Abstract
CD11c+ myeloid dendritic cells (MDCs) and CD11c– CD123+ plasmacytoid DCs (PDCs) have been identified as main human DC subsets. MDCs are professional antigen-presenting cells for T cells, and include Langerhans cells, dermal DCs, and interstitial DCs. They have been associated with HIV-1 capture and sexual transmission, whereas PDCs play an important role in the innate immune responses to different types of viruses, including HIV-1. To compare the influence of MDCs and PDCs on HIV-1 infection of T cells, we isolated donor-matched MDCs and PDCs from peripheral blood, activated them by adding different maturation-inducing compounds, and cocultured them with T cells and HIV-1. We found that MDCs enhance HIV-1 infection through capture of the virus and subsequent transmission to T cells, and that differently matured MDC subsets have different HIV-1 transmission efficiencies. These differences were not due to soluble factors, viral capture differences, or the expression of integrins ICAM-1, -2, -3, or LFA-1. In contrast, regardless of their state of maturation, PDCs inhibit HIV-1 replication in T cells through the secretion of IFNα and an additional, unidentified small molecule. This study shows that the 2 main types of DCs have opposing roles in HIV-1 infection of T cells.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells that play an important role in the initiation of immune responses. Immature DCs develop into mature effector DCs upon activation by microorganisms or inflammatory signals, and migrate to the draining lymph nodes where they encounter and stimulate naive Th cells.1,2 HIV-1 has been proposed to make use of this process, being captured by DCs in mucosal tissues and delivered to the lymph node, which then becomes the principal site of virus replication.3,4 In humans, 2 main DC subsets are found: CD11c+ myeloid DCs (MDCs) and CD11c– CD123+ plasmacytoid DCs (PDCs). MDCs include Langerhans cells, dermal DCs, and interstitial DCs, and are found in blood, skin, and mucosal tissues and have been associated with HIV-1 capture and sexual transmission. PDCs are located in blood and secondary lymphoid organs but they can be recruited to sites of inflammation.5-7 The difference between MDCs and PDCs is furthermore manifested by differential expression of Toll-like receptors (TLRs) and secretion of different cytokines: MDCs are known to secrete high levels of interleukin-12 (IL-12) whereas PDCs are thought to play an important role in innate immune responses to different types of viruses by producing IFNα.8-12 Several studies have shown that the numbers of both MDCs and PDCs are substantially reduced in the blood of patients infected with HIV-1 and that the DCs are functionally impaired with respect to T-cell proliferation and cytokine production.13-18 In addition, both types of DCs have been shown to be susceptible to HIV-1 infection, and to be able to transmit the virus to CD4+ T cells in vitro.19-21
In this study, we set out to compare the influence of differently matured MDCs and PDCs on HIV-1 infection of T cells. A large proportion of DC research is being performed with monocyte-derived DCs, which represent the MDC subset. However, several differences have been described for these in vitro–generated DCs and MDCs isolated from blood or skin.22,23 In addition, there is no in vitro PDC model available. Therefore, we isolated donor-matched MDCs and PDCs directly from blood and investigated their influence on HIV-1 infection by coculturing them with T cells. We found remarkable differences between MDCs and PDCs. MDCs enhance HIV-1 infection through capture of the virus and subsequent transmission to T cells. Differently matured MDC subsets exhibit distinct HIV-1 transmission efficiencies, and secreted factors by MDCs did not modulate the transmission of HIV-1 to T cells. In contrast, irrespective of their maturation status, PDCs inhibit HIV-1 replication in T cells by secretion of IFNα and an additional, heat-sensitive small molecule of smaller than 3 kDa. This study shows that the 2 main DC subsets found in blood have opposing roles in respect to HIV T-cell infection.
Materials and methods
Isolation and culturing of DCs
Peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation on Lymphoprep (Nycomed, Torshov, Norway). Subsequently, MDCs and PDCs were isolated using magnetic bead isolation and AutoMACS (Miltenyi Biotec, Bergisch Gladbach, Germany). For MDC isolation, PBMCs were depleted from B cells using anti-CD19 microbeads (Miltenyi Biotec) followed by positive selection with mouse anti–human BDCA-1-PE and anti-PE microbeads (Miltenyi Biotec). PDC isolation was performed with mouse anti–human BDCA-4-PE and anti-PE microbeads (Miltenyi Biotec). Prior to incubation with CD19/BDCA-1/BDCA-4 antibodies the cells were preincubated with FcR-block for 5 minutes (Miltenyi Biotec). Culturing was done in Iscove modified Dulbecco medium (IMDM; Life Technologies, Paisley, United Kingdom) with gentamicin (86 μg/mL; Duchefa, Haarlem, The Netherlands) and 10% fetal calf serum (HyClone, Logan, UT) at a density of 35 × 103 DCs/well. The medium was supplemented with granulocyte macrophage–colony-stimulating factor (GM-CSF; 500 U/mL; Schering-Plough, Uden, The Netherlands) or IL-3 (10 ng/mL; Strathmann Biotech AG, Hannover, Germany) for MDCs and PDCs, respectively. Maturation of the DCs was induced by culturing the cells for 24 hours with the following factors, provided alone or in combination as indicated in the “Results” section: maturation factors (MFs; LPS [100 ng/mL; Difco, Detroit, MI], IL-1β [10 ng/mL; Strathmann Biotec AG], and TNFα [50 ng/mL; Strathmann Biotec AG]), poly (I:C) (20 μg/mL; Sigma-Aldrich, St Louis, MO), IFNγ (1000 U/mL; Strathmann Biotec AG), R-848 (2 μg/mL; Invivogen, San Diego, CA), or fixed Staphylococcus aureus Cowan strain I bacteria (SAC; 25 μM; Calbiochem, La Jolla, CA). Each experiment in this study is a representative of 4 to 8 different donors.
Flow cytometry
All MDC and PDC fractions were tested for purity (> 90%) with CD11c-APC (BD Biosciences, San Jose, CA), lineage-markers (Lin-1-FITC; BD Biosciences), and CD19-FITC (Dakopatts, Glostrup, Denmark). After maturation, DCs were analyzed for the expression of cell-surface molecules by fluorescence-activated cell sorting (FACS). Mouse antihuman mAbs were used against the following molecules: BDCA-1-PE, BDCA-4-PE, CD11c-APC, CD11a-FITC (LFA-1; Pelicluster, Sanquin, Amsterdam, The Netherlands), CD83-APC, CD86-FITC, CD4-APC, ICAM-3-FITC (all BD Biosciences) or biotinylated ICAM-1 or ICAM-2 (R&D Systems, Abingdon, United Kingdom) followed by Streptavidin-PerCP-Cy5.5 (BD Biosciences). Samples were analyzed on a FACScan (BD Biosciences).
Virus stocks and T cells
C33A cervix carcinoma cells were transfected using calcium phosphate with 5 ng of the molecular clone of CXCR4-using HIV-1 LAI and CCR5-using JR-CSF. The virus containing supernatant was harvested 3 to 5 days after transfection, filtered, and stored at –80°C. The concentration of virus was determined by CA-p24 enzyme-linked immunosorbent assay (ELISA). PM1 cells, which are susceptible to CXCR4- and CCR5-using HIV-1,24 were maintained in Roswell Park Memorial Institute (RPMI) medium 1640 (Life Technologies), supplemented with 10% FCS, 2 mM sodium pyruvate, 10 mM HEPES, 2 mM l-glutamine, penicillin (100 U/mL) (Sigma-Aldrich), and streptomycin (100 μg/mL; Invitrogen, Breda, The Netherlands). The reporter cell line LuSIV was kindly donated by Janice E. Clements (Johns Hopkins University School of Medicine, Baltimore, MD). This CEMx174-derived cell line contains the firefly luciferase reporter gene downstream of the SIVmac239 LTR. Infection by HIV-1 results in Tat-mediated expression of luciferase, which can be measured 24 hours later.25 Cells were maintained in the same medium as PM1 cells, but with 300 μg/mL hygromycin B to maintain the luciferase construct. For experiments with DCs, hygromycin B–free medium was used.
Single-cycle replication assay with LuSIV cells
MDCs and PDCs (35 × 103) were stimulated with maturation-inducing compounds for 24 hours as indicated in the “Results” section. DCs were subsequently incubated with HIV-1 LAI (3 ng CA-p24/well) and 40 × 103 LuSIV cells for 24 hours. Alternatively, only the supernatant of 24-hour–stimulated MDCs or PDCs was incubated with LuSIV cells and HIV-1. After 24 hours of coculture, luciferase was measured as described previously.26 LuSIV cells (40 × 103) grown without DCs or HIV-1 were used to obtain the background luciferase value, which was subtracted from all data.
MDC transmission assay
Matured MDCs (35 × 103) were incubated for 2 hours with 5 ng CA-p24 HIV-1 LAI, followed by 2 washing steps in the plate to remove unbound virus. DCs were then cocultured with LuSIV cells for 24 hours, after which luciferase was measured. Control or blocking antibodies against ICAM-1 or -3 (20 μg/mL; Immunotech, Marseille, France) were preincubated with DCs and LuSIV cells for 30 minutes at 37°C before coculture. Background luciferase level of LuSIV cells grown without HIV-1 was subtracted from all data.
MDC CD40-ligation
MDCs (35 × 103) that were stimulated as indicated in the “Results” section were washed twice to remove all soluble factors. DCs were then cocultured with 35 × 103 CD40 ligand (CD40L)–expressing mouse plasmacytoma cells (J558 cells; a gift from Dr P. Lane, University of Birmingham, Birmingham, United Kingdom). Supernatants were harvested after 24 hours and added to 40 × 103 LuSIV cells and 3 ng CA-p24 of HIV-1 LAI. Luciferase levels were measured after 24 hours. Background luciferase level of LuSIV cells grown without HIV-1 was subtracted from all data.
HIV-1 capture by MDCs
MDCs that were stimulated with different maturation-inducing compounds for 24 hours as indicated in the “Results” section were incubated in a sterile FACS-tube (100 × 103 DC/100 μL) with 5 ng CA-p24 HIV-1 LAI for 4 hours at 37°C. After centrifugation at 400g, the DCs were washed with phosphate-buffered saline (PBS) to remove unbound virus. This step was performed 3 times, followed by lysis of the cells and CA-p24 ELISA to determine the amount of HIV-1 capture.
HIV-1 replication with PDC supernatant
PM1 T cells (60 × 103) were infected with 1 ng CA-p24 HIV-1 LAI or 2 ng JR-CSF with or without supernatant of SAC-stimulated PDCs in a final volume of 250 μL. We used 80 μL PDC supernatant per well, or made dilutions as indicated in the “Results” section. In some cases, supernatant and T cells were preincubated for 30 minutes with blocking antibodies against type I interferons27 (a kind gift of Dr I. Julkunen, National Public Health Institute, Helsinki, Finland) as indicated in the text. Viral replication was followed by CA-p24 ELISA of the supernatant.
Size fractionation of PDC supernatant
To separate supernatant of SAC-stimulated PDCs into fractions of 0 to 3 kDa, 3 to 10 kDa, 10 to 30 kDa, 30 to 100 kDa and more than 100 kDa, Microcon Centrifugal Filter Devices (Millipore, Bedford, MA) were used. A quantity of 80 μL of each fraction was incubated with 150 μL LuSIV cells and 3 ng CA-p24 HIV-1 LAI, followed by luciferase measurement after 24 hours. Background luciferase level of LuSIV cells grown without HIV-1 was subtracted from all data. The obtained fractions were analyzed for the presence of IFNα by ELISA with a detection limit of 10 pg/mL (coating antibody, detection antibody, and standard were obtained from R&D Systems, Endogen [Ettenleur, The Netherlands], and Roche [Nutley, NJ], respectively). Blocking antibodies against type I IFNs were used in a 1:100 dilution. Heat inactivation of the different fractions was done at 50, 75, or 100°C for 20 minutes.
Statistical analysis
Data were analyzed for statistical significance (GraphPad InStat, San Diego, CA) using analysis of variance (ANOVA). P values below .05 were considered to be significant.
Results
MDCs enhance, whereas PDCs inhibit, HIV-1 infection of T cells
To compare the influence of MDCs and PDCs on HIV-1 infection of T cells, donor-matched MDCs and PDCs were isolated from peripheral blood and matured by addition of several stimulating compounds. MDCs were cultured in medium only (immature DCs), or matured with poly(I:C), R-848, SAC or IFNγ plus MFs. PDCs were cultured in medium only, or with poly(I:C), R-848, and SAC. After 24 hours, DCs were cocultured with HIV-1 LAI and the reporter T-cell line LuSIV, which has an HIV-1–inducible promoter cloned upstream of the luciferase gene.25 HIV-1 infection of these cells results in luciferase production, which can be measured after 24 hours. Since no cell-to-cell spread of newly produced virus occurs, this single-cycle replication assay represents an effective way of determining HIV-1 infectivity.
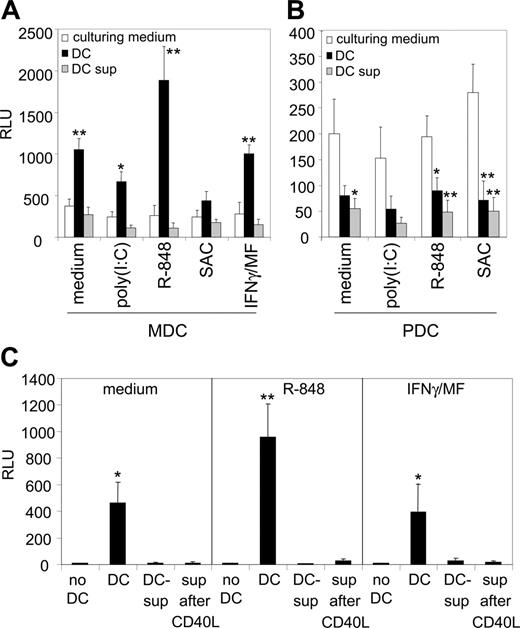
To determine the basal amount of luciferase production induced by HIV-1 in the absence of DCs, LuSIV cells were infected with HIV-1 with the different culture media. The luciferase levels were comparable for all different maturation-inducing compounds, meaning that they did not influence the induction of luciferase production by HIV-1 (Figure 1A-B, white bars). Please note that the scales of Figure 1A and 1B are different. The presence of DCs had opposite effects on HIV-1 infectivity: MDCs strongly stimulated HIV-1 infection, whereas PDCs reduced HIV-1 infection to below the basal levels (Figure 1A-B, black bars). Furthermore, MDCs matured by R-848 were significantly more efficient in HIV-1 stimulation than MDCs matured by other compounds (P < .01). To test whether the stimulating and inhibitory effects of MDCs and PDCs were mediated by secreted factors, LuSIV cells were infected with HIV-1 in the presence of the supernatant of matured DCs. Interestingly, the stimulating effect of MDCs was not reproduced with the supernatant of MDCs, but supernatant from PDCs was sufficient to reduce luciferase levels (Figure 1A-B, gray bars). This result demonstrates that both DC subsets have opposing effects on HIV-1 infection of T cells, mediated through different mechanisms.
MDCs do not stimulate HIV-1 infection through secreted factors
The results shown in Figure 1A-B demonstrate that the stimulating effect of MDCs is not mediated through a factor that is secreted during DC maturation. However, T cells can activate DCs via CD40 ligand (CD40-L)–CD40 signaling, leading to increased expression of costimulatory molecules CD80/CD86 and cytokine release.2 It is therefore still possible that the nature of the enhancing effect of MDCs is a factor that is secreted after T-cell engagement. To critically test this alternative explanation, we cocultured differently matured MDCs with a CD40L-expressing cell line to mimic T-cell encounter. After 24 hours, the supernatant was collected and incubated with LuSIV cells and HIV-1, followed by luciferase measurement one day later. We found that MDC supernatant after CD40 ligation did not stimulate HIV-1 infection (Figure 1C). This demonstrates that in order to enhance HIV-1 infection, MDCs must be present and that secreted factors do not play a role.
MDCs enhance HIV-1 infection of T cells, whereas PDCs inhibit it. (A, B) Donor-matched MDCs (A) and PDCs (B) from peripheral blood were differently stimulated with maturation-inducing compounds, as indicated on the x-axis. After 24 hours, the DCs were cocultured with HIV-1 and reporter LuSIV cells. HIV-1 infection of these cells results in luciferase production, which was measured after 24 hours (▪). Note the different scales of the graphs. LuSIV cells were also infected in the presence of only supernatant of stimulated DCs (▦). To determine basal luciferase induction by HIV-1 in the absence of DCs or DC supernatant, LuSIV cells were infected with the respective culturing media only (□). *P < .05; **P < .01 compared with culturing medium. (C) Factors secreted by MDCs following T-cell encounter are not responsible for the stimulation of HIV-1 infection. MDCs were cultured in medium only (left), or were matured with either R-848 (middle) or IFNγ plus MF (right), and were subsequently cocultured with CD40L-expressing J558 cells to mimic T-cell encounter. After 24 hours, the supernatant was collected and incubated with LuSIV cells and HIV-1, followed by luciferase measurement one day later. *P < .01; **P < .001 compared with “no DC.” Background luciferase level of LuSIV cells grown without HIV-1 was subtracted from all data. RLU, relative light units. Error bars represent standard deviations.
MDCs enhance HIV-1 infection of T cells, whereas PDCs inhibit it. (A, B) Donor-matched MDCs (A) and PDCs (B) from peripheral blood were differently stimulated with maturation-inducing compounds, as indicated on the x-axis. After 24 hours, the DCs were cocultured with HIV-1 and reporter LuSIV cells. HIV-1 infection of these cells results in luciferase production, which was measured after 24 hours (▪). Note the different scales of the graphs. LuSIV cells were also infected in the presence of only supernatant of stimulated DCs (▦). To determine basal luciferase induction by HIV-1 in the absence of DCs or DC supernatant, LuSIV cells were infected with the respective culturing media only (□). *P < .05; **P < .01 compared with culturing medium. (C) Factors secreted by MDCs following T-cell encounter are not responsible for the stimulation of HIV-1 infection. MDCs were cultured in medium only (left), or were matured with either R-848 (middle) or IFNγ plus MF (right), and were subsequently cocultured with CD40L-expressing J558 cells to mimic T-cell encounter. After 24 hours, the supernatant was collected and incubated with LuSIV cells and HIV-1, followed by luciferase measurement one day later. *P < .01; **P < .001 compared with “no DC.” Background luciferase level of LuSIV cells grown without HIV-1 was subtracted from all data. RLU, relative light units. Error bars represent standard deviations.
Differences in HIV-1 stimulation are not due to differences in HIV-1 capture
Interestingly, differently matured MDCs vary in their ability to stimulate HIV-1 infection: MDCs matured with R-848, a stimulator of TLR7 and TLR8,28 are most efficient in stimulation of HIV-1 infection in coculture experiments without washing steps (Figure 1A,C). All mature MDCs expressed comparable levels of maturation/activation markers CD83/86 (data not shown). Also in a transmission assay, in which the MDCs were washed after HIV-1 incubation to remove unbound virus and other soluble compounds, the R-848–matured MDCs are more efficient in HIV-1 stimulation (Figure 2A). This shows that MDCs stimulate HIV-1 infection of T cells through capture and transmission of the virus. To investigate whether differences in HIV-1 capture are responsible for this effect, differently matured MDCs were incubated for 4 hours with HIV-1, followed by extensive washing to remove unbound virus. Viral capture was subsequently determined by lysis of the cells and CA-p24 ELISA. We found no differences in the amount of HIV-1 capture (Figure 2B), showing that the increased transmission by R-848–matured MDCs must act at a later step.
MDCs matured with R-848 demonstrate enhanced HIV-1 transmission capacity, which is not due to increased viral capture. (A) MDCs were cultured in medium only, or with R-848 or IFNγ plus MF for 24 hours, followed by HIV-1 incubation and extensive washing to remove all factors. DCs were subsequently cocultured with LuSIV cells to allow HIV-1 transmission. Luciferase was measured after 24 hours. *P < .01, compared with medium and IFNγ/MF maturation. RLU, relative light units. (B) Differently matured MDCs as indicated on the x-axis were incubated for 4 hours with HIV-1, followed by extensive washing to remove unbound virus. Viral capture was subsequently determined by lysis of the cells and CA-p24 ELISA. Error bars represent standard deviations. NS, not significant.
MDCs matured with R-848 demonstrate enhanced HIV-1 transmission capacity, which is not due to increased viral capture. (A) MDCs were cultured in medium only, or with R-848 or IFNγ plus MF for 24 hours, followed by HIV-1 incubation and extensive washing to remove all factors. DCs were subsequently cocultured with LuSIV cells to allow HIV-1 transmission. Luciferase was measured after 24 hours. *P < .01, compared with medium and IFNγ/MF maturation. RLU, relative light units. (B) Differently matured MDCs as indicated on the x-axis were incubated for 4 hours with HIV-1, followed by extensive washing to remove unbound virus. Viral capture was subsequently determined by lysis of the cells and CA-p24 ELISA. Error bars represent standard deviations. NS, not significant.
ICAM-1 expression is required for MDC-mediated transmission
We have previously shown that the interaction between the integrins ICAM-1 on DCs and LFA-1 on T cells is crucial for HIV-1 transmission. Mature monocyte-derived DCs that express higher levels of ICAM-1 have an enhanced capacity to transmit HIV-1,29 and transmission to LFA-1–negative T cells is impaired (F.G., T.W. Kuijpers, B.B., and E.C.d.J., manuscript submitted). The ICAM-1–LFA-1 interaction plays a key role in the initiation of immune responses by strengthening the adhesion between DCs and T cells at the immunologic synapse.30,31 To explore the possibility of increased integrin expression on R-848–matured MDCs, differently matured MDCs were analyzed by FACS (Figure 3A-D). MDCs matured by R-848 do indeed express more ICAM-1, but MDCs matured with IFNγ/MF express even higher levels (Figure 3A). These data imply that there must be additional reasons for the superiority of R-848–matured MDCs. LFA-1 can also interact with ICAM-2 or -3,32,33 but no expression of ICAM-2 was found on MDCs, whereas ICAM-3 was not differently expressed on matured MDCs, suggesting that the latter is not responsible for the enhanced transmission by R-848 MDCs (Figure 3B-C). LFA-1 is expressed on T cells, but can also be found on DCs.34 However, we found no differences in LFA-1 expression either (Figure 3D). To confirm our previous work on the importance of the ICAM-1–LFA-1 interaction, blocking antibodies were used to test the importance of this molecule in HIV-1 transmission by blood MDCs. We preincubated HIV-1–loaded DCs with antibodies against ICAM-1 or -3 and cocultured them with LuSIV cells. Addition of blocking antibodies against ICAM-1 severely decreased transmission, whereas blocking antibodies against ICAM-3 did not (Figure 3E). These results show that ICAM-1 is still a prerequisite for DC-mediated HIV-1 transmission to T cells, but that there must be another factor responsible for the increased transmission by R-848–matured MDCs.
PDC supernatant inhibits replication of both CXCR4- and CCR5-using HIV-1
PDC supernatant inhibits HIV-1 in the single-cycle replication assay (Figure 1B). We next investigated whether this supernatant could inhibit HIV-1 over an extended period of time during a spreading infection in T cells. We therefore infected the PM1 T-cell line with HIV-1 in the presence of supernatant from SAC-stimulated PDCs. HIV-1 replication was followed by measuring CA-p24 accumulation in the supernatant by ELISA. Both CXCR4- and CCR5-using HIV-1 were significantly inhibited 10-fold by PDC supernatant in this spreading assay (Figure 4A; P < .01), and the supernatant could be diluted 10 times before it lost its inhibitory properties (Figure 4B). This spreading assay, however, could underestimate the actual inhibitory capacity of the supernatant, because the inhibitor is gradually lost due to possible degradation and necessary refreshment of the medium. We therefore tested the supernatant dilutions also in the single-cycle replication assay, and found that the supernatant could be diluted 100 times without losing its inhibitory properties (Figure 4C).
Type I interferons are partially responsible for the inhibition of HIV-1 replication
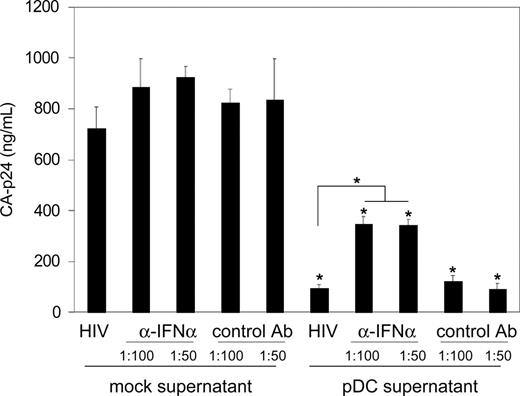
A very likely candidate HIV-1 inhibitor in the supernatant of PDCs is the family of type I interferons (IFNs), including IFNα. These antiviral molecules are produced in large amounts by PDCs and have been shown to inhibit replication of several viruses including HIV-1.12,35-37 With ELISA, we found that SAC- and poly(I:C)–stimulated PDCs secrete high levels of IFNα (3925 ± 75 pg/mL and 4295 ± 104 pg/mL, respectively). We therefore tried to counteract the inhibitory effect of PDC supernatant using well-described blocking antibodies against type I IFNs.27 Both PM1 T cells and PDC supernatant were preincubated with the antibodies before HIV-1 infection, followed by coculture for several days during which HIV-1 replication was monitored by measuring CA-p24 accumulation in the supernatant. Neutralization of type I IFNs partially restored virus replication, as shown for the day-5 sample of the replication curve (Figure 5). Even at twice the optimal antibody concentration, we found no further restoration of HIV-1 replication, suggesting that type I IFNs are only partially responsible for the inhibitory effect of PDC supernatant on HIV-1 replication.
ICAM-1 expression is required for MDC-mediated transmission. Differently matured MDCs as indicated on the left were analyzed by FACS for the expression of ICAM-1 (A), ICAM-2 (B), ICAM-3 (C), and LFA-1 (D). The mean fluorescence intensity is indicated. Open histograms represent isotype controls. (E) Blocking antibodies against ICAM-1 and -3 were preincubated with HIV-1–loaded, R-848–matured MDCs before coculture with LuSIV cells. Error bars represent standard deviations. *P < .01 compared with control antibody.
ICAM-1 expression is required for MDC-mediated transmission. Differently matured MDCs as indicated on the left were analyzed by FACS for the expression of ICAM-1 (A), ICAM-2 (B), ICAM-3 (C), and LFA-1 (D). The mean fluorescence intensity is indicated. Open histograms represent isotype controls. (E) Blocking antibodies against ICAM-1 and -3 were preincubated with HIV-1–loaded, R-848–matured MDCs before coculture with LuSIV cells. Error bars represent standard deviations. *P < .01 compared with control antibody.
PDC supernatant inhibits replication of both CXCR4- and CCR5-using HIV-1. (A) The PM1 T-cell line was infected with CXCR4-using LAI (filled markers) or CCR5-using JR-CSF (open markers) in the presence (triangles) or absence (diamonds) of supernatant from SAC-stimulated PDCs. HIV-1 replication was followed by measuring CA-p24 accumulation in the supernatant with ELISA. (B, C) PM1 T cells (B) or LuSIV cells (C) were infected with CXCR4-using HIV-1 LAI in the presence of PDCs, or PDC supernatant dilutions. RLU, relative light units. Error bars represent standard deviations. *P < .01 compared with medium control.
PDC supernatant inhibits replication of both CXCR4- and CCR5-using HIV-1. (A) The PM1 T-cell line was infected with CXCR4-using LAI (filled markers) or CCR5-using JR-CSF (open markers) in the presence (triangles) or absence (diamonds) of supernatant from SAC-stimulated PDCs. HIV-1 replication was followed by measuring CA-p24 accumulation in the supernatant with ELISA. (B, C) PM1 T cells (B) or LuSIV cells (C) were infected with CXCR4-using HIV-1 LAI in the presence of PDCs, or PDC supernatant dilutions. RLU, relative light units. Error bars represent standard deviations. *P < .01 compared with medium control.
PDCs secrete an additional inhibitory factor of less than 3 kDa
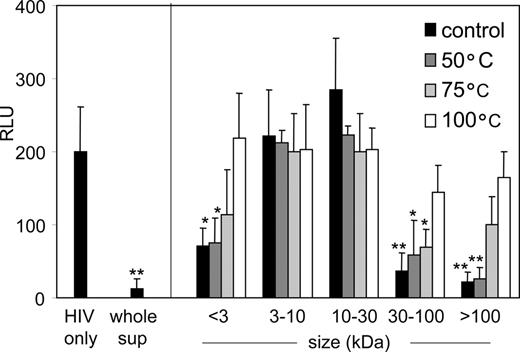
To confirm the presence of an additional inhibitory factor in PDC supernatant, a size fractionation of the supernatant was performed using centrifugal filters. The resulting fractions of less than 3, 3 to 10, 10 to 30, 30 to 100, and more than 100 kDa were subsequently tested for their capacity to inhibit HIV-1 infection in the single-cycle replication assay with LuSIV cells. The less than 3 kDa, 30 to 100 kDa, and more than 100 kDa fractions inhibited HIV-1 infection significantly, whereas the fractions between 3 and 30 kDa did not (Figure 6A, black bars). All fractions were tested for the presence of IFNα by ELISA (Figure 6A, gray bars). The 30- to 100-kDa fraction contained high levels of IFNα, as did the more than 100 kDa fraction, probably because this molecule (± 20 kDa) is present as dimers38 or complexed with other molecules or serum components. More importantly, the less than 3 kDa fraction contained no IFNα (Figure 6A), and addition of neutralizing antibodies against type I IFNs did not reverse the inhibitory properties of this fraction (Figure 6B). A full restoration of HIV-1 infectivity was obtained when the antibodies were used to neutralize the type I IFNs in the 30 to 100 kDa fraction, confirming the functioning of the antibodies (Figure 6B). Both IFNα and the small molecule inhibitor are heat-sensitive, since incubating the respective fractions at 50, 75, or 100°C resulted in loss of inhibitory properties (Figure 7). This suggests that the inhibitory factor is a small polypeptide.
Type I interferons are partially responsible for the inhibition of HIV-1 replication by PDC supernatant. PM1 T cells and PDC supernatant were preincubated with type I IFN blocking antibodies (or a control antibody), followed by HIV-1 infection and subsequent replication of the virus. A control experiment was performed with mock supernatant (DC culturing medium). Viral spread was followed by CA-p24 ELISA; the results of day 5 are shown. Antibodies were used in a recommended 1:100 dilution, or at twice that concentration (1:50). Error bars represent standard deviations. *P < .001 compared with respective controls with mock supernatant. #P < .001.
Type I interferons are partially responsible for the inhibition of HIV-1 replication by PDC supernatant. PM1 T cells and PDC supernatant were preincubated with type I IFN blocking antibodies (or a control antibody), followed by HIV-1 infection and subsequent replication of the virus. A control experiment was performed with mock supernatant (DC culturing medium). Viral spread was followed by CA-p24 ELISA; the results of day 5 are shown. Antibodies were used in a recommended 1:100 dilution, or at twice that concentration (1:50). Error bars represent standard deviations. *P < .001 compared with respective controls with mock supernatant. #P < .001.
PDCs secrete an additional HIV-1 inhibitory factor of less than 3 kDa. (A) PDC supernatant was size fractionated by filter centrifugation into fractions of less than 3 kDa, 3 to 10 kDa, 10 to 30 kDa, 30 to 100 kDa, and more than 100 kDa. These fractions were incubated with LuSIV cells and HIV-1 for 24 hours, followed by luciferase measurement (▪). All fractions were also tested for the presence of IFNα by ELISA (▦). *P < .001 compared with control (ie, HIV-1 only). (B) LuSIV cells and the less than 3 kDa and 30 to 100 kDa fractions were preincubated with type I IFN blocking antibodies, followed by coculture and HIV-1 infection. Luciferase was measured 24 hours later. *P < .01 compared with controls with mock supernatant. #P < .01. NS, not significant; RLU, relative light units. Error bars represent standard deviations.
PDCs secrete an additional HIV-1 inhibitory factor of less than 3 kDa. (A) PDC supernatant was size fractionated by filter centrifugation into fractions of less than 3 kDa, 3 to 10 kDa, 10 to 30 kDa, 30 to 100 kDa, and more than 100 kDa. These fractions were incubated with LuSIV cells and HIV-1 for 24 hours, followed by luciferase measurement (▪). All fractions were also tested for the presence of IFNα by ELISA (▦). *P < .001 compared with control (ie, HIV-1 only). (B) LuSIV cells and the less than 3 kDa and 30 to 100 kDa fractions were preincubated with type I IFN blocking antibodies, followed by coculture and HIV-1 infection. Luciferase was measured 24 hours later. *P < .01 compared with controls with mock supernatant. #P < .01. NS, not significant; RLU, relative light units. Error bars represent standard deviations.
Discussion
In the present study, we compared differently matured MDCs and PDCs from peripheral blood for their influence on HIV-1 infection of T cells. Opposing effects were scored: MDCs strongly promote HIV-1 infection, and PDCs severely inhibit HIV-1 replication. The former finding has been described before39 and we extended the findings here by showing that soluble factors secreted during MDC maturation or following T-cell encounter do not influence this process. To the best of our knowledge, no comparison has been made of differently matured blood MDCs and their ability to transmit HIV-1. We have previously shown that differently matured monocyte-derived DCs have different transmission efficiencies, which correlates with differences in ICAM-1 expression.29 Here we show that the same rule did not apply to blood MDCs: MDCs matured with R-848 enhance HIV-1 infection twice as efficient as MDCs matured with SAC, IFNγ/MF or poly(I:C), which does not perfectly correlate with ICAM-1 expression. Although we could confirm that ICAM-1 is a prerequisite for MDC-mediated HIV-1 transmission (Figure 3E), an additional factor seems to contribute to the increased HIV-1 transmission by R-848–matured MDCs. This factor is not another integrin like ICAM-2, ICAM-3, or LFA-1, and the amount of viral capture by R-848 MDCs was comparable to that of immature MDCs or IFNγ/MF-matured MDCs. One possible explanation is that HIV-1 is degraded to a smaller extent after capture by R-848 MDCs. Significant viral degradation occurs after viral capture,40 but a proportion of the virus survives by residing in a nonlysosomal compartment followed by transmission to T cells.41,42 Maturation of MDCs with R-848 could possibly change the balance between degradation and survival of HIV-1. Consequently, R-848–matured MDCs could direct HIV-1 more efficiently or in larger amounts toward the infectious synapse.42,43 More research on the differences between MDCs matured with R-848 and other compounds is necessary and may lead to the identification of novel factors that play a role in HIV-1 transmission by DCs.
The inhibitory factor of less than 3 kDa is heat-sensitive. Size fractions of PDC supernatant were incubated at 50, 75, or 100°C for 20 minutes, followed by incubation with LuSIV cells and HIV-1 for 24 hours. RLU, relative light units. Error bars represent standard deviations. *P < .05; **P < .01 compared with control (ie, HIV only).
The inhibitory factor of less than 3 kDa is heat-sensitive. Size fractions of PDC supernatant were incubated at 50, 75, or 100°C for 20 minutes, followed by incubation with LuSIV cells and HIV-1 for 24 hours. RLU, relative light units. Error bars represent standard deviations. *P < .05; **P < .01 compared with control (ie, HIV only).
R-848 is a synthetic compound that stimulates TLR7/8,28 for which the natural ligand has been proposed to be single-stranded RNA.44 HIV-1 is a single-stranded RNA virus and our results therefore suggest that HIV-1 may enhance its own transmission through engagement with TLR7/8. However, HIV-1 by itself does not fully activate monocyte-derived DCs or blood MDCs.20,45,46 For efficient HIV-1 transmission, MDCs need additional inflammatory factors or pathogens for full maturation.
In contrast to MDCs, PDCs inhibit HIV-1 replication. Previously, others have shown that PDCs are able to transmit HIV-1 to T cells,21 a finding that seems to contradict our results. However, our experiments involved DC–T-cell cocultures without washing of the DCs, such that all inhibitory factors remain present during the whole experiment. We identified IFNα as one of these factors, which is known to decrease the replication of several viruses, including HIV-1.12,35-37 Inhibition of HIV-1 by IFNα in PDCs supernatant has been described,47 but we now demonstrate that PDCs secrete at least one additional inhibitory factor that is smaller than 3 kDa. This factor is heat-sensitive, but remains to be identified. Some candidate inhibitors can be excluded. The group of chemokines that block the coreceptor, thereby preventing HIV-1 entry into T cells,48 are too large. Furthermore, PDCs do not secrete the SDF-1 ligand for CXCR4 and very little of the RANTES/MIP-1α/β ligands for CCR5.49 Indeed, the PDC supernatant inhibits CXCR4- and CCR5-using HIV-1 infection to the same extent (Figure 4A).
Another group of possible candidates are antimicrobial peptides. These small peptides (1-5 kDa) are secreted by different cell types and are active against a broad range of bacteria, fungi, and viruses.50,51 With respect to HIV-1, 3 inhibitory mechanisms of antimicrobial peptides have been described: direct virolysis, inhibition of transcription from the LTR promoter, and block of entry by binding to cell-surface molecules.52-55 The production of antimicrobial peptides by PDCs has not been studied extensively. One study reported small amounts of intracellular human β-defensin 1 (hBD-1) in PDCs from some donors.56 Another group showed that monocyte-derived MDCs harbor low amounts of mRNA for hBD-1 and hBD-2.57 hBD-1, and hBD-2 in particular, have been shown to inhibit HIV-1 replication by an unidentified mechanism.58 In our study, we found that PDCs of some donors express marginal amounts of mRNA for hBD-1 and hBD-2, and that no mRNA for another antimicrobial peptide, LL-37, was detected (Sylvia J.P. Bogaards, Peter H. Nibbering, unpublished results). For this reason, it is not very likely that hBD-1 or hBD-2 is the additional HIV-1 inhibitor in PDC supernatant. It cannot be excluded that other antimicrobial peptides are responsible for the inhibition. It would be of interest to perform a further search for this inhibitor and investigate whether the factor inhibits other viruses as well. Not only will this broaden our knowledge on the antiviral response of PDCs, but it could also create novel therapeutic options.
The differential impact of MDCs and PDCs on HIV-1 infection may reflect the different location and function of these cells in the human body. MDCs constantly sample the outside milieu to detect pathogens and form a direct link between innate and adaptive immunity.2 It has been known for several years that they stimulate HIV-1 infection,39 and it has been proposed that they transmit the virus to T cells following migration to secondary lymphoid organs.59 Their role in HIV-1 infection is therefore better understood than the role of PDCs. It is not very likely that PDCs are similarly involved in HIV-1 capture in mucosal tissues. PDCs are not found in high amounts at sites of pathogen entry; they do not capture, endocytose, and process antigens as effectively as MDCs; and their role in T-cell proliferation is less pronounced.11 In contrast, PDCs are involved in the innate immune response against many viruses, including HSV-1 and -2, influenza, VSV, and HIV-1.11 Their role in HIV-1 infection may be to control the virus in order to delay disease progression. The numbers of both MDCs and PDCs in blood of HIV-1–infected patients are reduced.13,14,16,18 Since both types of DCs can be infected by HIV-1,19,20,60 this reduction can be the result of depletion. Alternatively, they may have relocated to secondary lymphoid tissue as a consequence of HIV-1 infection.61,62 Both types of DCs isolated from HIV-1 patients were reported to be functionally impaired with respect to T-cell stimulation and cytokine production.15-17 It remains to be established whether this impairment is a cause or an effect of progressing HIV-1 infection, but at least the various data suggest that MDCs may have a dual role in HIV-1 infection: initially, MDCs may help to establish HIV-1 infection, but later on, progression toward AIDS is correlated with a loss of MDC numbers and a decreased antigen-presenting capacity to T cells.
In conclusion, we have shown that MDCs and PDCs have opposing effects on HIV-1 infection, and subsequent replication in T cells. MDCs enhance HIV-1 infection by facilitating transmission, PDCs inhibit infection by secreting factors that inhibit HIV-1 replication. These differences should be taken into account when studying the role of DCs in HIV-1 pathogenesis.
Prepublished online as Blood First Edition Paper, May 16, 2006; DOI 10.1182/blood-2006-03-010918.
Supported by grant 7008 from Aids Fonds Netherlands, Amsterdam.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Sylvia J.P. Boogaards and Peter H. Nibbering (Department of Infectious Diseases, Leiden University Medical Centre, The Netherlands) for the RT-PCR study and their permission to cite the unpublished results.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal