Abstract
The cardiovascular safety of COX-2 selective and nonselective nonsteroidal anti-inflammatory drugs (NSAIDs) has recently been called into question. The factors that predispose to adverse events by NSAIDs are unknown. Because patients with arthritis have decreased nitric oxide (NO) bioavailability, the in vivo effects of NSAIDs on murine vascular tone and platelet activity in the presence or absence of NO were examined. Here, we show that acute hypertensive and prothrombotic activities of the COX-2–selective inhibitor celecoxib are revealed only after in vivo inhibition of NO generation. The nonselective NSAID indomethacin was hypertensive but antithrombotic when NO was absent. In vitro myography of aortic rings confirmed that vasoconstriction required inhibition of both NOS and COX-2 and was abolished by supplementation with exogenous NO. These data indicate that NO suppresses vascular side effects of NSAIDs, suggesting that risk will be greatest in patients with impaired vascular function associated with decreased NO bioavailability.
Introduction
Recently, concerns were raised over elevated cardiovascular risks following administration of selective COX-2 inhibitors and nonselective NSAIDs.1-9 However, factors that interact with COX and modulate risk of adverse events are currently unknown. Prostacyclin (PGI) synthesis is elevated in patients with cardiovascular disease and arthritis.10-13 Also, decreased large-vessel NO bioactivity is observed.11,14-18 Indeed, because of the lack of NO, it is possible PGI may play an even more important role in maintaining vascular homeostasis and preventing adverse events in these groups than in healthy subjects. This led us to hypothesize that the ability of NSAIDs to mediate undesirable vascular events would be revealed or magnified in the absence of NO. In support, previous studies have found multiple complex interactions between NO and COX, including studies showing that NO inhibition can alter PGI signaling, consistent with this hypothesis.19-23 In this study, we examined acute effects of NSAID administration in healthy mice in vivo, with or without simultaneous NO blockade, specifically to examine whether NO influenced the ability of NSAIDs to mediate vascular side effects. The results suggest that NO bioactivity may be a determinant of susceptibility to adverse events of NSAIDs in patients with inflammatory diseases.
Materials and methods
Animal studies
Isometric tension functional studies
Male mice (10-12 weeks old) were killed by cervical dislocation. The thoracic aorta was dissected, cut into rings (2-3 mm), and suspended in an isometric tension myograph (DMT, Aarhuis, Denmark) containing Krebs buffer at 37°C and gassed with 5% CO2/95% O2. Cumulative concentration-response curve to phenylephrine (1 nM-1 μM) or acetylcholine (1 nM-10 μM) were constructed with or without 300 μM L-nitroarginine-methyl ester (L-NAME), 30 μM diethyenetriamineNONOate (DETA NONOate), 10 μM celecoxib, 10 μM indomethacin, or 100 μM aspirin. In some experiments, endothelium was removed by gentle rubbing before myography. Responses were expressed as percentage of baseline tension (vasoconstriction) or contracted tension (vasodilation). Responses from 3 to 4 rings of each animal were combined to produce an average.
Hypertension
Male 10- to 12-week-old wild-type C57BL/6 mice were administered L-NAME (100 mg/kg per day in drinking water) with or without celecoxib (400 mg/kg per day in chow) or indomethacin (6 mg/L in drinking water). Systolic blood pressure was monitored daily for 3 days before drug administration (training) and 6 days after drug administration by tail cuff plethysmography (World Precision Instruments, Hertfordshire, United Kingdom) in unanesthetized mice.
Whole-blood FACS analysis of platelet P-selectin expression
Mice were killed at day 3 after drug administration, and whole blood was collected as described.26 Antibody (5 μL; anti–P-selectin-FITC; Emfret Analytics, Heidelberg, Germany), anti–mouse αIIb-FITC or rat IgG1-FITC (Santa Cruz Biotechnology, Santa Cruz, CA) was added to 26 μL diluted blood and incubated 15 minutes at room temperature, before fluorescence-activated cell sorting (FACS) analysis. Platelets were identified based on forward and side-scatter characteristics and αIIb expression, then P-selectin expression was determined on the gated αIIb-positive platelet population.26
Immunohistochemistry of COX-2
Aortic ring sections (10 μm) were methanol fixed, permeabilized using 0.1% (wt/vol) Triton X-100/PBS, blocked using 1% (wt/vol) bovine serum albumin/PBS. COX-2 was visualized using goat anti–COX-2 (Santa Cruz Biotechnology) and anti–goat IgG-Alexa 568. Negative controls used equivalent concentrations of isotype control IgG. Images were acquired using a × 10 air lens, with excitation at 568 nM and emission 595/35 nM.
GC/MS determination of TX and PGI metabolites in urine
Mice were administered celecoxib or L-NAME (doses as above, under “Hypertension”) with 24-hour urine collections on day 3. Metabolites were quantified using a precise and accurate gas chromatography–mass spectrometry (GC/MS)/stable isotope dilution method.27
Results and discussion
Celecoxib and indomethacin mediate vasoconstriction in vivo, when NO generation is inhibited
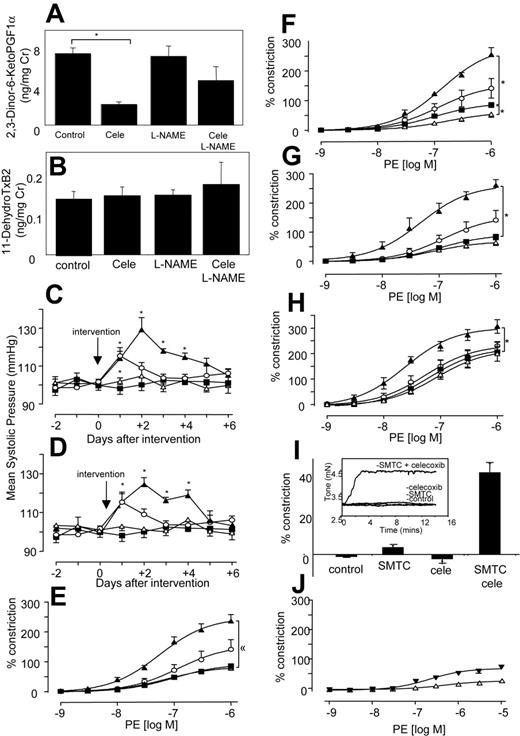
Because elevated blood pressure has been reported as a side effect of NSAIDs, even as early as a month for some selective COX-2 inhibitors, we determined the effect of COX inhibitors with and without NO blockade in vivo in healthy mice.7-9,28 Oral celecoxib (400 mg/kg per day) reduced urinary excretion of the COX-2–derived PGI metabolite 2,3-dinor-6-KetoPGF1α by 71% by C57BL/6 mice, and having no effect on the COX-1–derived thromboxane metabolite 11-dehydroTxB2 (Figure 1A-B), confirming selective inhibition of COX-2 at this dose.29,30 There was no effect of L-NAME alone on excretion of either metabolite. This indicates that NO does not directly modulate COX-1 or -2 turnover in the vasculature. However, L-NAME appeared to partially blunt the inhibitory effect of celecoxib on COX-2, through an unknown mechanism. Celecoxib alone did not alter systolic blood pressure (BP) (Figure 1C). In contrast, L-NAME increased BP acutely, peaking at day 1 (Figure 1C). However, when L-NAME was given together with celecoxib, the resulting BP elevation was significantly higher and more prolonged than for L-NAME alone (Figure 1C). This indicates that the removal of NO reveals a prohypertensive action of celecoxib, which is not detected in healthy mice. Next, BP was measured following indomethacin administration at a dose that blocks excretion of both TX and PGI urinary metabolites by greater than 70%.31 Indomethacin did not raise BP, but, in combination with L-NAME, the resulting elevation was significantly higher and more prolonged than for L-NAME alone (Figure 1D). Importantly, BP elevations for celecoxib + L-NAME or indomethacin + L-NAME were not significantly different, indicating that both drugs had identical effects on tone. These elevations in BP were acute and peaked at day 3, then decreased thereafter. These are otherwise healthy mice, and so may overcome the hypertensive effects of NSAIDs over a period of days, by inducing alternative vasorelaxant pathways (eg, endothelium-derived hyperpolarizing factor). These studies show that celecoxib + indomethacin are without effect on basal blood pressure in healthy mice; however, they significantly increase the vasoconstriction mediated by inhibition of vascular NO generation.
Myography studies show that COX-2 inhibition is vasoconstrictive in the absence of NO
To determine whether effects of NSAIDs were due to COX inhibition, in vitro studies examined agonist-induced constriction and relaxation in isolated murine aortic rings. For all NSAIDs, a significant enhancement of phenylephrine-induced constriction was found, but only with L-NAME present (Figure 1E-G).
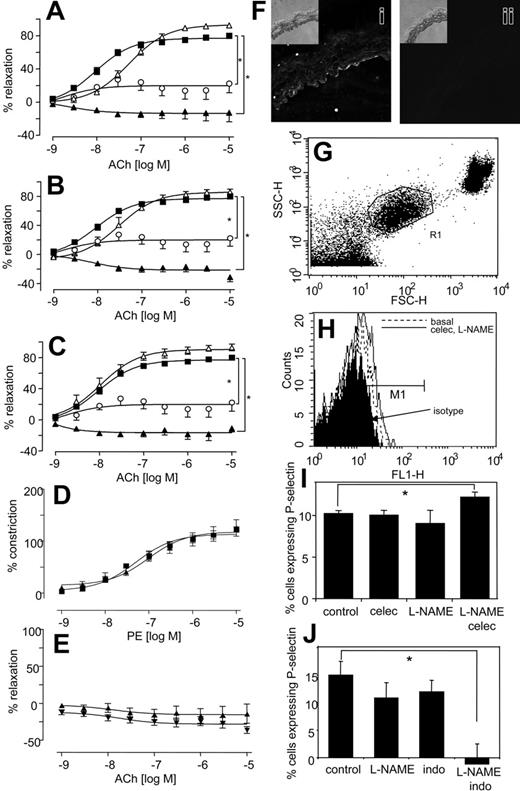
Similarly, aortic rings from COX-2–/– mice also constricted significantly more than wild-type mice when NO generation was blocked (Figure 1H). In separate experiments, it was found that incubation of aortic rings with a more potent NOS inhibitor, S-methyl-L-thiocitrulline (SMTC),32 led to a spontaneous and immediate increase in tone, but only when celecoxib was present (Figure 1I). Addition of an NO donor (DETA NONOate), generating NO at low levels that inhibited L-NAME–induced vasoconstriction, totally abolished the vasoconstrictive effect of celecoxib. Indeed, in the presence of exogenous NO, celecoxib mediated some vasorelaxation (Figure 1J). Although we do not know the molecular mechanism for this, it may include generation of vasoconstrictive prostanoids, such as PGF2α, with suppression of prostacyclin bioactivity by NO.33 Importantly, it suggests the therapeutic potential of prescribing NO donors, or NO-donating NSAID prodrugs in situations in which NO bioactivity is compromised. Finally, all NSAIDs increased the inhibitory activity of L-NAME on acetylcholine-induced relaxation (Figure 2A-C), while having little or no effect in its absence. The vascular expression of COX-2 was examined using immunohistochemistry and myography of endothelium-denuded rings. Predominant staining of COX-2 in endothelium (although some appears to also be localized to smooth muscle) and attenuation of the vasoconstrictive effect of celecoxib following endothelial removal indicate that COX-2 in aorta is largely endothelial (Figure 2D-F). In contrast, COX-1 expression was not detected in aorta of wild-type mice (not shown). In aggregate, these data indicate that the vasoconstrictive effects of NSAIDs revealed in the absence of NO most likely result from their action as COX-2 inhibitors.
Systolic blood pressure and vasoconstriction is significantly elevated in mice given both L-NAME and NSAIDs. (A) In vivo inhibition of COX-2 using celecoxib. Twenty-four–hour urine samples were obtained from mice administered celecoxib ± L-NAME for 3 days and analyzed using GC/MS for PGI metabolite (2,3-Dinor-6-KetoPGF1α) urinary excretion. (B) No inhibition of COX-1 in vivo using celecoxib. Twenty-four–hour urine samples were obtained as for panel A and analyzed using GC/MS for TX urinary metabolite (11-dehydroTxB2) urinary excretion (for both panels, n = 3-4 cages per group with 3 mice per cage, mean ± SEM). (C) Blood pressure elevations following celecoxib and L-NAME administration. Ten- to 12-week-old male C57BL/6 mice were administered L-NAME, celecoxib, or both. Systolic blood pressure was monitored daily. ▪, control mice; ○, L-NAME; ▵, celecoxib; ▴, celecoxib and L-NAME. (D) Blood pressure elevations following indomethacin and L-NAME administration. Ten- to 12-week-old male C57BL/6 mice were administered L-NAME, indomethacin, or both. Systolic blood pressure was monitored daily. ▪ indicates control mice; ○, L-NAME; ▵, indo; ▴, indo and L-NAME (for all blood pressure determinations, n = 5 animals per group, mean ± SEM, *P < .05 compared with day 0; using ANOVA test with Dunnett Post-Hoc Test to isolate differences). (E) Effect of celecoxib on in vitro constriction dose response. Aortic ring constriction responses to phenylephrine were determined (n = 5-9 per group). Control (▪), L-NAME (300 μM; ○), celecoxib (10 μM; ▵), or L-NAME (300 μM) and celecoxib (10 μM; ▴). (F) Effect of indomethacin on constriction dose response. Aortic ring constriction responses to phenylephrine were determined (n = 5-9 per group). Control (▪), L-NAME (300 μM; ○), indomethacin (10 μM; ▵), or L-NAME (300 μM) and indomethacin (10 μM; ▴) (G) Effect of aspirin on constriction dose response. Aortic ring constriction responses to phenylephrine were determined (n = 5-9 per group). Control (▪), L-NAME (300 μM; ○), indomethacin (10 μM; ▵), or L-NAME (300 μM) and aspirin (100 μM; ▴). (H) Effect of COX-2 deletion on constriction dose response. Aortic ring constriction responses to phenylephrine were determined (n = 5-9 per group). WT (▪), COX2–/– (○), WT + L-NAME (300 μM; ▵), and COX-2–/– + L-NAME (300 μM; ▴). (I) Aortic rings spontaneously constrict in the presence of SMTC and celecoxib. Aortic ring tone was determined using myography after 15 minutes of incubation (n = 4-6 per group) with SMTC (100 μM) with or without celecoxib (10 μM). Inset shows representative trace from myograph. (J) Exogenous NO abolishes the vasoconstrictive effect of celecoxib. Aortic ring constriction responses to phenylephrine were determined (n = 6 per group). WT + L-NAME (300 μM) + DETA NONOate (30 μM; ▾)WT + L-NAME + DETA NONOate + celecoxib (10 μM; ▴). For all aortic ring myography scans, data are expressed as mean ± SEM. *P < .05 compared with WT group, using 2-way ANOVA to isolate differences between groups.
Systolic blood pressure and vasoconstriction is significantly elevated in mice given both L-NAME and NSAIDs. (A) In vivo inhibition of COX-2 using celecoxib. Twenty-four–hour urine samples were obtained from mice administered celecoxib ± L-NAME for 3 days and analyzed using GC/MS for PGI metabolite (2,3-Dinor-6-KetoPGF1α) urinary excretion. (B) No inhibition of COX-1 in vivo using celecoxib. Twenty-four–hour urine samples were obtained as for panel A and analyzed using GC/MS for TX urinary metabolite (11-dehydroTxB2) urinary excretion (for both panels, n = 3-4 cages per group with 3 mice per cage, mean ± SEM). (C) Blood pressure elevations following celecoxib and L-NAME administration. Ten- to 12-week-old male C57BL/6 mice were administered L-NAME, celecoxib, or both. Systolic blood pressure was monitored daily. ▪, control mice; ○, L-NAME; ▵, celecoxib; ▴, celecoxib and L-NAME. (D) Blood pressure elevations following indomethacin and L-NAME administration. Ten- to 12-week-old male C57BL/6 mice were administered L-NAME, indomethacin, or both. Systolic blood pressure was monitored daily. ▪ indicates control mice; ○, L-NAME; ▵, indo; ▴, indo and L-NAME (for all blood pressure determinations, n = 5 animals per group, mean ± SEM, *P < .05 compared with day 0; using ANOVA test with Dunnett Post-Hoc Test to isolate differences). (E) Effect of celecoxib on in vitro constriction dose response. Aortic ring constriction responses to phenylephrine were determined (n = 5-9 per group). Control (▪), L-NAME (300 μM; ○), celecoxib (10 μM; ▵), or L-NAME (300 μM) and celecoxib (10 μM; ▴). (F) Effect of indomethacin on constriction dose response. Aortic ring constriction responses to phenylephrine were determined (n = 5-9 per group). Control (▪), L-NAME (300 μM; ○), indomethacin (10 μM; ▵), or L-NAME (300 μM) and indomethacin (10 μM; ▴) (G) Effect of aspirin on constriction dose response. Aortic ring constriction responses to phenylephrine were determined (n = 5-9 per group). Control (▪), L-NAME (300 μM; ○), indomethacin (10 μM; ▵), or L-NAME (300 μM) and aspirin (100 μM; ▴). (H) Effect of COX-2 deletion on constriction dose response. Aortic ring constriction responses to phenylephrine were determined (n = 5-9 per group). WT (▪), COX2–/– (○), WT + L-NAME (300 μM; ▵), and COX-2–/– + L-NAME (300 μM; ▴). (I) Aortic rings spontaneously constrict in the presence of SMTC and celecoxib. Aortic ring tone was determined using myography after 15 minutes of incubation (n = 4-6 per group) with SMTC (100 μM) with or without celecoxib (10 μM). Inset shows representative trace from myograph. (J) Exogenous NO abolishes the vasoconstrictive effect of celecoxib. Aortic ring constriction responses to phenylephrine were determined (n = 6 per group). WT + L-NAME (300 μM) + DETA NONOate (30 μM; ▾)WT + L-NAME + DETA NONOate + celecoxib (10 μM; ▴). For all aortic ring myography scans, data are expressed as mean ± SEM. *P < .05 compared with WT group, using 2-way ANOVA to isolate differences between groups.
NSAIDs enhance the inhibitory effect of L-NAME on aortic ring relaxation to acetylcholine, and platelet P-selectin expression is significantly elevated in mice given both L-NAME and celecoxib but decreased following L-NAME and indomethacin. (A) Effect of celecoxib on L-NAME inhibition of relaxation. Aortic ring relaxation responses to acetylcholine were determined. Control (▪), L-NAME (300 μM; ○), celecoxib (10 μM; ▵), or L-NAME (300 μM) and celecoxib (10 μM; ▴). (B) Effect of indomethacin on L-NAME inhibition of relaxation. Aortic ring relaxation responses to acetylcholine were determined. Control (▪), L-NAME (300 μM; ○), indomethacin (10 μM; ▵), or L-NAME (300 μM) and indomethacin (10 μM; ▴). (C) Effect of aspirin on L-NAME inhibition of relaxation. Aortic ring relaxation responses to acetylcholine were determined. Control (▪), L-NAME (300 μM; ○), aspirin (10 μM;▵), or L-NAME (300 μM) and aspirin (10 μM;▴). *P < .05 compared with wild-type group, using 2-wayANOVAto isolate differences between groups. For all experiments, n = 5-9 per group, mean ± SEM. (D) Effect of celecoxib on constriction of endothelium-denuded rings. Aortic ring constriction responses to phenylephrine were determined (n = 5-9 per group). WT denuded (▪), WT denuded + celecoxib (10 μM; ▴). (E) Effect of celecoxib on relaxation of endothelium-denuded rings. Aortic ring relaxation responses to acetylcholine were determined (n = 5-9 per group). WT denuded (▴), WT denuded + celecoxib (10 μM; ▾). (F) Immunohistochemistry of COX-2 in aortic rings. Rings were stained using anti–COX-2 (i) or isotype control IgG (ii). Inset shows corresponding transmission images. (G) Identification of platelets in murine whole blood. Mice were administered L-NAME, indomethacin, or both or celecoxib for 3 days. Platelets were gated in mouse whole blood by FACS analysis using anti–mouse αIIβ-FITC (shown as R1). (H) Representative data showing P-selectin expression following 3 days' administration with celecoxib and L-NAME. Platelets identified by αIIβ expression as in panel G were analyzed for P-selectin expression using anti–mouse P-selectin–FITC, or isotype rat anti–IgG-FITC. (I) Platelet P-selectin expression is significantly increased on day 3 following coadministration of celecoxib and L-NAME. Percentage of P-selectin–expressing cells were defined as those in M1, shown on Panel B with subtraction of percentage of isotype values from all samples. (J) Platelet P-selectin expression is significantly decreased on day 3 following coadministration of indomethacin and L-NAME. Percentage of P-selectin–expressing cells were defined as those in M1, shown on Panel B with subtraction of percentage of isotype values from all samples (for both panels, n = 5, mean ± SEM, *P < .05 compared with control using Student 2-tailed t test).
NSAIDs enhance the inhibitory effect of L-NAME on aortic ring relaxation to acetylcholine, and platelet P-selectin expression is significantly elevated in mice given both L-NAME and celecoxib but decreased following L-NAME and indomethacin. (A) Effect of celecoxib on L-NAME inhibition of relaxation. Aortic ring relaxation responses to acetylcholine were determined. Control (▪), L-NAME (300 μM; ○), celecoxib (10 μM; ▵), or L-NAME (300 μM) and celecoxib (10 μM; ▴). (B) Effect of indomethacin on L-NAME inhibition of relaxation. Aortic ring relaxation responses to acetylcholine were determined. Control (▪), L-NAME (300 μM; ○), indomethacin (10 μM; ▵), or L-NAME (300 μM) and indomethacin (10 μM; ▴). (C) Effect of aspirin on L-NAME inhibition of relaxation. Aortic ring relaxation responses to acetylcholine were determined. Control (▪), L-NAME (300 μM; ○), aspirin (10 μM;▵), or L-NAME (300 μM) and aspirin (10 μM;▴). *P < .05 compared with wild-type group, using 2-wayANOVAto isolate differences between groups. For all experiments, n = 5-9 per group, mean ± SEM. (D) Effect of celecoxib on constriction of endothelium-denuded rings. Aortic ring constriction responses to phenylephrine were determined (n = 5-9 per group). WT denuded (▪), WT denuded + celecoxib (10 μM; ▴). (E) Effect of celecoxib on relaxation of endothelium-denuded rings. Aortic ring relaxation responses to acetylcholine were determined (n = 5-9 per group). WT denuded (▴), WT denuded + celecoxib (10 μM; ▾). (F) Immunohistochemistry of COX-2 in aortic rings. Rings were stained using anti–COX-2 (i) or isotype control IgG (ii). Inset shows corresponding transmission images. (G) Identification of platelets in murine whole blood. Mice were administered L-NAME, indomethacin, or both or celecoxib for 3 days. Platelets were gated in mouse whole blood by FACS analysis using anti–mouse αIIβ-FITC (shown as R1). (H) Representative data showing P-selectin expression following 3 days' administration with celecoxib and L-NAME. Platelets identified by αIIβ expression as in panel G were analyzed for P-selectin expression using anti–mouse P-selectin–FITC, or isotype rat anti–IgG-FITC. (I) Platelet P-selectin expression is significantly increased on day 3 following coadministration of celecoxib and L-NAME. Percentage of P-selectin–expressing cells were defined as those in M1, shown on Panel B with subtraction of percentage of isotype values from all samples. (J) Platelet P-selectin expression is significantly decreased on day 3 following coadministration of indomethacin and L-NAME. Percentage of P-selectin–expressing cells were defined as those in M1, shown on Panel B with subtraction of percentage of isotype values from all samples (for both panels, n = 5, mean ± SEM, *P < .05 compared with control using Student 2-tailed t test).
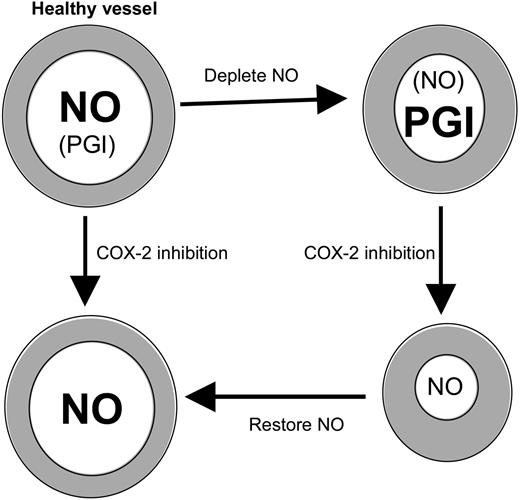
COX-2 inhibition potentiates vasoconstriction in the absence of NO. Healthy large vessels maintain tone predominantly using NO. If NO is depleted, COX-2–derived prostanoids (eg, PGI) compensate. Then, inhibition of COX-2 leads to constriction because PGI synthesis is blocked and may predispose to cardiovascular side effects. Repletion with NO prevents the increase in tone mediated by COX-2 blockade.
COX-2 inhibition potentiates vasoconstriction in the absence of NO. Healthy large vessels maintain tone predominantly using NO. If NO is depleted, COX-2–derived prostanoids (eg, PGI) compensate. Then, inhibition of COX-2 leads to constriction because PGI synthesis is blocked and may predispose to cardiovascular side effects. Repletion with NO prevents the increase in tone mediated by COX-2 blockade.
Inhibition of NO leads to platelet regulatory effects of NSAIDs in vivo
Because myocardial infarction is a side effect of selective COX-2 inhibition, the effects of NSAIDs on in vivo platelet function, with or without NO inhibition were examined by FACS analysis of whole-blood platelet P-selectin. Because there is variability between days (eg, as a result of sampling variations), each experiment used separate controls, conducted at the same time. Platelet P-selectin expression was not significantly altered from basal following 3 days of L-NAME or celecoxib administration (Figure 2G-I). In contrast, coadministration of L-NAME with celecoxib caused a 19% elevation of P-selectin–expressing platelets (Figure 2H-I). This indicates that PGI inhibition promotes platelet activity in vivo, but only in the absence of NO. Similar to celecoxib, indomethacin administration was without significant effect on P-selectin expression (Figure 2J). However, when coadministered with L-NAME, P-selectin expression was undetectable (Figure 2J). These data reveal a difference between the effects of selective COX-2 inhibitors versus nonselective NSAIDs in regulation of in vivo platelet activation in the absence of NO. Specifically, celecoxib slightly increases platelet P-selectin expression while indomethacin inhibits.
NO regulates bioactivity of NSAIDs; implications for their use in patients with compromised NO
Herein, we found that NO bioavailability regulates the vascular effects of COX-2 selective and nonselective NSAIDs in healthy mice. This idea is supported by previous studies using eNOS-deficient mice showing that COX-2 prostaglandins compensate for loss of NO in regulating coronary hemodynamics and flow-induced dilatation in vivo.34,35 The data indicate that a balance of NO and prostaglandins is required to maintain BP and platelet function at optimum levels in vivo and that altering this is undesirable. These data have implications for our understanding of PGI bioactivity in patients with arthritis and vascular disease who typically show 2-fold elevations in PGI, along with decreased large-vessel NO bioactivity in vivo.10-19 Specifically, the biologic role of PGI in regulating vessel tone and platelet function may be enhanced in these groups (Figure 3). As a direct result, the risk of cardiovascular events would be greater on administration of NSAIDs to persons with defects in vascular NO production. In summary, our study identifies an interaction between NO and COX that influences the risk of NSAID vascular side effects and may offer a strategy for reducing this, based on simultaneous elevation of NO levels using NO donors or NO-donating NSAID prodrugs.
Authorship
Contribution: P.B.A., B.C., J.M., H.W., J.U., and S.K.D. performed experiments; P.B.A., L.J.M., and V.B.O. designed experiments; and V.B.O. wrote the paper.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 24, 2006; DOI 10.1182/blood-2006-02-005330.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by Wellcome Trust (V.B.O., P.B.A., and B.C.), British Heart Foundation (J.M. and V.B.O.) and the National Institutes of Health (grants GM15431, CA77839, DK48831) (J.D.M.), (grant CA89450) (L.J.M.), and (grants HD12304, HD33994, and DA06668) (S.K.D.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal