Abstract
The absence of mutations in the IgV genes, together with the presence of ZAP-70 and CD38, are the most reliable negative prognostic markers for chronic lymphocytic leukemia (CLL) patients. Several lines of evidence indicate that CD38 may be not only a diagnostic marker but also a key element in the pathogenetic network in CLL. First, CD38 is a receptor that induces proliferation and increases survival of CLL cells. Second, CD38 signals start upon interaction with the CD31 ligand expressed by stromal and nurse-like cells. Third, CD38/CD31 contacts up-regulate CD100, a semaphorin involved in sustaining CLL growth. Fourth, evidence that nurselike cells express high levels of CD31 and plexin-B1, the high-affinity ligand for CD100, offers indirect confirmation for this model of receptor cross-talk. Elements of variation in the clinical course of CD38+ CLL patients include (1) potential intersection with ZAP-70, a kinase involved in the CD38 signaling pathway in T and natural killer (NK) cells, and (2) the effects of genetic polymorphisms of the receptors involved, at least of CD38 and CD31. Consequently, CD38 together with ZAP-70 appear to be the key elements of a coreceptor pathway that may sustain the signals mediated by the B-cell receptor and potentially by chemokines and their receptors. This would result in acquisition of increased survival potential, providing clues to the poorer prognosis of CD38+ patients.
Introduction
Chronic lymphocytic leukemia (CLL) is defined as a proliferation of B lymphocytes that express surface CD19 or CD20, CD5, CD23, and low levels of immunoglobulin (Ig), CD79β, and CD22.1 CLL is a heterogeneous disease: some patients experience a slowly progressive clinical course but most will eventually enter an advanced phase and require recurrent treatment. A significant number of CLL patients show an active form of the disease from the early stages, characterized by refractoriness to treatment, infectious and autoimmune complications, and a relatively rapid fatal outcome.2
Unlike most other B-lymphoproliferative disorders, little is known about the pathogenesis of CLL. The main challenge lies in determining the cellular origin of the disease and, consequently, the degree of immunocompetence of CLL cells. However, no common key cytogenetic abnormalities have been identified that might otherwise offer pathogenetic clues.3,4
Heterogeneity in the clinical behavior of CLL makes it difficult to identify which patients will benefit most from earlier or more aggressive treatment and those who should be treated with more conservative and less toxic approaches. Clinical researchers have long sought to identify a marker (or markers) for use as a prognostic tool.5
The earliest staging systems for CLL relied on disease burden parameters. The Rai and Binet systems were the first to determine a correlation between survival and different measures of disease burden.6,7 Histologic patterns of bone marrow (BM) involvement have also been used as a valid adjunct.8 However, these classifications fail to distinguish patients who will eventually progress to an aggressive form of the disease from those who have a more stable form. Likewise, lymphocyte doubling time (LDT), which is calculated by determining the number of months it takes the absolute lymphocyte count to double, shares the same drawback. Although it offers meaningful evidence about disease kinetics9 and is therefore widely used as a measure of disease aggressiveness, treatment decisions based solely on LDT may come too late for patients who eventually prove to have aggressive forms of the disease.
During the past 15 years, several soluble molecules have been used as prognostic tools in CLL, including thymidine kinase, CD23, and β2-microglobulin (reviewed in Shanafelt et al5 ). Although all of these molecules show some degree of correlation with tumor burden and disease stage in CLL patients, more studies are needed to determine the independence of these markers from stage and LDT as well as to define their role in the management of patients with early stage CLL.
A breakthrough in the identification of markers for the prediction of aggressive CLL came in 2 independent reports published at the end of 1999.10,11 It was shown that patients can be divided into 2 prognostic groups according to the presence (good prognosis) or absence (poor prognosis) of somatic mutations in the immunoglobulin variable region (IgV) genes.10,11 CD38 was initially proposed as a surrogate marker for the absence of IgV gene rearrangement.11 Although a subsequent study has failed to establish a clear-cut correlation with the absence of mutations in the IgV genes,12 CD38 expression can undisputedly be considered as an independent and reliable negative prognostic marker in CLL. CD38+ patients are characterized by an unfavorable clinical course with a more advanced stage of disease, poor responsiveness to chemotherapy, a shorter lapse in time before initial treatment is required, and a shorter survival rate. This conclusion holds after analyzing patients from a variety of different geographic origins and using different staining methods for the evaluation of CD38 expression (Table 1; last reviewed in Boonstra et al35 and Matrai36 ).
CD38 is a negative prognostic factor in CLL, independently of geographic origin or staining procedures
Geographic area . | No. of patients . | Cutoff system . | Reference . |
|---|---|---|---|
| New York, NY | 47 | % (30) | Damle et al11 |
| Houston, TX | 218 | % (20) | Ibrahim et al13 |
| Reggio Calabria, Italy | 161 | CD38 score | Morabito et al14 |
| Rome, Italy | 168 | % (30) | Del Poeta et al15 |
| San Giovanni Rotondo, Italy | 61 | % (30) | D'Arena et al16 |
| Rochester, MN | 131 | % (30) | Jelinek et al17 |
| Vienna, Austria | 81 | % (30) | Heintel et al18 |
| Essen, Germany | 133 | % (20) | Dürig et al19 |
| Nantes, France | 123 | % (30) | Chevallier et al20 |
| Ulm, Germany | 157 | % (20) | Krober et al21 |
| Barcelona, Spain | 155 | % (20) | Domingo-Domenech et al22 |
| Southampton, United Kingdom | 81 | % (30), ABC | Mainou-Fowler et al23 |
| Cleveland, OH | 131 | %, ABC | Hsi et al24 |
| Liverpool, United Kingdom | 140 | % (20) | Manocha et al25 |
| Cleveland, OH | 24 | % (30) | Chang et al26 |
| Genova, Italy | 52 | % (30) | Ottaggio et al27 |
| Essen, Germany | 79 | % (20) | Dürig et al28 |
| Essen, Germany | 252 | % (30) | Schroers et al29 |
| Roma, Italy | 242 | % (7, 20, 30) | Gentile et al30 |
| Cologne, Germany | 109 | % (30) | Mayr et al31 |
| Rochester, MN | 90 | MFI CD38/MFI control | Pittner et al32 |
| Surrey, United Kingdom | 201 | % (7, 30) | Del Giudice et al33 |
| Aviano, Italy | 137 | % (30) | Zucchetto et al34 |
Geographic area . | No. of patients . | Cutoff system . | Reference . |
|---|---|---|---|
| New York, NY | 47 | % (30) | Damle et al11 |
| Houston, TX | 218 | % (20) | Ibrahim et al13 |
| Reggio Calabria, Italy | 161 | CD38 score | Morabito et al14 |
| Rome, Italy | 168 | % (30) | Del Poeta et al15 |
| San Giovanni Rotondo, Italy | 61 | % (30) | D'Arena et al16 |
| Rochester, MN | 131 | % (30) | Jelinek et al17 |
| Vienna, Austria | 81 | % (30) | Heintel et al18 |
| Essen, Germany | 133 | % (20) | Dürig et al19 |
| Nantes, France | 123 | % (30) | Chevallier et al20 |
| Ulm, Germany | 157 | % (20) | Krober et al21 |
| Barcelona, Spain | 155 | % (20) | Domingo-Domenech et al22 |
| Southampton, United Kingdom | 81 | % (30), ABC | Mainou-Fowler et al23 |
| Cleveland, OH | 131 | %, ABC | Hsi et al24 |
| Liverpool, United Kingdom | 140 | % (20) | Manocha et al25 |
| Cleveland, OH | 24 | % (30) | Chang et al26 |
| Genova, Italy | 52 | % (30) | Ottaggio et al27 |
| Essen, Germany | 79 | % (20) | Dürig et al28 |
| Essen, Germany | 252 | % (30) | Schroers et al29 |
| Roma, Italy | 242 | % (7, 20, 30) | Gentile et al30 |
| Cologne, Germany | 109 | % (30) | Mayr et al31 |
| Rochester, MN | 90 | MFI CD38/MFI control | Pittner et al32 |
| Surrey, United Kingdom | 201 | % (7, 30) | Del Giudice et al33 |
| Aviano, Italy | 137 | % (30) | Zucchetto et al34 |
ABC indicates antibody binding capacity; and MFI, mean fluorescence intensity.
Our understanding of the role of CD38 as marker is evolving. Conceptual issues awaiting to be resolved include the degree of overlap between CD38 expression and (i) the absence of mutations in the IgV genes and (ii) the presence of ZAP-70, a cytoplasmic kinase that was recently introduced as a reliable tool in determining CLL prognosis.37 Another point of debate concerns the stability of CD38 expression over time, an important factor that might compromise clinical use of this marker.38 No less important are the technical aspects involved in staining and in determining the cutoff for positivity values. Both issues have great clinical relevance because they impact directly on the percentage of positive or negative patients calculated.
Premise
The aim of this review is to bring together all the available experimental evidence that suggests a causative correlation between CD38 expression and the clinical course of CLL. It differs from other reviews in the field in that it considers CD38 not only as a useful marker for identifying high-risk patients but also as a potential conduit for the delivery of potent positive signals controlling CLL cell proliferation and survival and, ultimately, contributing to the unfavorable prognosis of CD38+ patients. Before presenting this hypothesis in detail, we would like to review the key structural and functional characteristics of human CD38. We will therefore first present an outline of the main biologic features of human CD38. Next we will move on to the distribution and function of CD38 in normal B lymphocytes, and then we will review what is known about CD38 in CLL. Finally, we will turn to our hypothesis concerning the role of CD38 in CLL cell proliferation and survival.
Biology of human CD38
Structure
The protein encoded by the CD38 gene is a type II single-chain transmembrane molecule displaying a canonical molecular weight of approximately 45 kDa.39 The molecule may also exist in a soluble form present in biologic fluids in normal, para-physiologic, and pathologic conditions.40 High molecular weight forms of CD38 are reported in supernatants of cell lines from patients with X-linked agammaglobulinemia (XLA)41 and in myeloid leukemia cells.42 Complete details may be found in Mehta et al.43
Relevant to the hypothesis of CD38 as a signaling molecule (see below) is the observation that CD38 is located in critical areas of the plasma membrane in close physical proximity with professional signaling receptors,44 such as the T-cell receptor (TCR) in T lymphocytes,45 the B-cell receptor (BCR) complex in B cells,46,47 CD16 in natural killer (NK) cells,48 major histocompatibility complex (MHC) class II and CD9 in monocytes,49,50 and the CCR7 chemokine receptor CD83 and CD11b in mature dendritic cells.51 Recent papers have implied the existence of supramolecular complexes acting in conjunction with multiple partners.50 These complexes appear to reside within the cholesterol-rich areas of the plasma membrane52 and to be dependent on their integrity.53
Distribution
The tissue distribution of CD38 closely resembles that of ZAP-70. Although both molecules were initially considered as specific markers of T cells and thymocytes,54,55 additional studies showed that both are non–lineage-restricted. Major details are reviewed in Deaglio et al.56 CD38 expression in the B lineage is analyzed in the pertinent section (see below).
Functions
CD38 is a pleiotropic molecule that behaves simultaneously as an enzyme and as a receptor.
Ectoenzyme. The extracellular domain of CD38 contains an enzymatic site that can generate cyclic ADP ribose (cADPR) and ADPR from nicotine adenine dinucleotide (NAD+) and nicotinic acid adenine dinucleotide phosphate (NAADP) from NADP+.57 Thus, CD38 is one of the ADP-ribosyl cyclases, a family of multifunctional enzymes apparently ubiquitous in eukaryotic cells.58 cADPR, a universal second messenger with the ability to control calcium levels independently of inositol triphosphate (IP3), plays a key role in physiologic processes, including cell proliferation, muscle contraction, stem cell regeneration, and hormone secretion (reviewed in Lee59 and Guse60 ). CD38 attracted the attention of biochemists because of the apparent paradox of a reaction generating an essential second messenger (cADPR) in an antieconomical fashion. Indeed, the enzyme works in an environment with only trace amounts of substrate, and the final product is used inside the cell, as reviewed in De Flora et al.61 Recent data indicate that CD38 enzymatically controls the availability of extracellular NAD+ as a substrate for ADP-ribosyltransferase (ART)–catalyzed ADP ribosylation of cell surface proteins.62
Receptor. Agonistic monoclonal antibodies (mAbs) were first used to study the effects of CD38 engagement. The signaling events were analyzed in detail in distinct lineages and at discrete developmental stages (reviewed in Malavasi et al63 and Deaglio and Malavasi64 ). The emerging picture is complex and nonunivocal, although a few common threads have emerged.
CD38 engagement is followed by a signaling cascade typical of the canonical receptors, including tyrosine phosphorylation of a sequence of intracellular enzymes, nuclear events, and more long-term effects dependent on active protein synthesis. The individual steps of the signaling pathway vary according to the model studied; however, a common player in the CD38 pathway is the proto-oncogene c-cbl, which is reported to be tyrosine phosphorylated in every cell model tested, including T, B, NK, and myeloid cells.49,65-67 This observation suggests that CD38 may play a role in the fine-tuning of antigen receptor signaling. Downstream of cbl, the signal appears to be funneled through the ERK1/2 cross-point, at least in T and NK cells.48,68
An increase in the cytoplasmic levels of calcium ions is also a common theme following activation of CD38. The calcium wave is typically low, slow in rising, and long-lasting compared with the spikes obtained after signaling through the antigen receptors in T and B lymphocytes. This may indicate that the molecular mechanisms responsible for calcium mobilization are different. In several instances, it was possible to determine phospholipase C-γ (PLC-γ) tyrosine phosphorylation upon CD38 cross-linking, suggesting that the conventional IP3 pathway may account for calcium mobilization45,69 ; in other instances, and mostly using murine models, PLC-γ activation could not be detected.70 Whether cADPR produced extracellularly by CD38 contributes to the signaling processes is still a matter of debate. At present, it cannot be convincingly ruled out, as the deletion of the catalytically active site by means of site-directed mutagenesis is followed by a lack of binding by the available agonistic mAbs.71
What ignites the signal? The lack of correlation between CD38-mediated signals and the production of cADPR, ADPR, and/or nicotinamide prompted the search for nonsubstrate ligands capable of initiating the signaling process. The finding that CD38 could modulate CD4+/CD45RA+ naive T-lymphocyte adhesion to endothelial cells culminated in identification of CD31/PECAM-1, an Ig superfamily member mainly involved in the modulation of leukocyte adhesion to the vessels, as a ligand for CD38.72,73 In subsequent years, most of the signals recorded using agonistic mAbs were reproduced by using the CD31 ligand. These included mobilization of calcium signaling as well as more structured events, such as proliferation and cytokine induction.74
The structural requirements for signaling are still unclear and the role of the cytoplasmic tail still controversial at this point. On the one hand, Lund et al75 showed convincing evidence that the presence of the intracellular residues of CD38 is irrelevant for signals and enzymatic activities. Moreover, it is generally accepted that the tail does not contain signaling motifs. However, other studies have determined, at least in T lymphocytes, a direct association between the tail of CD38 and the SH2 domain of the kinase lck.76
As mentioned above, CD38 is laterally associated with professional signaling receptors in all the cell lineages thus far studied. Significantly, the association between CD38 and other signaling receptors was deemed to have a functional nature, as implied by the results of experiments with T, B, and NK cell line models genetically modified so as to lack functional signaling receptors.45,46,48 This hypothesis was also confirmed through the use of normal cells that lack functional receptors.77 Table 2 summarizes the major functional features of CD38 in distinct lineages.
Main functional features of human CD38 in distinct cell lineages and populations
Cell lineage . | Associated molecules . | Signaling pathway . | Functions . |
|---|---|---|---|
| Thymocytes | CD3ϵ | Unknown | Control of apoptosis |
| Circulating T cells | CD3/TCR complex | ZAP-70, PLC-γ1, ERK1/2 | Activation, proliferation, cytokine release |
| Residential T cells | Unknown (CD2?) | ERK1/2 | Activation, cytokine release |
| Circulating NK cells | CD16 | CD3ζ, ZAP-70, Cbl, ERK1/2 | Regulation of cytotoxicity |
| LAK cells | Unknown | Unknown | Cytokine release, IL-8—mediated migration |
| Monocytes | MHC class II, CD9 | c-cbl, Fgr, Hck | Modulation of superantigen presentation; cytokine release; chemotaxis to CXCR4, CCR1, and CCR5 ligands |
| Dendritic cells | CCR7, CD83, CD11b | Unknown | Maturation, IL-12 induction, chemotaxis, and transendothelial migration to CCL21 |
| Immature B cells | CD19 | c-cbl, PLC-γ2, PI-3K, ERK1/2 | Apoptosis |
| Mature B cells | sIgM, BCR complex | Bcl-2 upregulation | Rescue from apoptosis |
Cell lineage . | Associated molecules . | Signaling pathway . | Functions . |
|---|---|---|---|
| Thymocytes | CD3ϵ | Unknown | Control of apoptosis |
| Circulating T cells | CD3/TCR complex | ZAP-70, PLC-γ1, ERK1/2 | Activation, proliferation, cytokine release |
| Residential T cells | Unknown (CD2?) | ERK1/2 | Activation, cytokine release |
| Circulating NK cells | CD16 | CD3ζ, ZAP-70, Cbl, ERK1/2 | Regulation of cytotoxicity |
| LAK cells | Unknown | Unknown | Cytokine release, IL-8—mediated migration |
| Monocytes | MHC class II, CD9 | c-cbl, Fgr, Hck | Modulation of superantigen presentation; cytokine release; chemotaxis to CXCR4, CCR1, and CCR5 ligands |
| Dendritic cells | CCR7, CD83, CD11b | Unknown | Maturation, IL-12 induction, chemotaxis, and transendothelial migration to CCL21 |
| Immature B cells | CD19 | c-cbl, PLC-γ2, PI-3K, ERK1/2 | Apoptosis |
| Mature B cells | sIgM, BCR complex | Bcl-2 upregulation | Rescue from apoptosis |
LAK indicates lymphokine-activated killer.
Genetics
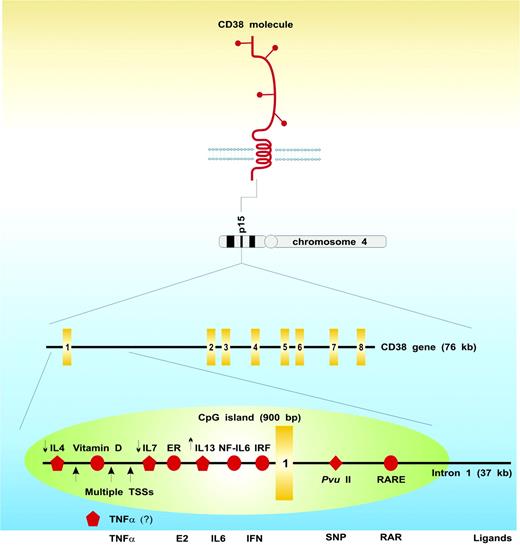
The gene encoding CD38 is located on chromosome 4 (4p15). CD38 consists of 8 exons, spans 70.6 kilobase pairs, and has a notably long first intron (∼ 37 kb78 ; Figure 1). CD38 has a well-characterized single-nucleotide polymorphism (SNP) located at the 5′ end of this intron (184C>G), which results in the presence (or absence) of a PvuII restriction site.79 The frequency of the 3 genotypes has been established in healthy Italian-born adults as 70% CC, 26% CG, and 4% GG. The SNP is located in an intronic hotspot, which is part of a CpG island located at the 5′ end of the gene and which also contains the functional CD38 retinoic acid responsive element responsible for the dramatic upregulation of CD38 expression induced by ATRA.80 In addition, evidence based on a novel truncated CD38 mRNA transcript suggests that one means of controlling CD38 gene expression involves control of transcriptional elongation, where a stop-or-go decision is taken at the 5′ end of intron 1.80
Comparison between human and murine CD38
The majority of the data discussed here were obtained using human cells. Of note, several differences between human and murine CD38 have been described, the most striking of which are listed in Table 3.
Comparison of human and murine CD38
. | Human . | Murine . |
|---|---|---|
| Structure | 45 kDa, presence of high-molecular-weight forms (p78 and p190), presence of a soluble form | 45 kDa, presence of homodimers, no data on the presence of a soluble form |
| Distribution | Predominantly naive T cells, absent on resting B cells (see below), NK cells, mature dendritic cells | Predominantly splenic and circulating B cells; absent on resting T cells, granulocytes, and immature and mature dendritic cells |
| Function | Activation of T and NK cells, suppression of B lymphopoiesis, rescue from apoptosis of germinal center B cells, cytokine and chemokine production, regulation of adhesion and extravasation | Activation of B cells, cytokine and chemokine production, granulocyte migration to inflammatory sites, dendritic cell trafficking. |
| Ligand | NAD+/NADP+, CD31 | NAD+/NADP+, CD31, unknown p66 and p130 |
| Gene | Chromosome 4, 70 kb; 8 exons; intron 1 ≈ × kb with RARE | Chromosome 5, 43 kb; intron 1 ≈ × kb |
. | Human . | Murine . |
|---|---|---|
| Structure | 45 kDa, presence of high-molecular-weight forms (p78 and p190), presence of a soluble form | 45 kDa, presence of homodimers, no data on the presence of a soluble form |
| Distribution | Predominantly naive T cells, absent on resting B cells (see below), NK cells, mature dendritic cells | Predominantly splenic and circulating B cells; absent on resting T cells, granulocytes, and immature and mature dendritic cells |
| Function | Activation of T and NK cells, suppression of B lymphopoiesis, rescue from apoptosis of germinal center B cells, cytokine and chemokine production, regulation of adhesion and extravasation | Activation of B cells, cytokine and chemokine production, granulocyte migration to inflammatory sites, dendritic cell trafficking. |
| Ligand | NAD+/NADP+, CD31 | NAD+/NADP+, CD31, unknown p66 and p130 |
| Gene | Chromosome 4, 70 kb; 8 exons; intron 1 ≈ × kb with RARE | Chromosome 5, 43 kb; intron 1 ≈ × kb |
The main differences between human and murine CD38 are reviewed in Deaglio and Malavasi.81
CD38 and normal human B lymphocytes
Expression
CD38 expression is tightly regulated during B-cell ontogenesis and is present at high levels in BM precursors, is downregulated in resting normal B cells, and then re-expressed in terminally differentiated plasma cells. This seesaw behavior suggests that CD38, though not a lineage marker, is expressed at times during B-cell development when cell-to-cell interactions are crucial.63 CD38 is also extremely useful in classifying functional mature B-lymphocyte subsets. The simultaneous evaluation of CD38 and IgD surface expression levels allowed identification of 5 discrete cell subsets corresponding to critical developmental stages of mature B lymphocytes.82 According to this model, CD38 expression is induced once naive B lymphocytes are activated, peaks when B cells enter the germinal center, decreases during centrocyte/centroblast differentiation, and is completely absent in memory B cells. Thus, CD38 expression is one of the early markers of mature naive B-cell activation, is upregulated before B cells enter the germinal center, and undergoes somatic mutations in the IgV genes.83-85
Schematic portrait of the human CD38 molecule and gene. Full details may be found in the pertinent sections of the paper. The yellow rectangles identify the single exons; the green oval, the CpG island; the red circles, specific responsive elements in the regulatory region of the gene; the red pentagons, regulatory interleukins for which no responsive element has been identified (the small arrows indicate whether they exert upmodulatory or downmodulatory effects); the red diamond, the polymorphic site; ER, estrogen-responsive element; IRF, interferon-responsive element; RARE, retinoic acid–responsive element; TSSs, transcription start sites (indicated by the big arrows); E2, estrogen; SNP, single-nucleotide polymorphism; and RAR, retinoic acid receptor. The modulatory role of TNFα on CD38 expression is unpublished (Mone Zaidi, personal communications, January 2006).
Schematic portrait of the human CD38 molecule and gene. Full details may be found in the pertinent sections of the paper. The yellow rectangles identify the single exons; the green oval, the CpG island; the red circles, specific responsive elements in the regulatory region of the gene; the red pentagons, regulatory interleukins for which no responsive element has been identified (the small arrows indicate whether they exert upmodulatory or downmodulatory effects); the red diamond, the polymorphic site; ER, estrogen-responsive element; IRF, interferon-responsive element; RARE, retinoic acid–responsive element; TSSs, transcription start sites (indicated by the big arrows); E2, estrogen; SNP, single-nucleotide polymorphism; and RAR, retinoic acid receptor. The modulatory role of TNFα on CD38 expression is unpublished (Mone Zaidi, personal communications, January 2006).
From a purely morphologic point of view, CD38+ B lymphocytes are found primarily within the germinal center of secondary lymphoid organs, whereas the marginal zone is predominantly CD38–,86 although some CD38dim/IgM+ cells may be found.87 The latter point is highly relevant to the context of CLL, given recent models that hypothesize that the CLL cell derives from T-cell–independent marginal zone B cells with different degrees of antigenic exposure and pressure. The finding of CD38 expression in selected cases of CLL is consistent with data suggesting that CLL cells exhibit features of activated and antigen-experienced B lymphocytes, regardless of IgV gene mutation.88
The study of CD38 expression during normal B-cell ontogenesis is relevant to the CLL framework only if one assumes that the phenotype of the neoplastic cell reflects its developmental history and antigenic exposure. An alternative is to consider CD38 expression by CLL cells as simply the result of the genetic storm that occurred during leukemic transformation. However, direct involvement of chromosome 4 (where the CD38 gene is located, in locus p15) has not yet been reported.
Role in signaling in B lymphocytes
Studies on the role of CD38 in human B lymphocytes are scarce and focus primarily on BM resident precursors (Table 2).
Coculture of CD19+ cells purified from normal BM samples and allogeneic BM stromal layers in the presence of anti-CD38 mAbs markedly reduces cell recovery after 7 days at all stages of development.89 This would seem to suggest that CD38 modulates critical interactions with stromal cells and does so by means of a mechanism that appears not to involve the enzymatic activity.89 Subsequent studies indicated that the mechanisms underlying these effects could be the implementation of a receptor-like signaling pathway rather than a mere blocking action.
Follow-up studies directly addressed the effects of CD38 ligation by means of agonistic mAbs on human B cells purified from the BM and derived line models. The results allowed the signaling pathway steps to be identified, including the coordinated activation of the tyrosine kinase syk, of PLC-γ, and of c-cbl.69 The latter event is followed by direct association of c-cbl with the p85 subunit of phosphatidylinositol-3 kinase (PI-3K), with a resulting increase in PI-3K activity.90 By using selective inhibitors, researchers were able to identify the key role played by the activation of PI-3K in CD38-mediated growth suppression of B-cell precursors.91 No information is available on the expression or activation of ZAP-70 in these cells.
Using human B-cell models, CD19 was shown to be markedly tyrosine phosphorylated and associated with lyn and PI-3K upon CD38 ligation. The conclusion is that CD19 is a major component of the CD38 signaling cascade in B-cell precursors, serving as a cell surface membrane docking site for cytoplasmic kinases.91
These data were later confirmed and extended to cases beyond the BM. Using lymphoblastoid cell lines, we showed that CD38 cross-linking induces a significant number of CD38 molecules to associate with CD19 and to enter the membrane lipid micro-domains. The physical association between CD38 and CD19, shown by cocapping experiments and confirmed by coimmunoprecipitation, is also functional: CD38-mediated Ca2+ fluxes are only apparent when CD19 is active, as demonstrated after silencing CD19.92
Results from studies on CD38 function in normal mature B cells are in net contrast to those obtained on B-cell precursors. CD38 ligation by means of agonistic mAbs results in protection from apoptosis through upregulation of bcl-2 proteins. The effects are similar to those mediated by the CD40-CD40L pathway, although the 2 systems appear to work independently.93
CD38 ligation in circulating mature B lymphocytes is followed by implementation of proliferative pathways acting in synergy with IL-2. Further, CD38 ligation induces de novo synthesis of a panel of cytokines, including IL-6, GM-CSF, and IFN-γ.94
The data available thus far do not permit any firm conclusions to be drawn; nonetheless, several inferences can be made. First of all, CD38 has the characteristics of a coreceptor. The physical and functional association with the BCR and CD19 suggests that CD38 may act as a fine modulator of the threshold of B-cell activation. Further, CD38 regulates the interactions with the external environment, which is probably the main agent in delivering signals that are negative in the BM and positive in secondary lymphoid organs. Lastly, there is no reported contribution by the products of CD38 enzymatic activities to the delivery of the signals, at least in humans.65
What happens after the neoplastic CLL transformation?
The view currently given most credence is that CLL represents a single disease presumably originating from the neoplastic transformation of marginal zone T-cell–independent B lymphocytes at different steps during activation.3,95 This working hypothesis has been substantiated by independent studies in which 2 types of CLL were shown to have largely overlapping genetic profiles,96,97 although they are distinguished by a different mutational status of the IgV genes that may account for phenotypic differences. The preferential presence of the activation marker CD38 in unmutated cases may imply that these cells are exposed to the antigen, although in such a way as to initiate the activation process without inducing somatic hypermutation. According to this hypothesis, the antigens recognized by these BCRs would likely be carbohydrate components of bacterial and viral origin and mimicking self-antigens (and would thus always be present at low concentrations). The findings of low affinities and restrictions in the usage of the IgV gene repertoire98 along with autoimmune manifestations in a significant percentage of CLL patients provide indirect confirmation of this view.
But what could be the potential role of preferential CD38 expression by this CLL subset? Given that CD38 ligation by means of agonistic mAbs is followed by proliferation and blast transformation of a subset of CLL cells, the molecule may perform as an active signaling receptor rather than a mere activation flag.47 We reported that the in vitro signaling properties mediated by CD38 are significantly enhanced by the simultaneous presence of IL-2. One possible interpretation is that IL-2 surrogates the role exerted by the microenvironment and by the interactions taking place in vivo between CLL cells and the surrounding stroma in BM and secondary lymphoid organs. Therefore, it would be reasonable to surmise that when they are exposed to IL-2 generated by a T-cell reaction, CLL cells in the BM or in lymph nodes upregulate CD38. This would be followed by increased signaling capacity, leading in turn to expansion of the CD38+ subset. A narrower interpretation would be that CLL cells modulate surface expression according to transit or localization in organs or districts (blood stream, BM, lymph nodes, among others). In line with these hypotheses, independent results have shown that surface CD38 expression in lymph nodes is significantly higher than in peripheral blood or BM neoplastic B cells simultaneously obtained from the same CLL patient, suggesting that this subset may constitute a reservoir of CLL cells that continuously contributes to the tumor burden.99 Data in support of this view can also be found in a recent report showing that CLL cells are characterized not simply by an apoptotic defect but also by the presence of an actively proliferating pool of cells residing in secondary lymphoid organs.100
To address the issue of which mechanism(s) maintains CD38+ cell proliferation, we showed that CD38-mediated signals may be activated upon interaction with the cell-based CD31 ligand. CD38+ CLL cells bind murine fibroblasts transfected with CD31 and exhibit increased growth and survival.101 Further, CD38/CD31 contacts upregulate the survival receptor CD100, a semaphorin family member involved in sustaining CLL growth and survival.102,103 There is also a concomitant loss of CD72, a low-affinity CD100 ligand and a negative regulator of immune responses.104 CD100 is upmodulated exclusively in the blast population of B-CLL cells obtained upon CD38 ligation either by an agonistic mAb or by the CD31 transfectants. Lastly, the model is indirectly confirmed by evidence that nurselike cells105,106 derived from CLL patients express high levels of functional CD31 and plexin-B1, the high-affinity ligand for CD100.101 The translational relevance is that CD38/CD31 cross-talk occurring in several districts of the body controls a critical activation pathway in CLL proliferation and survival. The importance of these observations is confirmed by the finding of a stepwise cooperation between CD38 and CD100 in determining the final effects. Indeed, interaction of CD100 with its plexin-B1 ligand extends the life span of CLL cells preactivated by combined CD38 and IL-2 signals. The absence of soluble CD100 suggests that the effects observed may be exerted through a direct interaction between membrane CD100 and plexin-B1.
CD38 and ZAP-70: is there any link?
Of the most recently identified markers for risk stratification of CLL cases, ZAP-70 and CD38 are perhaps the most promising for routine clinical applications because of their generally low cost and relative technical ease of use, at least compared with IgV mutation analyses (reviewed in Hamblin and Hamblin107 ). However, it is yet to be established whether CD38 and ZAP-70 are independent markers or whether they are functionally linked.
ZAP-70, a member of the syk family of tyrosine kinases, is involved in signal transmission to downstream pathways and is associated with the ζ-chain of the CD3 complex.108 ZAP-70 comprises 2 SH2 domains, the linker interdomain B and a kinase domain.109 The 3 different critical tyrosines thus far defined may functionally induce activation of positive signals or removal of the inhibitory effects of the c-cbl proto-oncogene.110
Although primarily involved in T-cell signaling, ZAP-70 does play a role in normal B-cell functions. It is expressed during the early phases of murine B-cell development and is necessary for the transition from pro-B to pre-B cells, a checkpoint controlled by signals from the pre-BCR, which monitors the successful rearrangement of IgV genes.111 In humans, the molecule is reported to be expressed by a subpopulation of normal, mature, and highly CD38+ B lymphocytes in spleen and tonsil.112
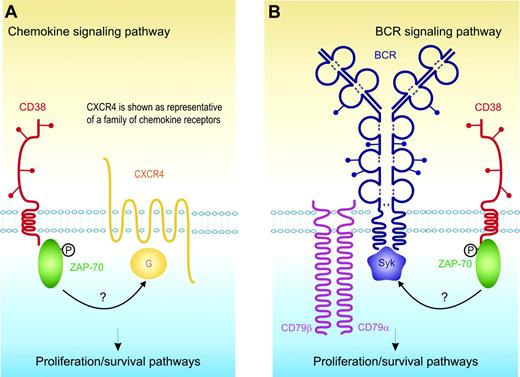
Preliminary and indirect confirmation of our working hypothesis that CD38 and ZAP-70 are functionally linked derives from the results of a clinical refinement of the CLL classification: coexpression of these 2 markers selectively identifies a subset of patients with the most aggressive disease.29 ZAP-70 has long been known to be an integral component of the CD38 signaling cascade in T and NK cells.48,68 We recently extended the analysis to CLL cells and anecdotally reported that CD38 ligation induces tyrosine phosphorylation of ZAP-70.113 This initial finding is currently undergoing confirmation in a wider and better classified cohort of CLL samples; however, it prompts several questions as to the functional implications of their simultaneous presence. The first possibility is that CD38 and ZAP-70 somehow synergize with the signals mediated by BCR. Independent studies have shown that BCR cross-linking results in ZAP-70 activation and, ultimately, in a stronger signal.114 According to this line of reasoning, CD38 might exert a coreceptor function, further sustaining the signal mediated by the BCR. The second possibility is that the presence and functions of ZAP-70 might be linked to the signaling pathways controlled by CXCR4, CXCR3, and CCR7. Chemokines are reported to significantly contribute to the delivery of growth signals to CLL cells that express functional receptors. Results obtained in T-cell models indicate that the signaling pathway driven by CXCR4 involves ZAP-70.115,116 In support of this view is evidence that ZAP-70+ CLL cells migrate better in response to CXCL12117 (Figure 2).
Other results obtained using mature dendritic cells indicate that CD38 engagement ensures efficient chemotaxis in response to CCL21 and point to the presence of lateral associations between CD38 and the CCR7 receptor as a potential modulatory mechanism.51 Another report shows that cADPR antagonists block chemotaxis of human monocytes to CXCR4, CCR1, and CCR5.118
The results summarized in the previous sections are multifarious and indicative of numerous uncertainties due to the still-limited experience with and availability of highly specific reagents. However, these reports provide preliminary evidence warranting a link between chemokines and their receptors on the one hand and CD38 and ZAP-70 on the other.
Model showing the functional link between CD38 and ZAP-70 and their role in activating proliferation/survival pathways in CLL cells. (A) CD38 activation leads to the direct tyrosine phosphorylation of ZAP-70 (indicated by the black P) and this event is in turn linked to the signaling pathways controlled by CXCR4, CXCR3, and CCR7 (only CXCR4 is shown, for sake of simplification). G indicates G protein. (B) CD38 activation leads to the direct tyrosine phosphorylation of ZAP-70 (indicated by the black P), further sustaining the signal mediated by the BCR.
Model showing the functional link between CD38 and ZAP-70 and their role in activating proliferation/survival pathways in CLL cells. (A) CD38 activation leads to the direct tyrosine phosphorylation of ZAP-70 (indicated by the black P) and this event is in turn linked to the signaling pathways controlled by CXCR4, CXCR3, and CCR7 (only CXCR4 is shown, for sake of simplification). G indicates G protein. (B) CD38 activation leads to the direct tyrosine phosphorylation of ZAP-70 (indicated by the black P), further sustaining the signal mediated by the BCR.
Another crucial question concerns the issue of why a T-cell marker such as ZAP-70 is detected in a disease immortalizing a mature B phenotype. Perhaps it reflects the complex genetic derailment secondary to the tumor hit. Or perhaps a normal CD38+/ZAP-70+ population marking a discrete step of normal B-cell ontogeny has gone undetected.112
Conclusions
This review is an attempt on the part of basic scientists to contribute experience that may prove helpful in improving the clinical management of CLL patients, keeping well in mind Pliny the Elder's principle of sutor ne ultra crepidam (“Cobbler, stick to thy last!”—or, more explicitly—“Basic scientists, stay in your own yard!”).
Our conclusions originate from the observation that a set of normal receptors and ligands ruling physiologic signaling pathways in B lymphocytes becomes detrimental when expressed in the context of CLL cells, ultimately leading to disease progression. Some tentative operational models can now be proposed. We are dealing with a surface receptor (CD38) and a cytoplasmic kinase (ZAP-70), which are both normal components of normal B-lineage differentiation. Their simultaneous expression by a B-lymphocyte hit by a neoplastic event gives rise to a deleterious synergy leading to the activation of a CD38-specific proliferation pathway. This implies that the CD38/CD31 cross-talk occurring in several districts of the body may induce CLL cells to switch on ZAP-70–dependent activation processes. The final result is that the cells respond poorly to conventional treatment and, consequently, act as a tumor reservoir. The lymph nodes would provide an ideal microenvironment and the appropriate set of receptors and signals for this scenario. According to this view, CD38 signaling in CLL would be initiated by close interactions with CD31, giving rise to a signal that induces overexpression of CD100. Preliminary evidence indicates that this signal relies specifically on ZAP-70 for transmission (S.D., T.V., and F.M., manuscript in preparation). The successive interplay of CD100 with its plexin-B1 ligand would account for a second set of signals, contributing to cell survival and proliferation.
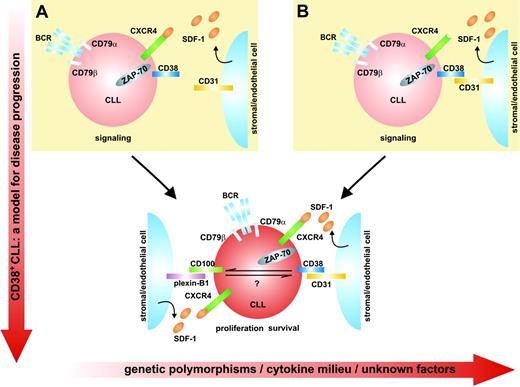
The role(s) of chemokines and their receptors in this intricate network is still only hypothetical. At this point, we surmise that these pathways intersect in 2 different ways. On the one hand, chemokines may generate molecular gradients that guide CD38+ cells to CD31+ stromal cells and thus initiate the CD38 signaling pathway. However, the reverse scenario cannot be ruled out, with CD38+ CLL cells being attracted to CD31+ cells, which in turn synthesize and secrete survival chemokines. Both mechanisms may operate in CLL cells, leading to the progressive implementation and exasperation of signaling pathways, ultimately causing disease progression and eventually acute transformation (eg, Richter syndrome). The model is summarized in Figure 3.
This model includes several oversimplifications. One is that the products of the enzymatic activities of CD38 do not contribute to the signaling pathway. Another is suggested by the clinical heterogeneity observed in homogeneously stratified patient samples and calls for a role for genetic polymorphisms. CD38 is genetically variable in the white population, with a biallelic polymorphism located in intron 1.79 Further, the regulation of this gene is extremely complex per se, with sensitivity to retinoids, cytokines, and hormones (Figure 1). CD31 is also genetically polymorphic and is considered a minor histocompatibility antigen.119 It is reasonable to ask whether selected matching between CD38 and CD31 allelic products may quantitatively modulate the resulting signals and hence the clinical effects. A third oversimplification concerns the fact that CD38 is only the first member of a family of NAD-metabolizing ectoenzymes whose newest addition is CD157.120 Although little is known about the role of CD157 in this field, it is of likely relevance that CD157 is expressed by nurselike and stromal cells121 and that the molecule is reported to participate in the delivery of growth signals to BM precursors.122
Model for disease progression of CD38+ CLL cells. (A) Chemokines may generate molecular gradients that guide CD38+cells to CD31+stromal cells and thus initiate the CD38 signaling pathway. (B) CD38+ CLL cells can alternatively be attracted to CD31+ cells, which in turn synthesize and secrete survival chemokines. These mechanisms accumulate in CLL cells, leading to the progressive implementation and exasperation of signaling pathways, ultimately causing disease progression and eventually acute transformation. The y-axis shows disease progression, whereas the x-axis lists potentially confounding elements.
Model for disease progression of CD38+ CLL cells. (A) Chemokines may generate molecular gradients that guide CD38+cells to CD31+stromal cells and thus initiate the CD38 signaling pathway. (B) CD38+ CLL cells can alternatively be attracted to CD31+ cells, which in turn synthesize and secrete survival chemokines. These mechanisms accumulate in CLL cells, leading to the progressive implementation and exasperation of signaling pathways, ultimately causing disease progression and eventually acute transformation. The y-axis shows disease progression, whereas the x-axis lists potentially confounding elements.
What is clear is that the CD38/CD31 axis governs a set of proliferation/survival signals that contribute to the increased aggressiveness of CD38+ CLL cells. Only field experience will tell whether this information will prove valuable in refining the processes of clinical diagnosis and prognosis of CLL. Further, the proposed model may serve as a template for the design of new pharmaceuticals capable of modulating the intricate network of surface molecules that regulate cell-cell contacts.123
Prepublished online as Blood First Edition Paper, April 18, 2006; DOI 10.1182/blood-2006-01-013003.
Supported by grants from Chronic Lymphocytic Leukemia Global Research Foundation (CLLGRF; S.D.); the Associazione Italiana Ricerca Cancro (AIRC) and the Regione Piemonte (Progetto CIPE; F.M.); and “PRIN” (MIUR) and Fondi Ateneo ex-60% (S.D., F.M.) projects. The Fondazione Internazionale Ricerche Medicina Sperimentale (FIRMS) and Compagnia di SanPaolo (Progetto Oncologia) also provided support and financial contributions. S.A. is the recipient of a European Society of Medical Oncology (ESMO) fellowship and is on leave of absence from the Department of Hematology, University Hospital Essen, Germany.
Thanks are given to Drs Luciana Bergui and Paola Omede' (Division of Hematology, University of Turin, Turin, Italy), Giovanni D'Arena (Fondazione Pascale, Naples, Italy), and Ozren Jaksic (Department of Hematology, Merkur University Hospital, Zagreb, Croatia) for providing patient samples and for helpful discussions. T.V. is a student of the PhD program in “Advanced techniques in localization of human tumors,” University of Turin, Turin, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal