B cells may be implicated in the pathophysiology of chronic graft-versus-host disease (GVHD), as evidenced by antibody production against sex-mismatched, Y chromosome–encoded minor HLA antigens in association with chronic GVHD. We therefore designed a phase 1/2 study of anti–B-cell therapy with rituximab in steroid-refractory chronic GVHD. Twenty-one patients were treated with 38 cycles of rituximab. Rituximab was tolerated well, and toxicity was limited to infectious events. The clinical response rate was 70%, including 2 patients with complete responses. Responses were limited to patients with cutaneous and musculoskeletal manifestations of chronic GVHD and were durable through 1 year after therapy. The median dose of prednisone among treated subjects fell from 40 mg/day to 10 mg/day, 1 year after rituximab therapy (P < .001). A chronic GVHD symptom score improved in the majority of treated patients. Antibody titers against Y chromosome–encoded minor HLA antigens fell and remained low, whereas titers against infectious antigens (EBV, tetanus) remained stable or rose during the treatment period. We conclude that specific anti–B-cell therapy with rituximab may be beneficial for patients with steroidrefractory chronic GVHD. This trial was registered at www.clinicaltrials.gov as #NCT00136396.
Introduction
Chronic graft-versus-host disease (GVHD) is the most important cause of late morbidity and mortality after allogeneic stem cell transplantation, occurring in 60% to 70% of long-term survivors.1 Chronic GVHD requires therapy for many months and often years,2,3 but, despite these efforts, chronic GVHD is the cause of death in up to one third of all long-term survivors after transplantation for leukemia.4 In addition to the effects on mortality, chronic GVHD is associated with a decreased quality of life.5 Attempts to identify novel agents or strategies to improve initial therapy for chronic GVHD have not improved on the combination of corticosteroids and a calcineurin inhibitor.3,6,7 In addition, strategies to reduce the incidence of chronic GVHD have not reproducibly had beneficial effects.8,9 Failure to improve chronic GVHD prophylaxis and therapy can be partly attributed to an incomplete understanding of the pathophysiology of chronic GVHD.2 Chronic GVHD is traditionally thought to be mediated by donor-derived, alloreactive T cells, although a recent large randomized trial has not demonstrated a reduced incidence of chronic GVHD with T-cell depletion.10 There is now mounting evidence implicating B cells in the pathophysiology of chronic GVHD. Antibodies to Y chromosome–encoded minor histocompatibility antigens are generated after sex-mismatched allogeneic transplantation,11 and the presence of these antibodies has been correlated with the occurrence of chronic GVHD and a decreased risk of relapse.12 The finding of a coordinated antibody response in the context of chronic GVHD generates the hypothesis that specific anti–B-cell therapy may be effective therapy for chronic GVHD. The efficacy of anti–B-cell therapy using the monoclonal anti-CD20 antibody, rituximab, for chronic GVHD has been previously reported.13-17 In this paper, we report the largest series of patients treated with monoclonal anti-CD20 antibody therapy and correlate allogeneic antibody responses with clinical responses.
Patients, materials, and methods
This study was an open-label, phase 1/2 study designed to test the safety and the efficacy of rituximab therapy for steroid-refractory chronic GVHD. The study was approved by the Human Subjects Committee of the Dana-Farber Cancer Institute Institutional Review Board, and all subjects signed informed consent at the time of enrollment. The trial design was approved by the Center for Biologics Evaluation and Research of the Food and Drug Administration (IND BB-IND-11103). Eligible subjects had chronic GVHD that was resistant or refractory to therapy with corticosteroids (equivalent of prednisone ≥ 0.5 mg/kg per day or 1 mg/kg every other day) for 30 days at any time within the previous 12 months and were on stable doses of other immunosuppressive medications. Current therapy with corticosteroids was not a requirement, and individuals were permitted to receive organ-specific topical therapy at trial initiation. Individuals who had received ablative or reduced-intensity conditioning were eligible, as were recipients of related and unrelated stem cell grafts. All recipients underwent transplantation at least 6 months prior to enrollment, none had received donor lymphocyte infusions in the preceding 100 days, and none were currently undergoing extracorporeal phototherapy. All patients continued to receive prophylaxis against Pneumocystis carinii and herpesvirus infection during study therapy.
Study evaluations
Prior to initiating rituximab, all patients were thoroughly evaluated by subspecialists trained in the care of patients with GVHD. Objective measurements of GVHD severity included ocular and modified oral Schirmer tests, oral mucosal scoring18 and symptomatic evaluation, pulmonary function tests, and laboratory and radiographic assessments. Cutaneous involvement was graded according to the body surface area involved with either lichenoid or sclerodermatous disease, with skin biopsies performed when indicated. Musculoskeletal GVHD involvement was assessed clinically by palpation of the corresponding subcutaneous, fascial, and muscular induration, but no validated tool exists for measurement of this induration, so Preston dynamometer measurements were used to objectively assess GVHD involvement of muscles, tendons, and tendon sheaths in the forearms. For any given patient, all subsequent examinations were performed by the same subspecialist. In addition, all subjects were required to complete a validated self-administered symptom scale assessment.19 All patients were reevaluated 8 weeks after commencing rituximab and were reevaluated 8 weeks after each subsequent course of rituximab, as well as 6 months and 1 year after study initiation. At each evaluation, subspecialists reevaluated their respective organ system, the subject completed the self-administered symptom scale, all current medications were recorded, and adverse events were documented.
A complete clinical response was defined as the objective resolution of all symptoms and signs attributable to chronic GVHD. Partial responses were defined as the presence of an objective response in one involved organ system without evidence of progression in another organ system and without the requirement for additional systemic therapy to treat any organ system. Stable disease was defined as a lack of objective response without the requirement for additional systemic therapy. Progressive disease was defined as the objective worsening of signs or symptoms in any organ system or the requirement for additional systemic therapy.
Study therapy
Rituximab (375 mg/m2 per week for 4 consecutive weeks) was administered according to the manufacturer's guidelines.20 Premedication with antihistamines and antipyretics, but not corticosteroids, was given. Patients with no response or an incomplete response to one cycle of therapy were eligible to receive a second 4-week course of rituximab 8 weeks after their initial therapy. Patients with a relapse of chronic GVHD after an initial response to one or two 4-week courses of therapy were offered a second or third course of therapy, respectively. Enzyme-linked immunosorbent assay (ELISA) for antibodies to H-Y antigens, EBV, and tetanus plasma samples were collected prior to rituximab infusion and at 9, 17, and 26 weeks and 1 year after their first rituximab infusion. Each sample was diluted in TBS 1:12.5, 1:50, 1:200, 1:800, and 1:3200 before testing by ELISA for the presence of IgG antibodies specific to tetanus toxoid and EBV EBNA1. EBV EBNA1 (Advanced Biotechnologies, Columbia, MD) and tetanus toxoid (kindly provided by D. Molrine, Massachusetts Department of Public Health)21 were diluted to 0.25 μg/well with carbonate binding buffer before coating 96-well ELISA plates (NUNC Scientific, Rochester, NY). Antibodies were detected with goat anti–human IgG conjugated to alkaline phosphatase (Pierce, Rockford, IL) and developed 30 minutes before measuring the absorbance at 450 to 550 nm. Titration curves for each patient's 5 sera samples were determined by plotting ELISA absorption (450-550 nm) results against sera log dilution for each antigen. A shift in the midpoint titration curve greater than 1 log dilution was considered significant.
Four male patients with female donors were similarly tested by ELISA for IgG antibody specific for Y chromosome–encoded (H-Y) antigens. DBY and UTY were recombinantly expressed in Escherichia coli and purified by nickel affinity chromatography as previously described.12,22 These H-Y antigens were diluted to 5.0 μg/mL in carbonate binding buffer before coating 96-well ELISA plates (NUNC Scientific) with 50 μL (0.25 μg antigen) per well. Antigen-specific antibody titration curves were plotted and revealed that the dynamic range of DBY and UTY IgG ELISA results were obtained with plasma diluted at 1:50.
Statistical analysis
Descriptive statistical methodology was used for most analyses. Outcome measures at all time points were compared with baseline measures using a 2-sided Wilcoxon signed-rank test.
Results
Between February 2004 and January 2005, 21 patients were enrolled, all of whom had clinically extensive chronic GVHD, with the exception of 1 patient with refractory limited hepatic chronic GVHD. Their demographic characteristics are found in Table 1. The median time from transplantation to the diagnosis of chronic GVHD was 10.2 months (range, 3-56.9 months). The median time from chronic GVHD diagnosis to trial enrollment was 13.8 months (range, 2.9-82.0 months), and the median time from transplantation to trial enrollment was 23.3 months (range, 8.5-114.6 months). In addition to corticosteroids, all subjects had been treated with at least 1 other form of systemic immunosuppression, with 6 subjects having progressed despite 2 other immunosuppressive medications. All but 3 subjects have been followed for 1 year from rituximab therapy.
Demographic and chronic GVHD characteristics at study entry
Characteristic . | Value . |
|---|---|
| Sample size, no. | 21 |
| Median age, y (range) | 42 (21-62) |
| Sex, no. (%) | |
| Male | 10 (48) |
| Female | 11 (52) |
| Time after transplantation, mo (range) | 23.3 (8.5-114.6) |
| Time after chronic GVHD diagnosis, mo (range) | 13.8 (2.9-82.0) |
| Stem cell donor, no. (%) | |
| HLA-matched sibling | 14 (67) |
| HLA-matched unrelated | 7 (33) |
| Stem cell source, no. (%) | |
| Peripheral blood stem cell | 15 (71) |
| Bone marrow | 6 (29) |
| Conditioning regimen, no. (%) | |
| Myeloablative* | 16 (76) |
| Nonmyeloablative† | 5 (24) |
| Acute GVHD prophylaxis, no. (%) | |
| Tacrolimus-methotrexate | 1 (5) |
| Tacrolimus-sirolimus-methotrexate | 4 (19) |
| Tacrolimus-sirolimus | 4 (19) |
| T-cell depletion with or without other | 7 (33) |
| Cyclosporine with or without methotrexate and/or other | 5 (24) |
| Prior acute GVHD, no. (%) | |
| Grades 0-I | 16 (76) |
| Grades II-IV | 5 (24) |
| End-organ involvement, no. (%) | |
| Skin | 17 (81) |
| Musculoskeletal | 12 (57) |
| Eye | 8 (38) |
| Oral mucosa | 7 (33) |
| Liver alone | 1 (5) |
| Immunosuppression at study entry, no. (%) | |
| Steriod alone | 4 (19) |
| Steroid with calcineurin inhibitor | 4 (19) |
| Steroid with calcineurin inhibitor and other | 3 (14) |
| Steroid with other | 9 (43) |
| Calcineurin inhibitor alone | 1 (5) |
Characteristic . | Value . |
|---|---|
| Sample size, no. | 21 |
| Median age, y (range) | 42 (21-62) |
| Sex, no. (%) | |
| Male | 10 (48) |
| Female | 11 (52) |
| Time after transplantation, mo (range) | 23.3 (8.5-114.6) |
| Time after chronic GVHD diagnosis, mo (range) | 13.8 (2.9-82.0) |
| Stem cell donor, no. (%) | |
| HLA-matched sibling | 14 (67) |
| HLA-matched unrelated | 7 (33) |
| Stem cell source, no. (%) | |
| Peripheral blood stem cell | 15 (71) |
| Bone marrow | 6 (29) |
| Conditioning regimen, no. (%) | |
| Myeloablative* | 16 (76) |
| Nonmyeloablative† | 5 (24) |
| Acute GVHD prophylaxis, no. (%) | |
| Tacrolimus-methotrexate | 1 (5) |
| Tacrolimus-sirolimus-methotrexate | 4 (19) |
| Tacrolimus-sirolimus | 4 (19) |
| T-cell depletion with or without other | 7 (33) |
| Cyclosporine with or without methotrexate and/or other | 5 (24) |
| Prior acute GVHD, no. (%) | |
| Grades 0-I | 16 (76) |
| Grades II-IV | 5 (24) |
| End-organ involvement, no. (%) | |
| Skin | 17 (81) |
| Musculoskeletal | 12 (57) |
| Eye | 8 (38) |
| Oral mucosa | 7 (33) |
| Liver alone | 1 (5) |
| Immunosuppression at study entry, no. (%) | |
| Steriod alone | 4 (19) |
| Steroid with calcineurin inhibitor | 4 (19) |
| Steroid with calcineurin inhibitor and other | 3 (14) |
| Steroid with other | 9 (43) |
| Calcineurin inhibitor alone | 1 (5) |
Myeloablative conditioning regimen was Cytoxan and total body irradiation.
Nonmyeloablative conditioning regimen was fludarabine and Busulfex.
Safety assessment
Thirty-eight courses of rituximab therapy were given to 21 subjects, all of whom were evaluable for toxicity assessments. Four subjects received only one course of therapy because of patient preference with lack of efficacy after one course (n = 2), malignant disease relapse (n = 1), and unassociated toxicity (n = 1). Overall, a total of 9 Common Toxicity Criteria (CTC) grade 3 to 4 adverse events were noted. Most events were infectious in nature and included one case of hepatitis B reactivation and one case of septic arthritis in a clinically uninvolved hip joint. In addition, there were 3 cases of infectious diarrhea (including one due to Mycobacterium avium intracellulare) and one case of viral conjunctivitis. Noninfectious adverse events included gastrointestinal hemorrhage in a patient without GI GVHD (n = 1) and nephrolithiasis with acute renal colic (n = 1). One acute infusional reaction to rituximab was noted but did not preclude further therapy.
Hematologic toxicity
No adverse hematologic events were noted on study. Total leukocyte counts decreased by 15.4% 26 weeks after rituximab administration but increased to within 11.5% of baseline values at 1 year (median, 0.0091 × 109/L [9.1 × 103/μL] at baseline, 0.0077 × 109/L [7.70 × 103/μL] at 26 weeks, and 0.008 05 × 109/L [8.05 × 103/μL] at 1 year). Platelet and red-cell parameters remained constant throughout the study period. When assessed by flow cytometry, circulating CD19+ B cells were undetectable 8 weeks after commencing rituximab therapy and remained undetectable in all patients until 1 year after rituximab. Levels of circulating immunoglobulin fell after rituximab therapy. The median level of circulating IgG fell from a baseline of 7.45 g/L (745 mg/dL) to 4.73 g/L (473 mg/dL) (36.5% decrease), and the median level of circulating IgM fell from a baseline of 0.74 g/L (74 mg/dL) to 0.175 g/L (17.5 mg/dL) (76% decrease) by week 16 after rituximab therapy. Immunoglobulin replacement therapy was permitted at individual investigators' discretion.
Clinical responses
Twenty patients are evaluable for response at a median of 15 months (range, 7.9-19.2 months) from study initiation. One patient with relapsed chronic myelogenous leukemia (CML) did not complete a course of therapy and is thus unevaluable for clinical response. Objective responses were noted in 14 (70%) of 20 patients, including 2 subjects (10%) with a complete clinical response (Table 2). These 2 subjects discontinued immune suppression entirely. In addition, 2 other subjects discontinued corticosteroids but remain on tapering courses of other immunosuppressants. In total, 68% of patients had a dose reduction of at least 50% in corticosteroid doses. The median dose of prednisone fell from 40 mg/d at trial initiation to 10 mg/d at the time of analysis (P < .001). This corresponded to a dose reduction in prednisone from 0.48 mg/kg per day (range, 0.13-1.83 mg/kg per day) to 0.13 mg/kg per day (range, 0-0.91 mg/kg per day). Of 16 patients who were using more than 0.25 mg prednisone/kg per day at trial entry, only 7 remained on doses more than 0.25 mg/kg per day at the time of analysis.
Summary of clinical results
Clinical parameter . | Study Entry . | 1 Year . | P . |
|---|---|---|---|
| Overall response rate, no./no. total (%) | NA | 14/20 (70) | |
| Complete response rate, no./no. total (%) | NA | 2/20 (10) | |
| Prednisone dose, median mg/d | 40 | 10 | < .001 |
| Discontinued prednisone, no./no total (%) | NA | 4/19 (21)* | |
| Prednisone reduction of at least 50%, no./no. total (%) | NA | 13/19 (68) | |
| No change, or higher dose, no./no. total (%) | NA | 6/19 (32) | |
| Cutaneous involvement, median % | |||
| Total body surface area | 42 | 20 | .02 |
| Sclerodermatous involvement | 35 | 20 | .19 |
| Lichenoid involvement | 19.5 | 3 | .17 |
| Rheumatologic involvement | |||
| VAS pain score, median | 4 | 1.5 | .02 |
| VAS fatigue score, median | 5 | 2 | .50 |
| Preston dynamometer, average left and right hand, lb | 62.5 | 71.5 | .13 |
| Oral involvement | |||
| Pathology score | 11.75 | 12.5 | .63 |
| Ocular involvement | |||
| Schirmer test, average left and right eye, mm | 1 | 3.75 | < .99 |
Clinical parameter . | Study Entry . | 1 Year . | P . |
|---|---|---|---|
| Overall response rate, no./no. total (%) | NA | 14/20 (70) | |
| Complete response rate, no./no. total (%) | NA | 2/20 (10) | |
| Prednisone dose, median mg/d | 40 | 10 | < .001 |
| Discontinued prednisone, no./no total (%) | NA | 4/19 (21)* | |
| Prednisone reduction of at least 50%, no./no. total (%) | NA | 13/19 (68) | |
| No change, or higher dose, no./no. total (%) | NA | 6/19 (32) | |
| Cutaneous involvement, median % | |||
| Total body surface area | 42 | 20 | .02 |
| Sclerodermatous involvement | 35 | 20 | .19 |
| Lichenoid involvement | 19.5 | 3 | .17 |
| Rheumatologic involvement | |||
| VAS pain score, median | 4 | 1.5 | .02 |
| VAS fatigue score, median | 5 | 2 | .50 |
| Preston dynamometer, average left and right hand, lb | 62.5 | 71.5 | .13 |
| Oral involvement | |||
| Pathology score | 11.75 | 12.5 | .63 |
| Ocular involvement | |||
| Schirmer test, average left and right eye, mm | 1 | 3.75 | < .99 |
VAS indicates visual analog scale.
One patient not using prednisone at study initiation and one patient with disease relapse are excluded.
In all subjects displaying evidence of response, signs of response were evident before the completion of 1 or 2 cycles of therapy, although the time to maximal response was variable. Subjective and objective responses were noted primarily in patients with cutaneous and musculoskeletal manifestations of chronic GVHD. The median body surface area involvement of cutaneous GVHD dropped significantly, from a median of 42% at study initiation to 35% after 2 cycles of therapy and down to 20% at 1 year (P = .01 and P = .02, respectively). The median body surface area involvement of sclerodermatous cutaneous GVHD fell from 35% at baseline to 24.5% after 2 cycles of therapy (P = .01) and down to 20% at 1 year after rituximab therapy (P = NS). The median body surface area involvement of lichenoid cutaneous GVHD fell from 19.5% at baseline to 4.5% after 2 cycles of rituximab and down to 3% at 1 year after rituximab (P = NS for both). Rheumatologic responses were quantified using rheumatologic visual analog scales (VASs) for pain and fatigue and Preston dynamometer measurements. VAS scores for pain decreased from a median of 4 (measured on a scale of 0 to 10) to 0 after 2 cycles of rituximab (P = .01) and to 1.5 at 1 year after rituximab (P = .02). A VAS score for fatigue decreased from a median of 5 to 4 after 2 cycles of rituximab (P = NS) and down to 2 at 1 year after rituximab (P = NS). The mean Preston dynamometer score was 62.5 lb (mean of left and right hand averages) at trial initiation and increased to 69.0 lb after 2 cycles of rituximab and increased further to 71.5 lb at 1 year after rituximab (P = NS). One individual with limited hepatic GVHD had a partial response, with normalization of the serum bilirubin, but with mild residual transaminitis. No patient with significant ocular or oral mucosal manifestations of GVHD had evidence of a clinical response, as measured by ocular Schirmer tests and oral mucosal pathology and pain scores (Table 2).
In comparison to chronic GVHD symptom scores at trial entry, a clinically relevant improvement in symptom scores was noted in 9 evaluable subjects (50%), 16 weeks after initiating rituximab therapy, of which 7 had clinical evidence of response to therapy. Six subjects had no difference noted, and 3 subjects were noted to have worsened symptom scores. Three subjects either were removed from study or did not complete the symptom score assessment at 16 weeks. Of those 9 subjects with improved scores at 16 weeks, 4 demonstrated continued or sustained improvement at 1 year when compared with the baseline, and 3 were not evaluable at the 1-year time point. The remaining 2 subjects had symptom scores that returned to their baseline assessments, and this correlated with a relapse in their chronic GVHD and requirement for further therapy. Of the 6 subjects with no clinically relevant differences noted at the 16-week time point, 2 went on to have meaningful improvements at 1 year, and the rest remained unchanged or unevaluable. Examining symptom scale subsets for patients with cutaneous involvement at the time of trial initiation revealed that 9 (60%) of 15 evaluable patients had improvements in their cutaneous subscale scores at week 16, whereas 7 (64%) of 11 evaluable patients with musculoskeletal involvement at the time of trial initiation had improvement in their musculoskeletal subscale 16 weeks after trial initiation.
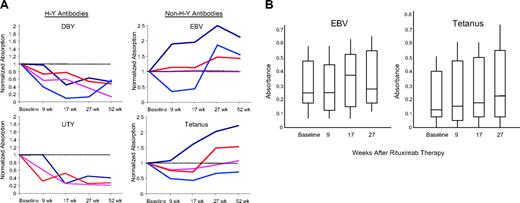
Serologic responses to rituximab. (A) Normalized antibody titers. The 2 left panels demonstrate the decreasing antibody titers against 2 H-Y antigens, DBY and UTY, in 4 male patients with female donors. Each subject has 5 measurements, including baseline (week 0) after 1 course of rituximab (week 9), after a second course of rituximab (week 17), 6 months after rituximab (week 27), and 1 year after rituximab (week 52). The 2 right panels demonstrate the stable or increasing titers against 2 non–H-Y antigens, EBV and tetanus. The dashed horizontal line represents the baseline level. (B) Box and whisker plot of EBV and tetanus titers in 12 patients. The boxes represent the 25th and 75th percentiles of the antibody titers measured. The whiskers represent the 5th and 95th percentiles of the measured values. The horizontal line within each box is the median antibody titer measured. All comparisons are P = NS.
Serologic responses to rituximab. (A) Normalized antibody titers. The 2 left panels demonstrate the decreasing antibody titers against 2 H-Y antigens, DBY and UTY, in 4 male patients with female donors. Each subject has 5 measurements, including baseline (week 0) after 1 course of rituximab (week 9), after a second course of rituximab (week 17), 6 months after rituximab (week 27), and 1 year after rituximab (week 52). The 2 right panels demonstrate the stable or increasing titers against 2 non–H-Y antigens, EBV and tetanus. The dashed horizontal line represents the baseline level. (B) Box and whisker plot of EBV and tetanus titers in 12 patients. The boxes represent the 25th and 75th percentiles of the antibody titers measured. The whiskers represent the 5th and 95th percentiles of the measured values. The horizontal line within each box is the median antibody titer measured. All comparisons are P = NS.
In total, 4 subjects had GVHD flares after initially having partial responses to therapy, including 1 subject who had discontinued all immune suppression and another who had discontinued corticosteroids at the time of their respective GVHD flares. No correlation with B-cell recovery was noted in clinically relapsing patients. Two subjects received a third course of rituximab, one of whom had a clinical response.
Antibody responses
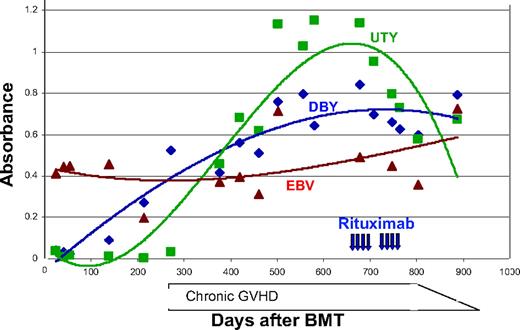
There were 4 male patients with female donors enrolled in this study, all of whom had one or more H-Y antibody responses. The specific reduction in H-Y antibody responses after rituximab administration is demonstrated in Figure 1A. All 4 patients with pretreatment DBY titers had significant decreases in the DBY titers of more than one log change and 3 of 3 patients with UTY titers likewise had significant decreases in their antibody response, which were sustained through 1 year of observation. In contrast, in the same 4 patients there were no significant decreases in the antibody titers against the infectious antigens, EBV and tetanus. In fact, titers against EBV and tetanus generally rose over the course of the year. Similarly, in 12 other individuals tested for EBV and tetanus titers before and after rituximab, there were no significant decreases in measurable titers (P = NS for both comparisons) (Figure 1B). Figure 2 shows a timeline of antibody measurements from a man who received female stem cells. In this subject, allogeneic antibodies developed concurrently with chronic GVHD, and DBY and UTY antibody titers rose for a year despite high-dose prednisone and tacrolimus therapy. The antibody titers decreased after rituximab therapy and remained low for a year after rituximab.
Representative time line of serologic response to rituximab in one patient. The trend in antibody titers against 3 antigens (DBY ♦, UTY ▪, and EBV ▴) before and after administration of rituximab (vertical arrows) is demonstrated by the trend lines (added for clarity). The clinical time course of chronic GVHD is depicted along the x-axis.
Representative time line of serologic response to rituximab in one patient. The trend in antibody titers against 3 antigens (DBY ♦, UTY ▪, and EBV ▴) before and after administration of rituximab (vertical arrows) is demonstrated by the trend lines (added for clarity). The clinical time course of chronic GVHD is depicted along the x-axis.
All 4 patients with demonstrable H-Y antibodies had clinical responses to rituximab therapy, including one patient who had a complete response and discontinued all immunosuppressants, and one other who was able to discontinue corticosteroids and remains on a tapering course of other immunosuppressants. In addition, one patient with a recurrence of symptoms responded to a third course of rituximab therapy approximately 8 months after his initial rituximab therapy.
Discussion
Effective prophylaxis against, and therapy for, chronic GVHD is limited by an incomplete understanding of chronic GVHD pathophysiology. Because chronic GVHD is thought to be mediated primarily by T cells, most therapeutic strategies thus far have focused uniquely on T-cell immunosuppression. However, our previous studies demonstrating that allogeneic H-Y antibody responses develop in association with chronic GVHD suggest a role for B cells in its pathogenesis. Our report documents the largest series of patients treated with rituximab, demonstrating evidence of selective activity in cutaneous and rheumatologic disease, as well as a correlation between the reduction of allogeneic antibodies directed against H-Y antigens and clinical response. Responses were durable and allowed for the reduction or discontinuation of corticosteroids in some patients for many months after rituximab therapy.
Treatment for chronic GVHD is limited. High-dose corticosteroid therapy is effective but often requires prolonged use, and many patients are unable to discontinue corticosteroid use even after months or years of therapy. The addition of a calcineurin inhibitor to initial therapy of chronic GVHD may reduce the requirement for high doses of corticosteroids but is not associated with an increased response rate or a reduction in transplant-related mortality.3 Similarly, the addition of other agents to the 2-drug combination of corticosteroids and calcineurin inhibitors have not improved chronic GVHD outcomes.6,7 As such, corticosteroids remain standard treatment for chronic GVHD, but their prolonged use is associated with a number of serious toxicities, including glucose intolerance, sleep disturbances, cushingoid changes, and osteoporosis and osteonecrosis. In our trial, rituximab therapy facilitated corticosteroid tapering, with the median dose of prednisone falling from 40 mg/day at trial initiation to 10 mg/day at the time of analysis (P < .001). As such, the addition of rituximab to prednisone for the initial treatment of chronic GVHD may both increase the overall response rate and enable a more rapid steroid taper while incurring less toxicity.
There is limited experience with rituximab therapy for chronic GVHD after allogeneic stem cell transplantation with only 3 small case series in the literature. Ratanatharathorn et al,15 after noting an improvement in chronic GVHD in a patient treated with rituximab for refractory autoimmune cytopenia, treated an additional 8 patients. All patients were treated with the standard regimen of 375 mg/m2 per week for 4 weeks. Four of 8 patients had sustained responses to therapy, even with recovery of B cells in 3 of these patients.16 Canninga-van Dijk et al14 treated 6 individuals with chronic GVHD that was refractory to corticosteroids. Patients received the standard course of rituximab and were permitted to receive an additional course of therapy in the case of incomplete response. Five of 6 patients had objective responses, with histologic correlation of responses noted.14 Most recently, Okamoto et al17 described favorable results in treating 3 individuals with sclerodermatous chronic GVHD. In addition to use specifically for therapy of chronic GVHD, several investigators have used rituximab as part of conditioning therapy and immediately after transplantation in an effort to reduce relapse in patients with B-cell malignancies.23-26 Anecdotally, some of these reports showed a reduction in the incidence of chronic GVHD in long-term survivors of transplantation.23,26 Rituximab has also been used as therapy for EBV reactivation and posttransplantation lymphoproliferative disease.27-34
In our study, responses were noted in patients with cutaneous and musculoskeletal disease, a finding that has previously been reported.14,16,17 Although it is possible that other involved organs (such as the eyes and oral mucosa) did not respond because of the destruction of target tissues (such as the lacrimal and salivary glands), or because our clinical measurement tools were inadequate to detect improvement, it is notable that rituximab is now being used successfully in autoimmune conditions resembling cutaneous and musculoskeletal chronic GVHD.35 This finding provides rationale for the use of rituximab in chronic GVHD, which can be considered a special case of autoimmunity. Other investigators have commented on the role of rituximab to selectively deplete autoreactive B cells in the context of autoimmunity,36 which may explain the relative selectiveness of rituximab against host minor antigen epitopes noted in our study. However, in contrast to true autoimmune conditions, in which the dysregulated immune system is anticipated to be detrimentally targeting autologous antigens, the novel allogeneic antigenic environment is anticipated to lead to recurrent antibody production against disparate minor histocompatibility antigens. In our experience, despite signs of B-cell reconstitution at 1 year after rituximab therapy, antibodies directed against H-Y antigens did not reappear.
It is also noteworthy that antibodies against infectious targets persisted or increased during the course of therapy. The allogeneic H-Y antibodies described in this study are thus likely the product of naive engrafted donor B cells proliferating in response to disparate host antigens, whereas patients' protective antitetanus and EBV antibodies may be the result of long-standing plasma cells and may not depend on evolving proliferative B-cell responses. Human studies have previously demonstrated the persistence of host antibodies years after myeloablative stem cell transplantation.37 Alternatively, these protective antibodies may develop from expansion and maturation of donor-derived memory B cells into plasma cells. Further evidence for the dynamic evolution of the H-Y antigen B-cell response includes the recognition of new H-Y antigen peptide epitopes over time,38 and that H-Y antibody responses demonstrate recurrent production of IgM isotypes after allogeneic transplantation (D.B.M., unpublished observation, 2005). Despite the association between these H-Y antibodies and relapse prevention, only one relapse was noted; this relapse was discovered during the first cycle of therapy and thus is likely not mechanistically related to the reduction in circulating H-Y antibodies.
B cells could be pathogenic through a variety of effector pathways, including antigen presentation to T cells, dysregulated autoimmune antibody synthesis, and allogeneic antibody induction.39 Antibodies may directly mediate chronic GVHD–associated tissue damage via antibody deposition with complement activation, antibody-dependent cell cytotoxicity, or may represent nonpathogenic biomarkers. The efficacy of rituximab in this trial does not clarify these possibilities and does not necessarily implicate these antibodies in the pathogenesis of chronic GVHD. In addition, the clinical improvement in patients without H-Y antibodies implicates non–Y-encoded minor histocompatibility antigens and antibodies to the same degree that H-Y antibodies are implicated in chronic GVHD pathophysiology.
In summary, we have demonstrated that anti–B-cell therapy with the monoclonal antibody, rituximab, is safe and effective as therapy in steroid-refractory chronic GVHD. The correlation of decreasing antibody titers and clinical responses supports the hypothesis that a coordinated B- and T-cell response is instrumental in chronic GVHD. There have been very few advances in the prevention and initial therapy of chronic GVHD, and, although novel therapeutics have demonstrated efficacy in treating steroid-refractory GVHD, treatment of advanced chronic GVHD remains a challenge. Novel prophylactic strategies that prevent chronic GVHD are warranted, and the use of monoclonal anti–B-cell therapy should be tested as a prophylactic and initial treatment strategy. Future studies should assess molecular and functional immune reconstitution, especially immunoglobulin repertoire, following rituximab therapy after allogeneic stem cell transplantation.
Prepublished online as Blood First Edition Paper, March 21, 2006; DOI 10.1182/blood-2006-01-0233.
Supported in part by Genentech; and the National Heart, Lung, and Blood Institute (P01 HL070149); and the Ted and Eileen Pasquarello Research Fund.
Genentech Inc, who sponsored this trial in part, is the manufacturer of rituximab, which is studied in this manuscript. C.C. has declared a financial interest in Biogen-IDEC, which comarkets rituximab with Genentech.
C.C., D.M., S.J.L., V.H., J.H.A., R.S., and E.A. designed the clinical trial; D.M., J.L., K.M., C.R., and J.R. performed the laboratory analysis; N.T., S.-B.W., D.B., and L.B.K. were clinical consultants; C.C., R.M., and M.P. performed the data management; C.C. and H.T.K. performed the statistical analysis; C.C. and D.M. drafted the manuscript; and all authors critically reviewed the manuscript.
C.C. and D.M. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Karen Kilgore for excellent administrative support and the patients and their families for their participation in this trial.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal