibrium to a more reduced state in normal T cells (Figure 6B). However, wogonin shows stronger shifts of ·O2– to H2O2 in the malignant Jurkat than in normal T cells (Figure 6B). We next investigated the redox status of malignant and normal T cells after treatment with a combination of TNFα and wogonin. TNFα treatment induces elevation of ·O2– and a decrease of H2O2 in both malignant and normal T cells (Figure 6C). Again, wogonin shows much stronger shifts of ·O2– to H2O2 in the malignant than in normal T cells (Figure 6D-E). Thus, wogonin has stronger influence on the redox status in malignant than in normal T cells.
Discussion
Resistance toward apoptosis is a key factor for survival of a malignant cell. Therefore, targeting the apoptotic pathway is one of the main strategies in anticancer therapy. Although TNFα shows broad cytotoxicity against a number of tumor cell lines, the clinical use of TNFα is limited because it induces a profound inflammatory response through activation of NF-κB. NF-κB also activates expression of various antiapoptotic genes that cause resistance to chemotherapy in many types of tumors.12-15,46 Thus, the decision between life and death in TNFα treatment is largely controlled by the activity of NF-κB. To overcome this problem, specific NF-κB inhibitors or drugs such as cycloheximide and actinomycin D, which block new protein synthesis, are usually used in combination with TNFα treatment to increase TNFα killing of tumor cells. However, these agents cause harmful side effects. Much effort has been devoted to search for agents that can specifically induce apoptosis in tumor cells, ideally with no or less toxicity to normal cells. Minimizing side effects and maximizing efficacy becomes a major goal in the development of apoptosis inducers. The principal active component of Huang-Qin, wogonin, has attracted our attention because it exerts cytotoxicity to several human cancer cell lines.30,31 In this study, we show that wogonin sensitizes TNFα-resistant malignant T cells toward TNFα-mediated apoptosis via attenuation of TNFα-induced NF-κB activity. Importantly, wogonin has no or little toxicity on peripheral blood mononuclear cells (Liu et al32 and our data). The wogonin-containing plant HuangQin is widely used in traditional Chinese medicine (TCM) to cure diseases associated with inflammation. Our data raise the potential to use wogonin as a sensitizer for TNFα anticancer therapy and to reduce the TNFα-induced inflammatory side effect.
NAC does not suppress wogonin-mediated apoptosis. (A) CEM cells were treated with either 20 μM wogonin or 5 μM parthenolide in the presence or absence of different concentrations of NAC as indicated. After 24 hours of treatment, apoptotic cells were quantified by FSC/SSC FACS analysis. (B) CEM cells were treated with a combination of TNFα (10 ng/mL) and wogonin (20 μM) in the presence or absence of NAC. Apoptotic cells were determined after 24 hours of treatment as described in panel A. Data show 1 representative experiment (in triplicate assays) of 3 independent experiments. Error bars indicate SD of triplicate assays.
NAC does not suppress wogonin-mediated apoptosis. (A) CEM cells were treated with either 20 μM wogonin or 5 μM parthenolide in the presence or absence of different concentrations of NAC as indicated. After 24 hours of treatment, apoptotic cells were quantified by FSC/SSC FACS analysis. (B) CEM cells were treated with a combination of TNFα (10 ng/mL) and wogonin (20 μM) in the presence or absence of NAC. Apoptotic cells were determined after 24 hours of treatment as described in panel A. Data show 1 representative experiment (in triplicate assays) of 3 independent experiments. Error bars indicate SD of triplicate assays.
Wogonin does not sensitize normal T cells to TNFα-induced apoptosis. (A) Freshly isolated peripheral blood T cells (day 0), T cells after 16 hours of PHA activation (day 1), or PHA-activated cells further cultured in the presence of IL-2 for 5 days (day 6) were treated with 100 ng/mL TNFα in the presence or absence of 100 μM wogonin. Apoptotic cell death was determined by FSC/SSC after 48 hours of treatment. Results in duplicate are representative of 3 healthy donors. Jurkat cells were treated with TNFα and wogonin as a control. (B) Day 0 and day 6 T cells were treated with wogonin in combination with different concentration of His- or LZ-TRAIL for 48 hours. Jurkat cells were treated with TRAIL and wogonin as a control. Data show 1 representative experiment (in triplicate assays) of 3 independent experiments. Error bars indicate SD of triplicate assays.
Wogonin does not sensitize normal T cells to TNFα-induced apoptosis. (A) Freshly isolated peripheral blood T cells (day 0), T cells after 16 hours of PHA activation (day 1), or PHA-activated cells further cultured in the presence of IL-2 for 5 days (day 6) were treated with 100 ng/mL TNFα in the presence or absence of 100 μM wogonin. Apoptotic cell death was determined by FSC/SSC after 48 hours of treatment. Results in duplicate are representative of 3 healthy donors. Jurkat cells were treated with TNFα and wogonin as a control. (B) Day 0 and day 6 T cells were treated with wogonin in combination with different concentration of His- or LZ-TRAIL for 48 hours. Jurkat cells were treated with TRAIL and wogonin as a control. Data show 1 representative experiment (in triplicate assays) of 3 independent experiments. Error bars indicate SD of triplicate assays.
The effects of wogonin on redox status of malignant and normal T cells. (A) Malignant Jurkat T cells produce ROSs at a greater level than normal T cells. The basal redox status of Jurkat, freshly isolated normal peripheral blood T cells (T0), or T cells further cultured for 6 days (T6) were monitored by the oxidation-sensitive fluorescent dyes for ·O2– and H2O2. The unfilled and light gray profiles are normal peripheral blood T0 and T6 cells, respectively. The dark gray profile is Jurkat T cells. The data are also presented as bar charts. (B) Wogonin has stronger effects on the redox status of malignant than normal T cells. T0,T6 and Jurkat T cells were treated with 50 μM wogonin for 60 minutes. The redox status was measured as in panel A. The data are also presented as bar charts. (C-D) Wogonin exerts stronger inhibition of TNFα-induced ROSs in malignant T cells. Normal peripheral blood T (T0) (C) and Jurkat T cells (D) were treated with 100 ng/mL TNFα in the presence or absence of 50 μM wogonin. The ·O2– and H2O2 levels were measured after 2 hours of treatment by the oxidation-sensitive fluorescent dyes as in Figure 2. (E) Data from panels C and D are compared by bar charts. The comparative analysis was carried out in the same experiment on the same day using the same instrument settings. Error bars indicate SD of triplicate experiments.
The effects of wogonin on redox status of malignant and normal T cells. (A) Malignant Jurkat T cells produce ROSs at a greater level than normal T cells. The basal redox status of Jurkat, freshly isolated normal peripheral blood T cells (T0), or T cells further cultured for 6 days (T6) were monitored by the oxidation-sensitive fluorescent dyes for ·O2– and H2O2. The unfilled and light gray profiles are normal peripheral blood T0 and T6 cells, respectively. The dark gray profile is Jurkat T cells. The data are also presented as bar charts. (B) Wogonin has stronger effects on the redox status of malignant than normal T cells. T0,T6 and Jurkat T cells were treated with 50 μM wogonin for 60 minutes. The redox status was measured as in panel A. The data are also presented as bar charts. (C-D) Wogonin exerts stronger inhibition of TNFα-induced ROSs in malignant T cells. Normal peripheral blood T (T0) (C) and Jurkat T cells (D) were treated with 100 ng/mL TNFα in the presence or absence of 50 μM wogonin. The ·O2– and H2O2 levels were measured after 2 hours of treatment by the oxidation-sensitive fluorescent dyes as in Figure 2. (E) Data from panels C and D are compared by bar charts. The comparative analysis was carried out in the same experiment on the same day using the same instrument settings. Error bars indicate SD of triplicate experiments.
Using free radical quenchers or lipid peroxidation inhibitors, several studies indicate that TNFα mediates its intracellular signaling through reactive oxygen intermediates.39 Recently, several reports described that TNFα can directly induce accumulation of H2O2 in fibroblasts.40-42 In those studies, H2O2 production, beginning 2 hours after TNFα treatment, progressively accumulated up to 12 hours after TNFα treatment.42 In contrast to those studies, we show that TNFα rapidly (less than 60 minutes) induces elevation of the free radical ·O2– in Jurkat and CEM T cells and simultaneously reduces the cellular basal levels of H2O2. The highest level of ·O2– was readily detected 1 hour after administration of TNFα, and this level was maintained for at least 4 hours. Thus, TNFα can shift the cellular redox potential to a more oxidative state (Figure 2A). However, treatment of cells with TNFα alone has little effect on cell death due to NF-κB activation.
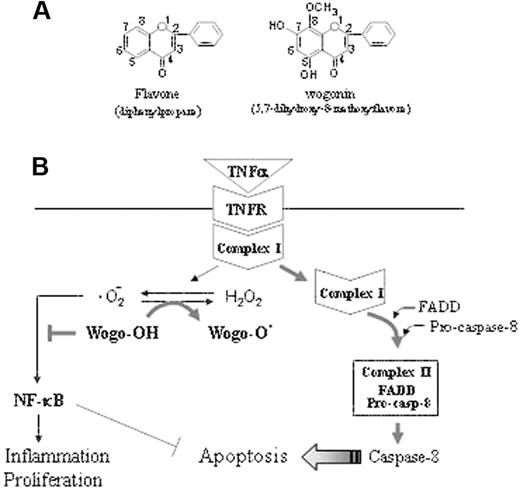
Schematic representation of the mechanism by which wogonin sensitizes TNFα-induced apoptosis. (A) The backbone of flavonoids. The activities of a flavonoid depend on the number of hydroxyl substitutions in its backbone structure. Wogonin contains 2 free 5,7,-OH groups. (B) The TNFR signaling pathway can either trigger activation of NF-κB crucial for TNFα-mediated immunity, inflammation, and proliferation or leads to programmed cell death. According to a recent model,2 TNFR signaling can be divided into 2 distinct stages that sequentially activate NF-κB and apical caspases. Within a few minutes after binding of TNFα to TNFR (stage 1), a signaling complex (complex I) containing the receptor itself and the adaptor proteins TRADD, TRAF2, and RIP1 but lacking the FAS/APO-1–associated death domain adaptor protein FADD forms and transduces signals that lead to activation of NF-κB and the JNK cascade. At later time points (stage 2), possibly after receptor internalization, RIP1, TRAF2, and TRADD dissociate from the receptor and recruit FADD and caspase-8 to form the second complex (complex II), which signals programmed cell death. Because of the long delay in the second complex assembly, NF-κB, activated at stage 1, has sufficient time to activate a variety of antiapoptotic factors that block the apoptotic pathway. We show that TNFα induces generation of ·O2– and shifts the cellular redox potential to a more oxidative state. Wogonin is able to transfer electron free radicals and thereby shift the cellular redox equilibrium to a more reduced state and thereby attenuate NF-κB activity and sensitize TNFα-induced apoptosis.
Schematic representation of the mechanism by which wogonin sensitizes TNFα-induced apoptosis. (A) The backbone of flavonoids. The activities of a flavonoid depend on the number of hydroxyl substitutions in its backbone structure. Wogonin contains 2 free 5,7,-OH groups. (B) The TNFR signaling pathway can either trigger activation of NF-κB crucial for TNFα-mediated immunity, inflammation, and proliferation or leads to programmed cell death. According to a recent model,2 TNFR signaling can be divided into 2 distinct stages that sequentially activate NF-κB and apical caspases. Within a few minutes after binding of TNFα to TNFR (stage 1), a signaling complex (complex I) containing the receptor itself and the adaptor proteins TRADD, TRAF2, and RIP1 but lacking the FAS/APO-1–associated death domain adaptor protein FADD forms and transduces signals that lead to activation of NF-κB and the JNK cascade. At later time points (stage 2), possibly after receptor internalization, RIP1, TRAF2, and TRADD dissociate from the receptor and recruit FADD and caspase-8 to form the second complex (complex II), which signals programmed cell death. Because of the long delay in the second complex assembly, NF-κB, activated at stage 1, has sufficient time to activate a variety of antiapoptotic factors that block the apoptotic pathway. We show that TNFα induces generation of ·O2– and shifts the cellular redox potential to a more oxidative state. Wogonin is able to transfer electron free radicals and thereby shift the cellular redox equilibrium to a more reduced state and thereby attenuate NF-κB activity and sensitize TNFα-induced apoptosis.
During aerobic metabolism, cells are constantly generating ROSs. Under physiologic conditions, ROSs, including free radicals such as ·O2–, hydroxyl radicals (·OH), and the nonradical H2O2, are a minor product of the mitochondrial oxidative respiratory chain. This occurs mostly in the form of ·O2–, which is metabolized to the nonradical H2O2 via a dismutation reaction catalyzed by the superoxide oxidoreductase dismutase (SOD), a cytoplasmic enzyme that defends against oxidative stress.47,48 Many flavonoids, depending on the number and position of OH groups in their backbone structure, are capable of transferring electron free radicals.49,50 Wogonin contains 2 free 5,7-OH groups (Figure 7A). We show that wogonin can scavenge ·O2– and shifts the cellular redox potential to a more reduced state (Figure 7B). In the presence of wogonin, attenuation of NF-κB activity and a synergistic increase in apoptotic cell death was seen in TNFα-treated malignant T-cell lines. Our data demonstrate that wogonin can promote TNFα-induced apoptosis via modulation of the cellular redox status. Interestingly, wogonin does not affect the redox status of normal T cells and therefore does not sensitize normal T cells to TNFα-induced apoptosis. In addition, the fact that wogonin suppresses TNFα-induced NF-κB activity indicates that it can reduce the proinflammatory side effects of TNFα.
Growing evidence indicates that ROSs can specifically activate certain intracellular signaling cascades and thus contribute to tumor development. It has been found that redox balance is impaired in cancer cells compared with normal cells.45 Human tumor cells have been shown to produce ROSs at a far greater rate than nontransformed cells.51,52 We also observed that Jurkat T cells produce much higher levels of the free radical ·O2– than normal T cells. In addition, increased expression or activity of the cellular antioxidant enzymes such as SOD, GSH peroxidase, catalase, peroxiredoxin, and malondialdehyde has been reported in tumor cell lines and tumor tissues.53 We show that wogonin can scavenge ·O2– in Jurkat and CEM tumor cells and shifts it to its breakdown product, H2O2, but has only a little effect on normal T cells. This may be explained by the fact that normal cells produce only very little ROSs and, therefore, only a small shift of redox status could be seen in normal T cells. In contrast, tumor cells produce abnormal higher levels of ROSs than normal cells and thus show strong influence by wogonin. This may explain why wogonin does not sensitize normal T cells to undergo TNFα-induced apoptosis.
Recently, it has been shown that TNFα may sensitize malignant cells to chemotherapeutic drugs in an NF-κB–independent manner via the mitochondrial apoptosis pathway.54 As shown in this study, wogonin alone can trigger malignant cells to undergo apoptosis. Wogonin induces cell death mainly through the mitochondrial pathway (M.L.-W., unpublished data, May 2006). Therefore, TNFα might also sensitize malignant cells to wogonin through an NF-κB–independent effect. Several studies indicate that TNFα-induced ROSs may promote TNFα-mediated cell death.41,42 In those studies, NF-κB activated by ROSs was demonstrated to up-regulate the antioxidant iron storage factor ferritin heavy chain, which in turn suppresses ROS accumulation and inhibits TNFα-induced apoptosis via prevention of ROS-induced sustained JNK activation.41 The role of ROSs in promoting TNFα-induced apoptosis was also demonstrated using NF-κB–deficient cells in which H2O2 production, beginning 2 hours after TNFα treatment, progressively accumulated up to 12 hours after TNFα treatment.42 However, because H2O2 accumulation occurred relatively late (after several hours) in those studies, it has been queried whether H2O2 accumulation is a cause or rather an effect of cell death.6 In our studies, we show that TNFα directly induces production of ·O2–. However, the TNFα-induced ROSs are not sufficient to induce cell death in malignant T cells.
In summary, we have shown that wogonin selectively sensitizes TNFα-induced apoptosis in malignant but not normal T cells. Wogonin is a potent antioxidant that can scavenge ROSs generated by TNFα treatment and can thereby down-regulate activation of NF-κB. Thus, wogonin enhances the cytotoxicity of TNFα on tumor cells and at the same time limits the proinflammatory effect of TNFα. In addition, wogonin significantly increases the toxicity of TRAIL to tumor cells. These data suggest that wogonin may serve as a TNFα or TRAIL adjuvant for cancer treatment.
Authorship
S.C.F., S.B., J.Y.Z., M.G., and M.K.T. performed research; U.M. contributed AML patient samples; P.H.K. analyzed data; and M.L.-W. designed research, analyzed data, and wrote the paper.
The authors declare no competing financial interests.
S.C.F., S.B., and J.Y.Z. contributed equally to this study.
Prepublished online as Blood First Edition Paper, August 24, 2006; DOI 10.1182/blood-2006-03-011973.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Dr T. Wirth for providing the Jurkat cells bearing the 3xkB-Luc reporter gene and Tobias L. Hass and Dr Henning Walczak for providing us LZ- and His-TRAIL.
This work was supported by the Deutsche Forschungsgemeinschaft, the Wilhelm Sander Stiftung, and the Tumor Center Heidelberg/Mannheim.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal