Abstract
The definitive hematopoietic cell lineages have been proposed to originate from hemogenic endothelial cells during mouse embryogenesis. c-Myb is a transcription factor that is essential for the development of definitive hematopoiesis. To investigate the functional role of c-Myb in hematopoietic cell development from endothelial cells, we introduced a c-myb transgene expressed under the control of a tetracycline-regulated promoter into the c-myb–/– embryonic stem (ES) cell line, with the aim of inducing c-Myb expression at any stage and at any level. Induction of c-Myb expression after replating c-myb–/– endothelial cells rescued the generation and proliferation of definitive hematopoietic progenitor cells, suggesting that c-Myb expression in developing endothelial cells is not a prerequisite for their hematogenic potential. Overexpression of c-Myb, however, prevented the terminal differentiation of erythrocytes and megakaryocytes and completely abolished B-lymphocyte development. Our results indicate that c-Myb is a major factor that controls differentiation as well as proliferation of hematopoietic progenitor cells derived from hemogenic endothelial cells, and that appropriate levels of c-Myb protein are strictly defined at distinct differentiation steps of each hematopoietic cell lineage.
Introduction
Definitive hematopoietic progenitor cells develop in the para-aortic splanchnopleural and aorta-gonad-mesonephros (AGM) regions of midgestational mouse embryos.1 Hematopoietic stem cells then colonize the developing fetal liver where definitive hematopoiesis takes place until the bone marrow takes over as a lifelong hematopoietic organ.2 The developmental origin of definitive hematopoietic progenitor cells has been a controversial issue. Lineage tracing experiments using a whole embryo culture system demonstrated that the definitive erythroid cell lineage was derived from endothelial cells.3 We and other research groups have reported that endothelial cells sorted from differentiating embryonic stem (ES) cells or the mouse conceptus were capable of generating definitive hematopoietic cells of multiple lineages.4-8 Therefore, it has been proposed that the endothelial cell lineage is one of the direct sources of definitive hematopoietic progenitor cells.9,10
A number of transcription factors are involved in the development of the definitive hematopoietic cell lineages.11 The protooncogene c-myb, which encodes a transcription factor belonging to the Myb family, is an important gene for development of the definitive hematopoiesis.12 Mice lacking a functional c-myb gene died at 15 days postcoitum of severe anemia resulting from a lack of fetal liver hematopoiesis.13 Despite the apparent requirement for the c-myb gene in the development of definitive hematopoiesis, the exact stage at which c-myb exerts its role in the developmental process of hematopoietic progenitor cells is not clear. We previously found that hemogenic endothelial cells expressed the c-myb gene, suggesting a putative role of c-Myb in the development of hematopoietic cells from endothelial cells.6,14 Mukouyama et al15 reported that explant cultures of the AGM region dissected from c-myb–/– embryos exhibited proliferation of endothelial cells but not hematopoietic cells. Retroviral-mediated transduction of c-myb rescued production of definitive hematopoietic cells in c-myb–/– AGM cultures, suggesting that c-Myb plays a role in the generation of hematopoietic cells in the AGM region.15 However, as the AGM explant cultures may consist of cells of diverse lineages, it remains to be clarified whether c-Myb is involved in the hematopoietic differentiation of endothelial cells.
The c-myb gene is abundantly expressed in immature hematopoietic cells.16-18 Early experiments reported that ectopic expression of c-Myb blocked the induced differentiation of hematopoietic cell lines.19,20 Furthermore, discrete threshold levels of c-Myb activity appear to be required for differentiation along individual hematopoietic cell lineages. This latter conclusion is based on recent observations indicating that suppression of c-Myb function exerts a distinct effect on each hematopoietic cell lineage. Mouse embryos homozygous for a knockdown allele of the c-myb gene exhibited an increase in phenotypically immature cells and megakaryocyte/macrophage colony-forming cells in the fetal liver.21 Maturation of the definitive erythroid progenitors was compromised, and the development of B- and T-lymphoid lineages was severely affected in these embryos.21 Essentially the same phenotype was observed in mice that have mutations in either the DNA-binding domain or the transactivation domain of c-Myb.22,23
Here, we report on the function of c-Myb in the development of hematopoietic cells from endothelial cells. With the aim of controlling the timing and level of c-Myb expression during the course of hematopoietic development, we have combined a tetracycline-regulated gene expression system with in vitro differentiation of c-myb–null ES cells. We find that induction of c-Myb expression in sorted endothelial cells rescued the generation and proliferation of definitive hematopoietic progenitor cells, including precursors of the erythroid and myeloid lineages. More importantly, we can conclude that the precise level of expression of c-Myb affects the efficiency of differentiation both into and along distinct hematopoietic cell lineages.
Materials and methods
Plasmid construction
To engineer c-myb-IRES-EGFP, a 2.7–kilobase pair (kbp) HindIII fragment encompassing the coding region of the c-myb cDNA from pACT–c-myb was subcloned into a NotI site of pUHD10-3, which contains a tetracycline-regulated promoter.24
Electroporation and drug selection
c-myb–/– ES cells25 were maintained on gelatin-coated culture dishes in Glasgow MEM (Invitrogen, Carlsbad, CA) supplemented with 10% Knockout SR (Invitrogen), 1% FCS, and leukemia inhibitory factor (LIF) (Chemicon, Temecula, CA). Cells were electroporated with linearized pUHD10-3-IRES-puromycin together with pCAG-tTA and selected for resistance to puromycin (2 μg/mL; Sigma, St Louis, MO). Resistant ES cell clones were further screened for resistance to puromycin under differentiation conditions in the absence of tetracycline (Tet). The selected tTA-expressing parental ES cell clones were electroporated with linearized pUHD10-3-c-myb-IRES-EGFP together with pPyCAGZeocinpA and selected for resistance to zeocin (10 μg/mL; Sigma). Resistant ES cell clones were allowed to differentiate on OP9 stromal cells, and transgene expression was induced by withdrawal of Tet from day 4 onward. At day 6, expression of c-Myb and EGFP was analyzed by immunoprecipitation and fluorescence-activated cell sorting (FACS), respectively.
Antibodies
Monoclonal antibodies (mAbs) against Flk-1 (AVAS12),26 VE-cadherin (VECD1),27 and CD41 (MWReg30)28 were purified from hybridoma culture supernatants using CELLine (BD Biosciences, Bedford, MA). The mAbs were labeled with allophycocyanin (APC; ProZyme, San Leandro, CA) or biotin (Pierce, Rockford, IL) using standard procedures. APC-conjugated mAb against B220 (RA-6B2); phycoerythrin (PE)–conjugated mAbs against CD19 (MB19-1), CD43 (S7), and CD31 (MEC13.3); biotin-labeled mAb against CD71, streptavidin-APC, and streptavidin-PE-Cy7 were purchased from BD Biosciences. Biotin-labeled mAb against IL7Rα (A7R34), APC-conjugated mAb against Mac1 (M1/70), PE-conjugated mAbs against Gr1 (RB6-8C5), Ter119 (Ter119), and CD45 (30-F11) were purchased from eBioscience (San Diego, CA).
In vitro differentiation of ES cells
In vitro differentiation of ES cells was performed as described previously.29 In brief, 5 × 104 ES cells were inoculated into a 25-cm2 culture flask preseeded with OP9 stromal cells30 and cultured for 5 days in alpha-MEM supplemented with 10% FCS and 5 × 10–5 mol/L 2-mercaptoethanol (induction medium) in the absence of LIF. Tet (1 μg/mL; Sigma) was added in the medium when expression of c-Myb/EGFP was to be suppressed. Cultured cells were harvested by incubating with cell dissociation buffer (Invitrogen), blocked in mouse serum, and washed with Hanks balanced salt solution (Invitrogen) containing 1% BSA (Sigma). Cells were stained with PE–anti-CD31 and APC–anti-VE-cadherin mAbs, followed by sorting of VE-cadherin+CD31+ endothelial cells using a FACS Vantage-SE equipped with a DiVa option or a FACSAria (BD Biosciences). Sorted endothelial cells were inoculated at a density of 1 × 104 cells/well into either 6-well culture plates or 2-well culture slides preseeded with OP9 stromal cells. Cells were cultured for 3 days in the induction medium and subjected to immunostaining of endothelial cell colonies.
Differentiation of hematopoietic cells
Sorted endothelial cells were cultured on OP9 stromal cells in the presence of SCF (20 ng/mL), IL3 (20 ng/mL), and EPO (2 IU/mL) for erythroid/myeloid cells or SCF (50 ng/mL), Flt3L (20 ng/mL), and a culture supernatant containing IL7 (2.5%) for B lymphocytes. Recombinant growth factors, except for EPO and IL7, were purchased from R&D Systems (Minneapolis, MN). The rhEPO was provided by Kirin Brewery (Gunma, Japan). Tet was removed from some cultures to induce c-Myb/EGFP expression. Hematopoietic cells were harvested and analyzed by flow cytometry after 9 days for erythroid/myeloid cells or 13 days for B lymphocytes.
For hematopoietic colony formation assay, sorted endothelial cells were put into a 6-well plate (3000 cells/well) preseeded with OP9 stromal cells and incubated in the presence of cytokines. After 15 hours, medium was replaced with fresh semisolid medium that consisted of the induction medium, mixture of cytokines, and 1.2% methylcellulose. Cells were further cultured for 5 days, and hematopoietic cell colonies generated were scored. Colonies were picked and morphologically examined following Giemsa staining.
RT-PCR
Total RNA was isolated using Trizol (Invitrogen) and reverse-transcribed using SuperScript II reverse transcriptase (RT; Invitrogen). Semiquantitative polymerase chain reaction (PCR) was performed using Ex Taq DNA polymerase (Takara Shuzo, Otsu, Japan). The primer pairs used were as follows: c-myb, 5′-CAC CAT TCT GGA CAA TGT TAA GAA C-3′ and 5′-GTA AGG TAG GTG CAT CTA AGC-3′; Gata1, 5′-CAG CAC TGG CCT ACT ACA G-3′ and 5′-TCA AGG TGT CCA AGA ACG TG-3′; Gata2,5′-GAC ACA CCA CCC GAT ACC CAC CTA T-3′ and 5′-GCC TAC GCC ATG GCA GTC ACC ATG CT-3′; Actb (β-actin), 5′-TCG TGC GTG ACATCAAAG AG-3′ and 5′-TGG ACA GTG AGG CCA GGATG-3′.
Immunofluorescent staining
For the immunologic detection of c-Myb protein on endothelial cell colonies, 2-well culture slides were fixed with 4% PFA and stained with rat mAb against VE-cadherin (VECD1) and rabbit polyclonal antibody against c-Myb (C-20; Santa Cruz Biotechnology, Santa Cruz, CA). The presence of VE-cadherin and c-Myb were revealed by Alexa-647–labeled goat anti–rat IgG and Alexa-555–labeled goat anti–rabbit IgG antibodies (Molecular Probes, Eugene, OR), respectively. Confocal microscope images were taken with an IX70 microscope with UplanF1 objective (60×/1.25 NA oil) and FV500 software (all from Olympus, Tokyo, Japan). Subsequent image processing was performed using Adobe Photoshop version 7.0 (Adobe Systems, San Jose, CA) for Windows XP.
Immunoprecipitation and Western blotting
Cells were lysed in a lysis buffer (20 mM Tris-HCl, pH7.4, 150 mM NaCl, 1% Triton X-100, 100 μM sodium vanadate, 1 mM dithiothreitol, 5 mg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride). The supernatant was immunoprecipitated with anti–c-Myb antibody and incubated with protein A–Sepharose beads. Beads were washed with PBS, and proteins were solubilized in a sample buffer. Immunoprecipitates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA). After blocking in TBST (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% Tween20) containing 5% dry-fat skim milk, the membrane was probed with anti–c-Myb antibody and HRP-conjugated protein A. To normalize the amount of protein in cell lysates, total-cell lysates were blotted with antiactin antibody (I-19; Santa Cruz Biotechnology) and HRP-conjugated antirabbit antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). The protein bands were visualized by enhanced chemiluminescence (ECL; Pierce). Recombinant DNA experiments were approved by Kumamoto University.
Results
Tetracycline-regulated expression of c-Myb protein in c-myb–deficient ES cells
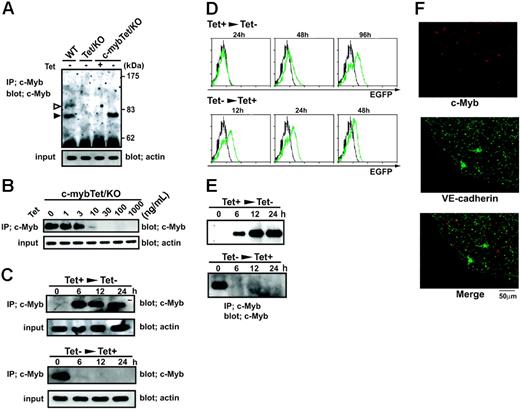
To determine the c-Myb–dependent processes as well as the role of c-Myb during the course of the development of definitive hematopoiesis, we produced c-myb–null ES cell clones in which expression of c-Myb protein can be induced by using a Tet-regulated gene-expression system. In this system, a Tet-Off–specific promoter drives expression of c-myb-IRES-EGFP transcripts when activated by Tet transactivator (tTA) in the absence of Tet or suppresses it in the presence of Tet.
Tet-regulated expression of c-Myb protein in c-myb knockout ES cells. (A) Wild-type ES cells (WT), c-myb–/–parental ES cells expressing tTA (Tet/KO), and c-myb–/–ES cells carrying the Tet-regulated c-myb transgene (c-mybTet/KO) were cultured for 2 days in the presence (1 μg/mL) or absence of Tet. Cells were lysed and subjected to immunoprecipitation and Western blotting with an anti–c-Myb antibody. Two isoforms of the c-Myb protein, p75 (filled arrow) and p89 (open arrow), were detected in wild-type cells, whereas only p75 was detected in c-mybTet/KO cells cultured in the absence of Tet. Total-cell lysates were blotted with antiactin to normalize the initial amount of protein. (B) c-mybTet/KO ES cells were cultured for 2 days in the presence of indicated concentrations of Tet (0-1000 ng/mL). Cells were lysed and subjected to immunoprecipitation and Western blotting as described under “Materials and methods.” (C) c-mybTet/KO ES cells were cultured in the presence (top panels) or absence (bottom panels) of Tet (1 μg/mL) for 2 days. The culture conditions were then shifted to either with Tet (1 μg/mL) (bottom panels) or without Tet (top panels), and incubation continued for another 6 to 24 hours. Cells were lysed and subjected to immunoprecipitation and Western blotting. (D) c-mybTet/KO ES cells were cultured in the presence (top panels) or absence (bottom panels) of Tet (1 μg/mL) for 2 days. The culture conditions were then shifted to either with Tet (1 μg/mL) (bottom panels) or without Tet (top panels), and incubation continued for another 12 to 96 hours. Cells were then analyzed for EGFP expression by flow cytometry. Histograms show intensity of EGFP fluorescence on the cells harvested at indicated time points (green) together with cells that were kept in the presence of Tet as a negative control (black). (E) c-mybTet/KO ES cells were allowed to differentiate on OP9 stromal cell layers in the presence of Tet (1 μg/mL) for 4 days. Flk-1+cells were sorted by FACS and recultured with OP9 cells in the presence (top panel) or absence (bottom panel) of Tet (1 μg/mL). After 24 hours, the culture conditions were shifted to either with Tet (1 μg/mL) (bottom panel) or without Tet (top panel), and incubation continued for another 6 to 24 hours. Cells were then lysed and subjected to immunoprecipitation and Western blotting with an anti–c-Myb antibody. (F) VE-cadherin+CD31+cells were sorted from differentiating c-mybTet/KO ES cells and recultured on OP9 layers in the absence of Tet. After 3 days, the cultures were stained in situ with antibodies against VE-cadherin (green) and c-Myb (red). VE-cadherin staining was localized to cell-cell junctions and perinuclear regions, whereas c-Myb staining was localized to nucleus. Results shown are representative of at least 3 independent experiments. Original magnification, ×600 (objective, 60×/1.25 NA oil).
Tet-regulated expression of c-Myb protein in c-myb knockout ES cells. (A) Wild-type ES cells (WT), c-myb–/–parental ES cells expressing tTA (Tet/KO), and c-myb–/–ES cells carrying the Tet-regulated c-myb transgene (c-mybTet/KO) were cultured for 2 days in the presence (1 μg/mL) or absence of Tet. Cells were lysed and subjected to immunoprecipitation and Western blotting with an anti–c-Myb antibody. Two isoforms of the c-Myb protein, p75 (filled arrow) and p89 (open arrow), were detected in wild-type cells, whereas only p75 was detected in c-mybTet/KO cells cultured in the absence of Tet. Total-cell lysates were blotted with antiactin to normalize the initial amount of protein. (B) c-mybTet/KO ES cells were cultured for 2 days in the presence of indicated concentrations of Tet (0-1000 ng/mL). Cells were lysed and subjected to immunoprecipitation and Western blotting as described under “Materials and methods.” (C) c-mybTet/KO ES cells were cultured in the presence (top panels) or absence (bottom panels) of Tet (1 μg/mL) for 2 days. The culture conditions were then shifted to either with Tet (1 μg/mL) (bottom panels) or without Tet (top panels), and incubation continued for another 6 to 24 hours. Cells were lysed and subjected to immunoprecipitation and Western blotting. (D) c-mybTet/KO ES cells were cultured in the presence (top panels) or absence (bottom panels) of Tet (1 μg/mL) for 2 days. The culture conditions were then shifted to either with Tet (1 μg/mL) (bottom panels) or without Tet (top panels), and incubation continued for another 12 to 96 hours. Cells were then analyzed for EGFP expression by flow cytometry. Histograms show intensity of EGFP fluorescence on the cells harvested at indicated time points (green) together with cells that were kept in the presence of Tet as a negative control (black). (E) c-mybTet/KO ES cells were allowed to differentiate on OP9 stromal cell layers in the presence of Tet (1 μg/mL) for 4 days. Flk-1+cells were sorted by FACS and recultured with OP9 cells in the presence (top panel) or absence (bottom panel) of Tet (1 μg/mL). After 24 hours, the culture conditions were shifted to either with Tet (1 μg/mL) (bottom panel) or without Tet (top panel), and incubation continued for another 6 to 24 hours. Cells were then lysed and subjected to immunoprecipitation and Western blotting with an anti–c-Myb antibody. (F) VE-cadherin+CD31+cells were sorted from differentiating c-mybTet/KO ES cells and recultured on OP9 layers in the absence of Tet. After 3 days, the cultures were stained in situ with antibodies against VE-cadherin (green) and c-Myb (red). VE-cadherin staining was localized to cell-cell junctions and perinuclear regions, whereas c-Myb staining was localized to nucleus. Results shown are representative of at least 3 independent experiments. Original magnification, ×600 (objective, 60×/1.25 NA oil).
We first characterized the inducibility of the c-myb transgene in the established ES clones. Expression of c-Myb protein was detected in the undifferentiated wild-type ES cells but not in the c-myb–/– parental ES cells (Figure 1A). The c-myb–/– ES cell clones carrying the exogenous c-myb transgene showed expression of 75-kDa c-Myb when Tet was removed from the culture medium (Figure 1A). The c-Myb protein was not detected when cells were cultured in the presence of Tet at doses of 30 to 1000 ng/mL (Figure 1B). A low-level expression of c-Myb became detectable when the dose of Tet was reduced to 10 ng/mL (Figure 1B). The level of c-Myb protein increased significantly when the dose was further reduced (Figure 1B). Therefore, the level of c-Myb protein induced was dependent on the concentration of Tet.
We next examined the kinetics of transgene induction. c-Myb became detectable as early as 6 hours after removal of Tet (Figure 1C). Because the half-life of c-Myb is very short,31 expression of the protein could also be abolished rapidly. We observed that c-Myb protein decreased to undetectable levels after reexposure of the cells to Tet for 6 hours (Figure 1C). In contrast to c-Myb protein, fluorescence of EGFP derived from the bicistronic transcripts responded more slowly to Tet. FACS analyses revealed that EGFP fluorescence became detectable 48 hours after removal of Tet (Figure 1D). A significant level of EGFP fluorescence remained after reexposure to Tet for even 48 hours (Figure 1D).
Differentiation of ES cells toward mesodermal cell lineages can be induced by cultivation with OP9 stromal cells.32 Endothelial cells are derived from Flk-1+ mesodermal cells that arise during such in vitro ES cell differentiation. We detected expression of c-Myb protein in the cells differentiating from Flk-1+ cells that were derived from wild-type ES cells but not from the c-myb–/– parental ES cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The c-myb–/– ES cell clones carrying the transgene showed expression of 75-kDa c-Myb in differentiating cells in response to Tet. Kinetic analyses showed that c-Myb protein became detectable as early as 6 hours after removal of Tet and reached a plateau at 12 hours (Figure 1E). The c-Myb protein decreased to undetectable levels after reexposure of the cells to Tet for 6 hours as observed in undifferentiated ES cells (Figure 1E).
We further tested induction of c-Myb expression in individual differentiating c-myb–/– ES cells. VE-cadherin+CD31+ endothelial cells were sorted from ES cells differentiating in the presence of Tet. Endothelial cells were allowed to form colonies by replating on OP9 cells. Immunohistologic analysis revealed c-Myb protein expression in 40% of total colonies generated in the absence of Tet (data not shown). c-Myb protein was detected homogeneously in most of the cells in the positive colonies (Figure 1F), suggesting a clonal activation of the transgene.
Taken together, our results indicate that the expression of exogenous c-Myb can be controlled tightly in c-myb–/– ES cells using the Tet-regulated gene expression system.
c-Myb–dependent hematopoiesis initiated by endothelial cells
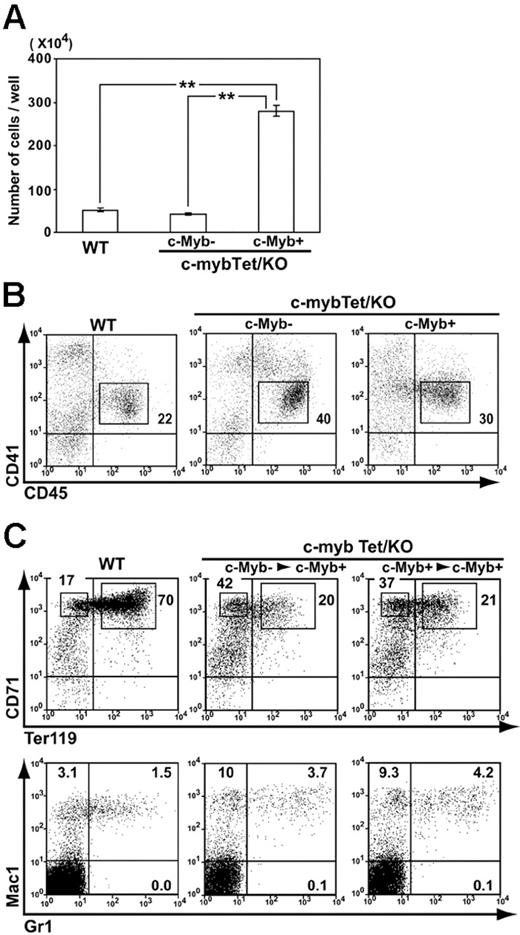
We next examined the potential of endothelial cells derived from wild-type and c-myb–/– ES cells to generate hematopoietic cells in vitro. VE-cadherin+CD31+ endothelial cells sorted from differentiating wild-type ES cells gave rise to hematopoietic cells when recultured with OP9 cells for 4 days (Figure 2A). FACS analysis on the recovered cells revealed that almost all of the CD45+ cells expressed CD41 (Figure 2B), a phenotype representing committed hematopoietic progenitor cells.33-36 CD45+CD41+ cells were capable of forming hematopoietic cell colonies in semisolid cultures with soluble cytokines (data not shown). A comparable number of cells with the same CD45+CD41+ phenotype appeared transiently in the cultures of endothelial cells derived from c-myb–/– ES cells in the absence of c-Myb activation (Figure 2A-B), but these cells did not last as long as wild-type cells (data not shown). However, when c-Myb expression was induced after sorting of endothelial cells, production of hematopoietic cells was dramatically enhanced (Figure 2A). CD45+CD41+ cells also increased, taking the increased cell recovery into account (Figure 2B). Thus, it is suggested that, although c-Myb may not be an absolute requirement for commitment of endothelial cells to hematopoietic lineages, it can promote the process of commitment and/or serves to drive subsequent expansion of committed hematopoietic progenitor cells.
When the generation of hematopoietic cells was assessed at 9 days after replating of endothelial cells, the cultures were almost dominated by erythroid lineage cells (Figure 2C). A large number of CD71high erythroblasts were recovered from cultures of c-myb–/– endothelial cells in which c-Myb expression had been induced after replating, although the ratio of CD71highTer119+ cells to CD71highTer119– cells was lower than that observed in wild-type cell cultures (Figure 2C). The same shift was observed when c-Myb induction was initiated 2 days before sorting of endothelial cells. The frequency of Mac-1+Gr-1+ myelomonocytes was comparable between wild-type cell cultures and c-Myb–rescued c-myb–/– cultures (Figure 2C).
Hematopoietic cell generation from c-myb knockout endothelial cells restored by c-Myb induction. (A-B) Wild-type ES cells (WT) and c-myb–/–ES cells carrying the Tet-regulated c-myb transgene (c-mybTet/KO) were allowed to differentiate on OP9 stromal cell layers in the presence of Tet (1 μg/mL) for 5 days. VE-cadherin+CD31+CD45–CD41– cells were sorted by FACS and recultured (70 000 cells/well) with OP9 cells and cytokines (SCF, IL3, Epo) in the presence (c-Myb–) or absence (c-Myb+) of Tet (1 μg/mL). After 4 days, hematopoietic cells were harvested and counted (A). Mean ± SEM from 3 independent experiments are shown (**P < .01). Cells were then analyzed by flow cytometry for expression of CD45 and CD41 (B). Numbers indicate the percentage of cells in rectangular area. (C) VE-cadherin+CD31+ cells were sorted from differentiating ES cells as described under “Materials and methods.” In some cultures, c-myb transgene expression was induced from 2 days prior to sorting (right panels; c-mybTet/KO; c-Myb+> c-Myb+). Sorted cells were cultured for 9 days with OP9 cells and cytokines (SCF, IL3, Epo) in the absence of Tet. Hematopoietic cells generated were analyzed by flow cytometry for expression of lineage markers. Results shown are representative of at least 3 independent experiments. Numbers indicate the percentage of cells in population.
Hematopoietic cell generation from c-myb knockout endothelial cells restored by c-Myb induction. (A-B) Wild-type ES cells (WT) and c-myb–/–ES cells carrying the Tet-regulated c-myb transgene (c-mybTet/KO) were allowed to differentiate on OP9 stromal cell layers in the presence of Tet (1 μg/mL) for 5 days. VE-cadherin+CD31+CD45–CD41– cells were sorted by FACS and recultured (70 000 cells/well) with OP9 cells and cytokines (SCF, IL3, Epo) in the presence (c-Myb–) or absence (c-Myb+) of Tet (1 μg/mL). After 4 days, hematopoietic cells were harvested and counted (A). Mean ± SEM from 3 independent experiments are shown (**P < .01). Cells were then analyzed by flow cytometry for expression of CD45 and CD41 (B). Numbers indicate the percentage of cells in rectangular area. (C) VE-cadherin+CD31+ cells were sorted from differentiating ES cells as described under “Materials and methods.” In some cultures, c-myb transgene expression was induced from 2 days prior to sorting (right panels; c-mybTet/KO; c-Myb+> c-Myb+). Sorted cells were cultured for 9 days with OP9 cells and cytokines (SCF, IL3, Epo) in the absence of Tet. Hematopoietic cells generated were analyzed by flow cytometry for expression of lineage markers. Results shown are representative of at least 3 independent experiments. Numbers indicate the percentage of cells in population.
We quantified hematopoietic progenitor cells by overlaying semisolid medium containing methylcellulose 15 hours after replating endothelial cells on OP9. Figure 3A shows that a wide range of myeloid and erythroid cell colonies were generated in the wild-type cell cultures, whereas only macrophage colonies and a few macrophage/granulocyte colonies were observed in the c-myb–/– cell cultures. As expected from the results shown in Figure 2, induction of c-Myb expression in the c-myb–/– cultures restored formation of hematopoietic cell colonies. The c-Myb–rescued cells showed a tendency to generate more colonies on average than wild-type cells, although we could demonstrate a statistically significant increase only in granulocyte/erythrocyte/macrophage colonies and megakaryocyte colonies (Figure 3A).
It is known that ES cells are able to differentiate into mature megakaryocytes when cultured on OP9 cells.37 These mature megakaryocytes are typically large cells with polylobulated nuclei and are positive for CD41 and AChE staining. The final phase of differentiation of these megakaryocytes involves projection of long-beaded proplatelets and release of platelets into the culture medium. We found that megakaryocyte colonies generated in the c-Myb–rescued c-myb–/– cultures consisted of large cells with a homogeneous morphology that were rarely observed in wild-type megakaryocyte colonies (Figure 3B). These cells showed high EGFP fluorescence under the microscope (Figure 3B), and May-Grunwald Giemsa staining of picked cells demonstrated large chromatin-condensed polylobulated nuclei, confirming that they are mature megakaryocytes (Figure 3B). Although proplatelet processes budding off platelets were frequently observed in wild-type megakaryocyte colonies, no such extension of membrane was observed on the EGFPhigh megakaryocytes (Figure 3B), suggesting a block to terminal differentiation of the c-Myb–rescued cells.
Hematopoietic cell colony formation by c-myb knockout endothelial cells is restored by c-Myb induction. (A) VE-cadherin+CD31+ cells were sorted from differentiating wild-type ES cells and c-mybTet/KO ES cells as described in Figure 2. Cells were recultured (3000 cells/well) for 6 days with OP9 cells and cytokines (SCF, IL3, Epo) in the presence (c-Myb–) or absence (c-Myb+) of Tet (1 μg/mL). To quantify hematopoietic cell colonies, the cultures were overlaid with a semisolid medium from the second day on. Ery indicates erythroid colony; G, granulocyte colony; GM, granulocyte/macrophage colony; M, macrophage colony; GEM, granulocyte/erythrocyte/macrophage colony; GEMMeg, granulocyte/erythrocyte/macrophage/megakaryocyte colony; GMMeg, granulocyte/macrophage/megakaryocyte colony; Meg, megakaryocyte colony. Mean ± SEM from 3 independent experiments are shown (*P < .05). (B) Phase-contrast microscopy images of representative megakaryocyte and GM colonies derived from wild-type (i) and c-Myb–rescued c-myb–/– endothelial cells (iii) are shown (Nikon Eclipse TE300). Rectangular areas indicated by dashed lines were further magnified (ii,iv). A fluorescence microscopy image (v) of the same megakaryocyte colony (iii) is shown. The colony was picked and stained with May-Grunwald Giemsa solution (vi) (Leica DMLS). Meg indicates megakaryocyte; P, proplatelet; GM, granulocyte/macrophage. Original magnifications: panels i, iii, and v, 100× (Plan Fluor 10×/0.30 NA objective); panel vi, 1000× (C Plan 100×/1.25 NA oil objective).
Hematopoietic cell colony formation by c-myb knockout endothelial cells is restored by c-Myb induction. (A) VE-cadherin+CD31+ cells were sorted from differentiating wild-type ES cells and c-mybTet/KO ES cells as described in Figure 2. Cells were recultured (3000 cells/well) for 6 days with OP9 cells and cytokines (SCF, IL3, Epo) in the presence (c-Myb–) or absence (c-Myb+) of Tet (1 μg/mL). To quantify hematopoietic cell colonies, the cultures were overlaid with a semisolid medium from the second day on. Ery indicates erythroid colony; G, granulocyte colony; GM, granulocyte/macrophage colony; M, macrophage colony; GEM, granulocyte/erythrocyte/macrophage colony; GEMMeg, granulocyte/erythrocyte/macrophage/megakaryocyte colony; GMMeg, granulocyte/macrophage/megakaryocyte colony; Meg, megakaryocyte colony. Mean ± SEM from 3 independent experiments are shown (*P < .05). (B) Phase-contrast microscopy images of representative megakaryocyte and GM colonies derived from wild-type (i) and c-Myb–rescued c-myb–/– endothelial cells (iii) are shown (Nikon Eclipse TE300). Rectangular areas indicated by dashed lines were further magnified (ii,iv). A fluorescence microscopy image (v) of the same megakaryocyte colony (iii) is shown. The colony was picked and stained with May-Grunwald Giemsa solution (vi) (Leica DMLS). Meg indicates megakaryocyte; P, proplatelet; GM, granulocyte/macrophage. Original magnifications: panels i, iii, and v, 100× (Plan Fluor 10×/0.30 NA objective); panel vi, 1000× (C Plan 100×/1.25 NA oil objective).
Arrested maturation of c-Myb–rescued erythroblasts
As indicated in the previous section, maturation of erythroid lineage cells appeared to be arrested in c-Myb–rescued cultures initiated from c-myb–/– endothelial cells. Erythroid colonies were less hemoglobinized in c-Myb–rescued cultures compared with wild-type cultures (data not shown). We closely examined erythroid maturation of the cells cultured on OP9 for 9 days by monitoring expression of CD71 and Ter119 surface antigens and GATA transcription factors. Wild-type cell cultures showed a high ratio of CD71highTer119high to CD71highTer119–/low cells (Figure 4A). A significant number of CD71lowTer119high cells were also present, in agreement with a previous report that maturation toward early erythrocytes in Ter119+ erythroblasts is accompanied by down-regulation of CD71 expression.38 In contrast, up-regulation of Ter119 expression was perturbed in CD71high cells present in c-Myb–rescued c-myb–/– cultures (Figure 4A). Few CD71low Ter119+ cells were detected in these cultures.
RT-PCR analyses demonstrated a down-regulation of c-myb expression on transition of CD71highTer119– cells to CD71high Ter119+ in wild-type cell cultures (Figure 4B). Therefore, it is possible that overexpression of c-Myb affected maturation of erythroblasts in c-Myb–rescued cultures. Ectopic expression of c-Myb in CD71highTer119+ cells increased Gata2 mRNA that would otherwise be down-regulated on normal differentiation of erythroblasts (Figure 4B). Compared with Gata2, Gata1 expression was not as significantly influenced by c-Myb activation. We tried to rescue erythroid differentiation of c-myb–/– endothelial cells by conditioning the concentration of Tet. However, development of erythroid lineage cells was diminished in conditions leading to the lower activation of the c-myb transgene (Figure 4A). We observed that CD71lowTer119– cells were still present under conditions of lower activation of c-Myb. Differential counts of the harvested cells demonstrated the presence of macrophages, megakaryocytes, and erythroblasts, suggesting heterogeneity in this population (Table S1).
Disturbance of erythrocyte maturation by forced expression of c-Myb. (A) VE-cadherin+CD31+cells were sorted from differentiating wild-type ES cells and c-mybTet/KO ES cells as described in Figure 2. Cells were recultured for 9 days with OP9 cells and cytokines (SCF, IL3, Epo) in the presence of different concentrations of Tet. Cells were harvested and analyzed by flow cytometry for expression of Ter119 and CD71. Numbers indicate the percentage of cells in rectangular area. (B) CD71highTer119– and CD71highTer119+ cells were sorted from wild-type cell cultures and subjected to RT-PCR analysis for c-myb expression (left panels). CD71highTer119+ cells were sorted from wild-type and c-Myb–rescued (0 ng/mL Tet) cultures and subjected to RT-PCR analyses for expression of c-myb, Gata1, and Gata2 genes (right panels). Results shown are representative of at least 3 independent experiments. (C) VE-cadherin+CD31+ cells sorted from differentiating wild-type ES cells and c-mybTet/KO ES cells were recultured for 5 days with OP9 cells and cytokines in the absence of Tet. Tet (1 μg/mL) was then added in some cultures (Tet–> Tet+) and incubation continued, whereas others were kept in the absence of Tet (WT and Tet– > Tet–). After 3 days of incubation, cells were harvested and analyzed by FACS for expression of Ter119 and CD71. CD71highTer119+ cells were further sorted and subjected to RT-PCR analyses for expression of c-myb and Gata2 genes. Numbers indicate the percentage of cells in rectangular area.
Disturbance of erythrocyte maturation by forced expression of c-Myb. (A) VE-cadherin+CD31+cells were sorted from differentiating wild-type ES cells and c-mybTet/KO ES cells as described in Figure 2. Cells were recultured for 9 days with OP9 cells and cytokines (SCF, IL3, Epo) in the presence of different concentrations of Tet. Cells were harvested and analyzed by flow cytometry for expression of Ter119 and CD71. Numbers indicate the percentage of cells in rectangular area. (B) CD71highTer119– and CD71highTer119+ cells were sorted from wild-type cell cultures and subjected to RT-PCR analysis for c-myb expression (left panels). CD71highTer119+ cells were sorted from wild-type and c-Myb–rescued (0 ng/mL Tet) cultures and subjected to RT-PCR analyses for expression of c-myb, Gata1, and Gata2 genes (right panels). Results shown are representative of at least 3 independent experiments. (C) VE-cadherin+CD31+ cells sorted from differentiating wild-type ES cells and c-mybTet/KO ES cells were recultured for 5 days with OP9 cells and cytokines in the absence of Tet. Tet (1 μg/mL) was then added in some cultures (Tet–> Tet+) and incubation continued, whereas others were kept in the absence of Tet (WT and Tet– > Tet–). After 3 days of incubation, cells were harvested and analyzed by FACS for expression of Ter119 and CD71. CD71highTer119+ cells were further sorted and subjected to RT-PCR analyses for expression of c-myb and Gata2 genes. Numbers indicate the percentage of cells in rectangular area.
We next examined whether reintroduction of Tet into the arrested CD71highTer119–/low cells restored maturation of erythroid cells. Sorted endothelial cells were cultured for 5 days in the absence of Tet to induce erythroid differentiation, followed by reexposure to Tet for 3 days. RT-PCR analyses revealed that re-addition of Tet completely abolished expression of c-myb transcripts (Figure 4C). Interestingly, Gata2 transcripts were still detected even after suppression of the c-myb transgene. Nevertheless, further maturation of the arrested CD71highTer119–/low cells was not restored by reintroduction of Tet (Figure 4C).
Failure of B-cell differentiation from c-Myb–rescued endothelial cells
B lymphocytes are generated from ES cell–derived endothelial cells by culturing with OP9 cells in the presence of IL7, Flt3L, and SCF.39 We investigated whether B-cell development could be rescued by inducing c-Myb expression in c-myb–/– endothelial cells. Although wild-type endothelial cells gave rise to B220+CD19+CD43+IL7Rα+/– pro/pre-B cells in this culture condition, only a few B220+CD43+ cells were generated from c-myb–/– endothelial cells even under the c-Myb–rescued condition (Figure 5A). These B220+CD43+ cells expressed neither CD19 nor IL7Rα (Figure 5A). Induction of c-Myb initiated 2 days prior to sorting still failed to restore differentiation of B lymphocytes from c-myb–/– endothelial cells (data not shown). We also tried to rescue B-cell development by titrating the concentration of Tet. As shown in Figure 5B, however, B-cell differentiation could not be rescued in conditions leading to the lower activation of the c-myb transgene. Although B220+CD43+CD19– cells were still detected, these cells did not express EGFP. These results suggest that the level of c-Myb should be strictly regulated on commitment and differentiation of B lymphocytes.
Discussion
In vitro differentiation of ES cells has been used to investigate the development of the hematopoietic system. However, studies comparing wild-type and genetically modified ES cells are often hampered by epigenetic variation between clones, which can have a profound influence on differentiation potential. The tetracycline-regulated system used in this study provides the advantage that a single ES cell clone can be used to assess the effect of specific gene expression through the simple addition or removal of tetracycline to control the place, level, and time of that expression. By enabling tetracycline-regulated c-myb gene expression in cells derived from c-myb knockout ES cells (Figure 1) we have been able to clarify the stage-specific function of c-Myb during the course of hematopoietic cell development from endothelial cells.
Failure of c-Myb–rescued endothelial cells to generate B lymphocytes. (A) VE-cadherin+CD31+ cells were sorted from differentiating wild-type ES cells and c-mybTet/KO ES cells as described in Figure 2. Cells were recultured for 13 days with OP9 cells and cytokines (SCF, Flt3L, IL7) in the presence (c-Myb–) or absence (c-Myb+) of Tet (1 μg/mL). Floating cells were harvested and analyzed by flow cytometry for expression of B220, CD19, and CD43. The B220+CD43+ population was gated and further analyzed for IL7Rα expression. (B) VE-cadherin+CD31+ cells sorted from differentiating wild-type ES cells and c-mybTet/KO ES cells were recultured for 13 days with OP9 cells and cytokines in the presence of different concentrations of Tet. Floating cells were harvested and analyzed by flow cytometry for expression of B220, CD19, CD43, and EGFP. Results shown are representative of at least 3 independent experiments. Numbers indicate the percentage of cells in population.
Failure of c-Myb–rescued endothelial cells to generate B lymphocytes. (A) VE-cadherin+CD31+ cells were sorted from differentiating wild-type ES cells and c-mybTet/KO ES cells as described in Figure 2. Cells were recultured for 13 days with OP9 cells and cytokines (SCF, Flt3L, IL7) in the presence (c-Myb–) or absence (c-Myb+) of Tet (1 μg/mL). Floating cells were harvested and analyzed by flow cytometry for expression of B220, CD19, and CD43. The B220+CD43+ population was gated and further analyzed for IL7Rα expression. (B) VE-cadherin+CD31+ cells sorted from differentiating wild-type ES cells and c-mybTet/KO ES cells were recultured for 13 days with OP9 cells and cytokines in the presence of different concentrations of Tet. Floating cells were harvested and analyzed by flow cytometry for expression of B220, CD19, CD43, and EGFP. Results shown are representative of at least 3 independent experiments. Numbers indicate the percentage of cells in population.
We detected CD45+CD41+ cells transiently differentiated from c-myb–/– endothelial cells even when the c-myb transgene was kept silent (Figure 2). Studies have identified CD45+CD41+ cells as committed hematopoietic progenitor cells both arising during the differentiation of ES cells and in early mouse embryos.33-36 The CD45+CD41+ cells derived from wild-type endothelial cells have the potential to give rise to definitive hematopoietic cell colonies (data not shown). Clarke et al25 detected increased proportions of cells expressing CD34 and Sca-1, which phenotypically represented early precursors of definitive hematopoietic cells, in embryoid bodies generated from c-myb–/– ES cells.25 Together, these results suggest that c-Myb is not an absolute requirement for hematopoietic commitment from endothelial cells.
Despite the observation of commitment, robust production of hematopoietic cells, as observed in cultures initiated from wild-type endothelial cells, was never achieved in cultures of differentiating c-myb–/– ES cells, suggesting that c-Myb is essential for proliferation or differentiation of committed hematopoietic progenitor cells. In agreement with this latter conclusion, we were able to rescue stromal cell–assisted in vitro hematopoiesis by activating the c-myb transgene in c-myb–/– cells (Figures 2, 3). More noteworthy is the fact that c-Myb induction after sorting and replating endothelial cells was enough to rescue the efficient generation of hematopoietic cells. Activation of the c-myb transgene in differentiating c-myb–/– ES cells resulted in higher levels of 75-kDa c-Myb protein expression compared with that seen in wild-type ES cells (Figure 1). Probably as a consequence of this above normal c-Myb expression, CD45+CD41+ hematopoietic progenitor cells were increased in the rescued endothelial cell cultures compared with the wild-type controls (Figure 2). We previously reported that the c-myb gene is expressed in hemogenic endothelial cells.6,14 However, although the c-Myb protein preexisting in hemogenic endothelial cells may not be a prerequisite for initiation of hematopoiesis, it remains difficult to definitively conclude from our results whether c-Myb exerts its function only after the commitment of hematopoietic cells from endothelial cells.
Forced expression of c-Myb appeared to inhibit terminal maturation of megakaryocytes and erythrocytes. We found that megakaryocyte colonies in c-Myb–rescued cultures consisted of large polylobulated cells with no sign of platelet production (Figure 3). Furthermore, differentiation of erythroid cells was disturbed at the stage between CD71highTer119– and CD71highTer119low proerythroblasts in c-Myb–rescued c-myb–/– cultures (Figure 4). Therefore, activation of expression to the correct level as well as down-regulation at the appropriate time during differentiation seems to be important for c-Myb to regulate differentiation of hematopoietic cells. In light of these observations, it is interesting that colony assays of fetal liver cells from mouse embryos bearing a knock-down allele of c-myb showed an increase in colonies containing a high proportion of megakaryocytes compared with wild type, whereas erythroid progenitors were significantly reduced.21 Recently, several ENU-induced mutations in c-Myb have been described that lead to a range of hematopoietic effects, including increases in bone marrow megakaryocytes and peripheral platelet numbers.22,23 One of these c-Myb mutations is in the transactivation domain and disrupts the protein's interaction with the transcriptional coactivator p300.23 The latter mutation was also shown to inhibit erythroid differentiation of megakaryocyte/erythrocyte progenitors (MEPs),23 and it was proposed that c-Myb drives erythroid differentiation and suppresses megakaryocyte differentiation of MEPs. Our data indicate that forced expression of c-Myb does not affect proliferation of MEPs nor their commitment to the erythrocyte and megakaryocyte lineages (Figure 3). However, the terminal differentiation of megakaryocytes appeared to be perturbed by c-Myb overexpression (Figure 3), suggesting that c-Myb needs to be down-regulated for maturation of megakaryocytes. A similar conclusion has been obtained from experiments in which megakaryocyte differentiation was stimulated when the c-myb gene was conditionally inactivated in megakaryoblasts arrested by a knockdown mutation in the Gata1 gene (Oscar Berlanga and J.F., in preparation).
The erythroid population generated ex vivo from fetal liver cells of c-myb knockdown embryos was shown to be arrested at the CD71highTer119– stage, corresponding to the CFU-E, whereas wild-type cells progressed more effectively through the proerythroblast to basophilic erythroblast stages.21 Forced expression of c-Myb protein in ES cell–derived hematopoietic progenitors resulted in a similar phenotype to the c-myb knockdown fetal liver cells (Figure 4). This suggests that the amount of c-Myb protein should be kept at an appropriate level or, alternatively, that down-regulation of c-Myb is necessary to allow proerythroblasts to differentiate further. Constitutive expression of c-Myb has been reported to block erythrocyte maturation.19,40 We found that c-myb expression was down-regulated on transition from the CD71highTer119– to CD71highTer119+ stage (Figure 4). Ectopic expression of c-Myb in CD71highTer119low cells resulted in an activation of Gata2 which has been proposed to preserve immaturity of hematopoietic precursor cells.41 However, it is an open question as to whether the arrest in maturation of c-Myb–rescued erythroblasts can be attributed to the elevation of Gata2 expression, because forced expression of Gata2 was reported to increase the production of red blood cells in the OP9-assisted ES-cell differentiation system.42 Nevertheless, abolition of ectopic c-Myb by the reintroduction of Tet to the arrested CD71highTer119–/low cells did not diminish Gata2 expression nor did it restore the arrest of differentiation (Figure 4). Hence, other molecules that are induced by c-Myb activation are also suggested to be responsible for the inhibition.
Forced expression of c-Myb failed to rescue B-lymphocyte differentiation in c-myb–/– endothelial cells (Figure 5). Although we detected generation of a few B220+CD19–CD43+ cells in c-myb–/– cultures, this population was EGFP– even under the c-Myb–rescued conditions, indicating that development of even the earliest progenitor cells of B-lymphoid lineage could not be restored. Mice homozygous for the c-myb knockdown allele showed an arrest in B-cell differentiation at the B220+CD43+ pro-B cell stage in their bone marrow.21 Similarly, disruption of the interaction between c-Myb and p300 resulted in a decrease in the numbers of intramarrow pro-B and pre-B cells,23 indicating that B-cell differentiation beyond the pro-B cell stage is dependent on a threshold level of c-Myb activation. In contrast, the most immature B-lymphoid precursors were not affected in c-myb mutant mice. We could not detect any cells overexpressing c-Myb protein in the B220+ population generated from wild-type endothelial cells in which c-Myb was additionally induced from the same Tetregulated transgene (data not shown). These observations suggest that a high level of c-Myb is not essential for, or may even be deleterious to, the differentiation of the earliest progenitors of B-cell lineage. This is reminiscent of the role of PU.1, another transcription factor essential for B-cell development. Mice lacking PU.1 fail to generate mature myeloid or B-lineage cells.43,44 Although high levels of PU.1 favor differentiation of precursors into myeloid lineage cells, lower levels in contrast favor generation of B-lineage cells by activating IL7Rα expression.45,46 It can be proposed therefore that a combined dosage of transcription factors is required for the progression of early B-cell differentiation.
The c-Myb protein is known to cooperate with a variety of other transcription factors in the transcriptional transactivation of target promoters.47 For instance, c-Myb activates the mim-1 gene only in cooperation with Ets2 or C/EBPβ.48,49 Formation of a multiprotein complex of transcription factors often results in competition with other complexes for a common component, as appears to be the case for the involvement of CBP in distinct complexes with Gata1 and c-Myb.50 This type of situation probably underlies indirect effects of transcription factor levels on gene regulation. Our results presented here illustrate the importance of achieving the correct levels of c-Myb protein during hematopoietic cell development and support the hypothesis that different levels of c-Myb are required for progression of distinct steps of differentiation.
Prepublished online as Blood First Edition Paper, April 4, 2006; DOI 10.1182/blood-2005-09-3846.
Supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (no. 14081204) and the Japan Society for the Promotion of Science (no. 15790495); by a postdoctoral fellowship from the 21st Century COE, Kumamoto University (T.F.); and by a Wellcome Trust Senior Research Fellowship (J.F.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr S. Ishii for the pACT–c-myb plasmid; Dr H. Niwa for the pPyCAGZeocinpA plasmid; Dr T. Era for the pUHD10-3-puromycin, pUHD10-3-IRES-EGFP, and pCAG-tTA plasmids; and Dr D. Maennel for the anti-CD41 mAb. We thank Kirin Brewery Co, Ltd, for the generous gift of recombinant human erythropoietin. We also thank Dr S. Fraser for critical comments on the manuscript and Y. Shimoda for technical assistance.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal