Abstract
All 3 hematopoietic GATA transcription factors, GATA-1, GATA-2, and GATA-3, are acetylated, although the in vivo role of this modification remains unclear. We examined the functions of an acetylation-defective mutant of GATA-1 in maturing erythroid cells. We found that removal of the acetylation sites in GATA-1 does not impair its nuclear localization, steady-state protein levels, or its ability to bind naked GATA elements in vitro. However, chromatin immunoprecipitation (ChIP) experiments revealed that mutant GATA-1 was dramatically impaired in binding to all examined cellular target sites in vivo, including genes that are normally activated and repressed by GATA-1. Together, these results suggest that acetylation regulates chromatin occupancy of GATA-1. These findings point to a novel function for transcription factor acetylation, perhaps by facilitating protein interactions required for stable association with chromatin templates in vivo.
Introduction
GATA transcription factors regulate distinct steps during the development of the hematopoietic system. GATA-1 is expressed in erythroid cells, megakaryocytes, mast cells, and eosinophil granulocytes where it controls cellular functions both by activating and repressing gene transcription.1-3 GATA-1 contains 2 highly conserved zinc fingers. The C-terminal zinc finger mediates DNA binding to typical GATA elements (WGATAR). The N-terminal zinc finger stabilizes binding to DNA and is required for GATA-1 binding to more complex palindromic GATA sites.
GATA-1 undergoes multiple posttranslational modifications, including phosphorylation, sumoylation, and acetylation. Acetylation of GATA-1 by the acetyltransferases CBP and p300 occurs predominantly at clustered lysine residues that reside at the C-terminal tails of both zinc fingers. These clusters are conserved among human, mouse, chicken, and zebra fish GATA-1 and also among GATA-2 and GATA-3 of various species.4-7 Additional, less prominent acetylation sites have been observed.4,5,8 How acetylation regulates GATA-1 function is unclear. Although acetylation of chicken GATA-1 is reported to strongly increase its affinity for DNA in vitro,4 no effects on DNA binding were observed upon acetylation of mouse GATA-1.5 Although in vitro acetylation of zebra fish GATA-1 slightly stimulated DNA binding,8 this did not appear to involve the conserved lysines near the zinc fingers, implying that additional acetylation sites contribute to these effects. Similarly, acetylation of GATA-2 by p300 moderately increased DNA binding but required the presence of multiple lysines, including those distinct from the lysines next to the C-terminal zinc finger.6 These data suggest that acetylation of GATA-1 and GATA-2 might exert mild effects on DNA binding in vitro when multiple lysines are modified. These subtle effects, if any, on DNA binding are in stark contrast to the substantial effects of acetylation site mutations on GATA-1 activity in erythroid cells in vivo,5 suggesting that acetylation might serve functions other than directly modulating the affinity of GATA-1 for DNA. Here, we show that acetylation of GATA-1 is dispensable for DNA binding in vitro but essential for binding to chromatin in vivo.
Materials and methods
Cell cultures and viral infections
G1E cells were cultured as described.9 Cells were exposed to 1 μM tamoxifen where indicated.
Alanine substitutions were introduced at the acetylated lysine residues (underlined) of GATA-1 near the N-terminal zinc finger (RPKKRMI) and the C-terminal zinc finger (SGKGKKKRGS). GATA-1 fused to the ligand-binding domain of the estrogen receptor (GATA-1–ER) or an acetylation-defective mutant of GATA-1(NC)–ER were introduced into MIG-R1 containing an IRES element followed by the GFP gene. Cells were infected as described9 and selected for GFP expression by FACS.
ChIP assay
The chromatin immunoprecipitation (ChIP) assay was performed exactly as described10 and analyzed by real-time PCR on an ABI Prism 7000 or 7900 system (AME Bioscience, Foster City, CA). Antibodies included anti–GATA-1 (N6; Santa Cruz Biotechnology, Santa Cruz, CA); and anti-ER (Ab10; Lab Vision Neomarkers, Fremont, CA). All primer sequences are described in Document S1 (available at the Blood website; see the Supplemental Materials link at the top of the online article).
Additional methods
Gel mobility shift assays and indirect immunofluorescence microscopy were performed according to standard protocols.
Results and discussion
G1E erythroid cells that lack GATA-1 are arrested at the proerythroblast stage, but they mature upon activation of stably expressed GATA-1 fused to the ligand-binding domain of the estrogen receptor (GATA-1–ER).9 To generate a GATA-1 mutant that is defective for acetylation, we substituted the acetylated lysine residues near each of the 2 zinc fingers with alanines (GATA-1(NC)–ER). These mutations markedly reduce GATA-1 acetylation in vitro and in vivo.5 Constructs were introduced into G1E cells via a retroviral vector containing the GFP cDNA. GFP-positive pools of cells were found to contain similar amounts of GATA-1–ER and GATA-1(NC)–ER (Figure S1A), suggesting that both proteins have comparable stability. Results shown here derive from pools of cells although individual clones produced the same results (not shown).
Equal DNA binding by GATA-1–ER and acetylation defective GATA-1(NC)–ER. EMSA with an α-globin–derived GATA site with nuclear extracts from G1E cells expressing GATA-1–ER and GATA-1(NC)–ER. As control, extracts from mouse erythroleukemia (MEL) cells and parental G1E cells were included. Anti–GATA-1 antibodies were added where indicated.
Equal DNA binding by GATA-1–ER and acetylation defective GATA-1(NC)–ER. EMSA with an α-globin–derived GATA site with nuclear extracts from G1E cells expressing GATA-1–ER and GATA-1(NC)–ER. As control, extracts from mouse erythroleukemia (MEL) cells and parental G1E cells were included. Anti–GATA-1 antibodies were added where indicated.
Because the GATA-1 acetylation sites conform to nuclear localization sequences, we examined whether GATA-1(NC)–ER accumulates normally in the nucleus by immunofluorescence microscopy. GATA-1(NC)–ER and GATA-1–ER exhibited the same nuclear staining intensity with little cytoplasmic signal (Figure S1B). GATA-1(NC)–ER failed to induce maturation of G1E cells and did not induce or repress any of the examined target genes (Figure S2).
Parallel electrophoretic mobility shift assays (EMSAs) and ChIP experiments were performed to compare in vitro DNA binding and in vivo occupancy of GATA-1 constructs. When assayed by EMSA with the TGATAA element from the murine α-globin promoter, both GATA-1–ER and GATA-1(NC)–ER bound with equal avidity (Figure 1), consistent with our previous findings.5 To examine whether potential differences in binding affinity might be revealed in the context of select nucleotides flanking the core GATA sequence, we examined DNA binding to probes containing the sequences TGATAG, AGATAA, and AGATAG that are found at certain GATA-1 target genes. No differences in DNA binding were observed between GATA-1–ER and GATA-1(NC)–ER at any of these sites (Figure S3). The residues near the C-terminal zinc finger that are targeted for acetylation do not make direct contact with DNA11 and fall outside the basic region that plays a role in discriminating between distinct GATA elements.12 This might explain why mutation or acetylation of these residues has no effect on DNA binding.
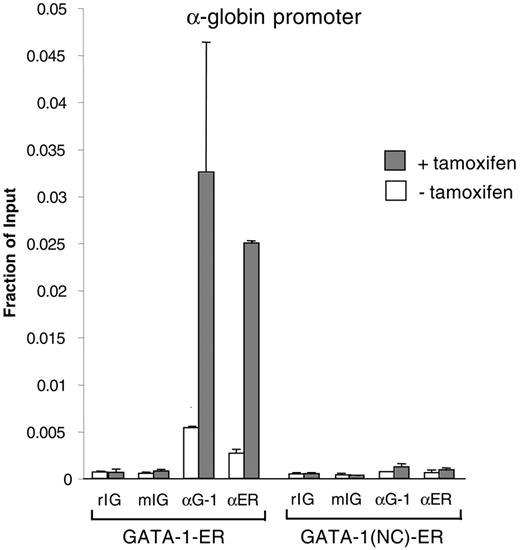
In vivo occupancy of GATA-1 at its native targets was measured by quantitative ChIP with primers for the α-globin promoter. Although GATA-1–ER occupancy was high when compared with controls, GATA-(NC)–ER was virtually undetectable (Figure 2). Similar results were obtained at several additional GATA-1 targets, including the β-major, Fog1, Band3, Ahsp, and Eklf genes, and HS3 of the LCR (Figure S4A-F). We also considered the possibility that the determinants for GATA-1 association with its targets might be different at genes that are silenced by GATA-1.13,14 Thus, we examined GATA-1 occupancy at GATA elements of the Gata2 and c-Kit genes, both of which are repressed by GATA-1. Again, GATA-1(NC)–ER failed to bind to these genes (Figure S4G-H).
Negative ChIP experiments need to be viewed with caution because disparate crosslinking efficiencies or epitope exposure between wild-type and mutant GATA-1 constructs might account for differences in detectability. Thus, we performed ChIP experiments with antibodies against the ER portion of the GATA-1 fusion proteins and obtained the same results (Figure 2; Figure S4A-H).
Acetylation of transcription factors has been shown to influence DNA binding, nuclear localization, protein stability, and protein-protein interactions.15 The present work suggests that mutations at the acetylation sites disrupt stable association with chromatin. Note that other mutations in GATA-1 that do not affect the residues required for DNA binding also show diminished chromatin occupancy to some sites in vivo. These include mutations that impair binding to FOG-113,14 and select deletions outside the zinc finger domain of GATA-1 (Kirby D. Johnson, Shin-Il Kim, and Emery H. Bresnick, manuscript submitted). This suggests that protein-protein interactions are important to stabilize GATA-1 at its target sites perhaps through the formation of a stable enhanceosome-like structure.16 Disruption of any single protein interaction might destabilize the enhanceosome, leading to diminished association of GATA-1 with its target site in vivo. We speculate that acetylation of GATA-1 promotes its association with one or more proteins critical for enhanceosome stability. Candidate proteins for acetylation-dependent GATA-1 binding include chromatin-modifying enzymes such as CBP/p300, which contain an acetyllysine binding bromodomain.17 This might increase the stability of the GATA-1–chromatin interaction by linking it to other DNA-binding proteins. Alternatively, these interactions might increase access of GATA-1 to chromatinized targets by directly regulating the fluidity of chromatin.
Mutations of residues that included the acetylation sites near the C-terminal zinc finger of zebra fish GATA-1 reduced self-association and transcriptional activity.8 This finding was interpreted to suggest that self-association is required for GATA-1 activity in vivo. However, on the basis of the present work, it is possible that loss of chromatin occupancy caused the reported loss of GATA-1 activity. More generally, our findings emphasize that binding of transcription factors to naked DNA in vitro and chromatinized templates in vivo are functionally distinct and can be uncoupled by mutations.
GATA-1(NC)–ER fails to bind GATA-1 target genes in vivo. ChIP assay using anti–GATA-1 (αG-1) and anti-ER (αER) antibodies and control rat and mouse IgG (rIG and mIG). Error bars indicate SD.
GATA-1(NC)–ER fails to bind GATA-1 target genes in vivo. ChIP assay using anti–GATA-1 (αG-1) and anti-ER (αER) antibodies and control rat and mouse IgG (rIG and mIG). Error bars indicate SD.
Authorship
G.A.B., J.M.L., and C.R.V. designed the research and prepared the manuscript; and J.M.L. and C.R.V. performed the research.
The authors declare no competing financial interests.
J.M.L. and C.R.V. contributed equally to this study.
Prepublished online as Blood First Edition Paper, August 3, 2006; DOI 10.1182/blood-2006-07-032847.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Mitchell Weiss and Emery Bresnick for critical comments on the manuscript.
This work was supported by National Institutes of Health grants DK58044 and DK54937 (G.A.B.).


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal