Abstract
The role for IL-7R expression in the differentiation of effector T cells into resting memory remains controversial. Here, using a conditional IL-7R transgenic model, we were able to test directly whether CD8 effector T cells require IL-7R expression for their differentiation into resting memory cells. In the absence of IL-7R expression, effector cells transferred into “full” hosts underwent a protracted and unremitting contraction compared with IL-7R–expressing control cells and were unable to develop into long-term resting memory cells. Surprisingly, when the same effector cells were transferred into empty T-cell–deficient hosts, they could generate long-lived fully functional resting memory cells independently of IL-7R expression. Formation of these latter cells was found to be dependent on IL-15, because the same IL-7R–deficient effector cells were rapidly lost from IL-15–deficient hosts, having a half-life of less than 40 hours. Therefore, our data suggest that, under physiological conditions, both IL-7 and IL-15 synergize to promote the formation of memory cells directly by limiting the contraction of effectors that occurs following an immune response and that reexpression of IL-7R is a key checkpoint in the regulation of this process.
Introduction
The number of T cells in the immune system is maintained at a remarkably constant level throughout life. This is despite variable dynamics in T-cell production by both the thymus and during immune responses, both of which contribute to the heterogeneous composition of the peripheral T-cell compartment. This homeostatic equilibrium is achieved by strict control of lymphocyte survival and proliferation. While there are differences in the factors and signals that control survival and proliferation of naive versus memory T cells for both CD4 and CD8 lineage T cells,1 the end result in terms of controlling their absolute cell numbers is the same. The differences in mechanisms of homeostasis that exist between these subsets most likely permit a degree of independence in their regulation.2
During immune responses, however, activation of naive T cells by foreign peptide/major histocompatibility complex (MHC) uncouples the cells from these homeostatic constraints so that they can undergo a rapid expansion in numbers as part of the ongoing adaptive response. The effector cells generated in these responses are short lived, and once the pathogen has been cleared there is a rapid contraction in their number. Crucially, however, some of the effector cells are able to revert to a resting state and once again become subject to homeostatic regulatory mechanisms but this time as memory cells. The regulation of resting memory by the cytokines IL-153-5 and IL-76-8 has been well studied. In contrast, the mechanisms that permit entry of some effector cells into the memory pool are not well understood, and how T-cell memory is generated remains a key question in immunology.
Given the key role of cytokines in the maintenance of CD8 memory, it is possible they also play a role in the effector-to-memory transition. In support of this idea, IL-7R–deficient mice have been shown to make defective immune responses.9,10 More recent studies have described a correlation between reexpression of IL-7R by T cells during immune responses and the generation of memory,11,12 and in at least one study the size of this emergent IL-7R–expressing population was comparable to that of the memory population that was eventually formed, suggesting a precursor relationship between the populations.11 Expression of CD8αα homodimers that can specifically recognize the nonclassical class I MHC molecule TL has also been suggested to provide a differentiation signal to effectors cells.13 Interestingly, these cells also expressed high levels of IL-7R. More recently, however, studies have questioned these models14,15 and specifically the relationship between IL-7R expression and memory formation.15
Thus, the specific role of IL-7R in the formation of CD8 memory remains controversial. Do effector cells express IL-7R as they differentiate into long-term memory cells and, if so, what is the role of IL-7R itself in this process? If, as suggested by others, expression of IL-7R is indicative of memory formation, is it simply a marker for effector cells whose fate it is to generate memory cells, or do signals through the receptor actually play a role in the process? To address these key questions directly, we generated mice with a tetracycline-inducible IL-7R transgene to ask whether expression of IL-7R by effector cells was specifically required for their development into memory cells. We show that under physiological conditions, reexpression of IL-7R by effector cells is a crucial checkpoint in their differentiation into resting memory but that this process also depends on a potent synergy with IL-15. We further show that these 2 cytokines together play a key role in controlling the contraction that occurs after an immune response in the absence of antigen.
Materials and methods
Mice
Inducible IL-7R transgenic mice were generated using the tetracycline regulatory system. Il7r cDNA was subcloned into a Tet reporter construct featuring upstream TRE and minimal CMV promoter and downstream SV40 splice donor and acceptor sites and polyA signals. Fragments were prepared and injected into pronuclei of fertilized oocytes from (CBA × C57BL/10)F1 mice.16,17 Inducible IL-7R transgenic mice (TreIL-7R) were intercrossed with Il7r–/– mice bearing the reverse tetracycline transactivator domain (rtTA) transgenes under control of huCD2 promotor (rtTAhuCD2). The resultant TreIL-7R rtTAhuCD2Il7r–/– mice were further intercrossed with F5 Rag1–/–Il7r–/– mice to generate experimental F5 Rag1+/–Il7r–/– TreIL-7R rtTAhuCD2 mice (F5 TreIL-7R). Breeders and weaned pups were fed doxycycline (dox) in food (3 mg/g) to induce IL-7R expression. (F5 Rag1–/– × C57BL/6J Ly5.1)F1 mice were used as controls throughout. These strains and Rag1–/–, Ly5.1 F5 Rag1–/–, Il7–/–, Il15ra–/– (The Jackson Laboratory, Bar Harbor, ME), and Rag1–/–Il15ra–/– mice were bred in a conventional colony free of pathogens at the National Institute for Medical Research, London. All lines used were of H-2b haplotype. Animal experiments were done according to institutional guidelines and Home Office regulations.
Flow cytometry
Flow cytometry was carried out using lymph node, thymus, spleen cells, or peripheral blood lymphocytes (PBLs). Cell concentrations were determined using a Scharfe Instruments Casy Counter (Scharfe System, Reutlingen, Germany). Erythrocytes in PBL samples were lysed prior to staining. Monoclonal antibodies used in this study were as follows: APC- and PE-CD4 (GK1.5) (PharMingen, San Diego, CA); APC- and FITC-TCR (H57-597) (PharMingen); APC-CD44 (Leinco Technologies, St Louis, MO); FITC- and PE-CD44 (PharMingen); FITC-, PerCP-, and APC-CD8 (PharMingen); FITC- and PE-CD122 (PharMingen); FITC-Ly5.2 (PharMingen); APC-Cy7-Ly5.2 (eBioscience, San Diego, CA); biotin- and PE-Ly5.1(PharMingen); and PE–IL-7 receptor α (eBioscience). Cell viability was determined by 7-AAD (Sigma, St Louis, MO) exclusion and labeling at 10 μg/mL. Four- and 5-color cytometric staining was analyzed on a FACSCalibur and LSR instruments (Becton Dickinson, San Jose, CA), respectively, and data analysis performed with FlowJo V6.3 software (TreeStar, Ashland, OR).
In vitro culture, labeling, and adoptive transfer of T cells
Lymphocytes were teased from lymph nodes and spleen of donor mice and single-cell suspensions were prepared. For survival assays, F5 T splenocytes were purified using anti-CD8 microbeads and magnetic-activated cell separation (MACS) columns (Miltenyi Biotec, Bergisch Gladbach, Germany) and cultured (106/mL) in complete RPMI 1640 media (Sigma) supplemented with 10% FCS (Sigma), glutamine (Sigma), 2-mercaptoethanol (Sigma), and antibiotics (Sigma). Dox (Sigma) was supplemented at 2 μg/mL and IL-7 at 50 ng/mL. For stimulations, T cells were cultured at 106/mL with 10 nM NP68 peptide18 for 72 hours in complete media. Blasts were isolated after 72 hours by centrifugation over Ficoll gradient. Cells were further cultured (105/mL) in recombinant mouse IL-2 (10 ng/mL) (Peprotech, Rocky Hill, NJ) for a further 96 hours. Cells were labeled with 2 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) in Dulbecco PBS (GibcoBRL, Carlsbad, CA) for 10 minutes at 37°C and washed twice.
Cells were transferred into various recipient mice via tail vein injections. Tolerant hosts, lymphoreplete with Ly5.1 C57BL6/J–derived cells, were generated by making radiation chimeras using F5 TreIL-7R mice as hosts. Irradiated (5 Gy [500 rad]) recipients were reconstituted with T-depleted bone marrow cells from Ly5.1 C57BL6/J donors. Chimeras were used as recipients of F5 effector cells 8 to 10 weeks following reconstitution.
Cellular expansion predicted by the CFSE profile of labeled cells was calculated by determining the frequency (F) of donor cells that had undergone different numbers of divisions (d). Adjusted frequencies (f) were calculated by dividing F by 2d for each division. The predicted expansion was determined by the calculation ΣF/Σf. Mean divisions were determined by the calculation ΣF.d/Σf.
In vivo killing assay
Splenocyte targets from Ly5.1 C57BL/6J were pulsed with either 10–6 M, 10–7 M, 10–8 M, 10–9 M NP68, or no peptide as control for 2 hours at 37°C and then labeled with 4 μM, 1 μM, 250 nM, 62 nM, and 16 nM CFSE, respectively. A total of 4 × 106 of each population of cells was mixed and injected together with 2 × 107 F5 blasts in Rag1–/– recipients. Rag1–/– and F5 Rag1–/– recipients received targets alone as controls. Twenty-four hours later, target cells in host spleens were stained with Ly5.1 and analyzed by fluorescence-activated cell sorting (FACS). Percent killing was determined by comparison of target populations remaining in experimental hosts with control hosts that received targets alone and normalized to the unpulsed population.
Results
Inducible IL-7R overcomes the thymic developmental block in IL-7R–deficient mice
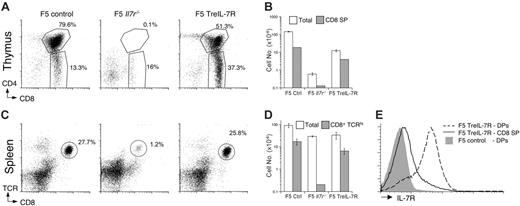
To study the role of IL-7R during CD8 memory development, we used the F5 T-cell receptor (TCR) transgenic mouse that expresses a class I–restricted TCR specific for the peptide epitope 366-374 (NP68 peptide) of the nucleoprotein of influenza virus (A/NT/60/68).18 To ask whether expression of IL-7R is important for the differentiation of effector cells into resting memory, we wished to test the ability of IL-7R–deficient F5 effector cells to generate resting memory cells. However, generation and analysis of F5 Il7r–/– mice revealed a profound block in thymic development (Figure 1A) at the double-negative 2 stage (data not shown), and these mice had virtually no mature peripheral T cells (Figure 1C-D). The few F5 T cells that emerge do so independently of IL-7R signaling, overcoming a very profound selection pressure in the thymus for IL-7R independence, and subsequently reside in a lymphopenic peripheral compartment. Thus, the physiological and functional significance of these cells is unclear. Therefore, to specifically address these problems, we generated F5 Il7r–/– mice bearing a tetracycline-inducible IL-7R transgene (F5 TreIL-7R) (see “Materials and methods”).
Analysis of thymus from dox-fed F5 TreIL-7R mice revealed successful restoration of thymic development. CD4+CD8+ double-positive (DP) and mature CD8+CD4– single-positive (SP) compartments were substantially restored compared with F5 Il7r–/– controls both in terms of cell frequency (Figure 1A) and absolute numbers (Figure 1B), although neither quite reached wild-type (WT) levels. Restored thymic production of F5 T cells was also reflected in the frequency (Figure 1C) and number (Figure 1D) of F5 T cells present in spleen of dox-fed F5 TreIL-7R mice compared with controls. The tissue distribution of TreIL-7R transgene expression driven by rtTAhuCD2 does not exactly match that of the endogenous Il7r gene, most notably in its ability to target IL-7R expression to DP thymocytes (Figure 1E). However, no adverse effects of this ectopic expression on thymic selection were observed in F5 TreIL-7R mice, consistent with other reports of constitutive IL-7R transgenic mice.19
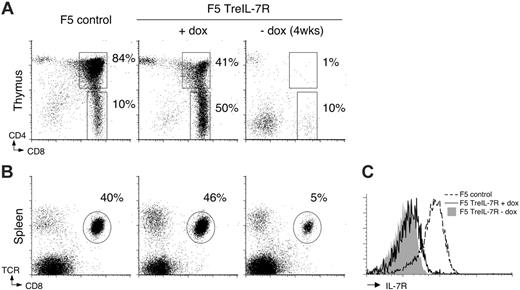
We next tested the full reversibility of IL-7R transgene expression. Groups of F5 TreIL-7R mice were taken off their doxcontaining diet (F5 TreIL-7ROFF) while controls were maintained on dox food. Four weeks later, thymus and spleen were examined. In the absence of dox, thymi atrophied and displayed a developmental block similar to that of F5 Il7r–/– controls (Figures 1A and 2A). Analysis of spleen from F5 TreIL-7ROFF mice revealed a marked reduction in F5 T-cell frequencies (Figure 2B) and numbers, containing less than 1.9 × 106 (± 1.3 × 105) T cells compared with more than 12 × 106 (± 3 × 104) in dox-fed controls, consistent with the importance of IL-7R expression for naive T-cell survival.10,20 Examination of IL-7R expression by T cells from F5 TreIL-7R mice revealed that, in contrast to the high expression observed in thymus (Figure 1E), transgene expression by peripheral F5 T cells was much lower (Figure 2C). This appears to be a feature of some Tet-inducible transgenes20,21 and may be a particular issue for proteins that have a naturally high turnover. Nevertheless, expression was found to be functional in vitro and only in the presence of continued dox (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). Therefore, the conditional expression of IL-7R in this inducible transgenic model does successfully overcome the problems of the F5 Il7r–/– parent strain, because we can generate IL-7R–deficient F5 T cells whose thymic development is still IL-7R expression dependent and that reside in a more normal lymphoreplete peripheral T-cell compartment.
TreIL-7R expression restores thymic development in F5 Il7r–/– mice. Single-cell suspensions were prepared from thymus and spleen of 6-week-old F5 control, F5 Il7r–/–, and dox-fed F5 TreIL-7R mice. (A) Dot plots show CD4 versus CD8 expression in the thymus for representative mice of the indicated strain. (B) The bar chart shows total thymocyte (□) and CD8 SP thymocyte (▦) cell numbers of mice from panel A. (C) Dot plots show TCR versus CD8 expression by splenocytes from the corresponding mice in panel A. (D) The bar chart shows total spleen (□) and CD8+TCRhi (▦) cell numbers. (E) The histograms show IL-7R expression by CD4+CD8+ SP (broken line) and CD4–CD8+ DP (solid line) thymocytes from F5 TreIL-7R mice and CD4+CD8+ DP thymocytes from control F5 mice (gray fill) as negative control, because DP thymocytes are known not to express IL-7R. Data are representative of 3 or more independent experiments.
TreIL-7R expression restores thymic development in F5 Il7r–/– mice. Single-cell suspensions were prepared from thymus and spleen of 6-week-old F5 control, F5 Il7r–/–, and dox-fed F5 TreIL-7R mice. (A) Dot plots show CD4 versus CD8 expression in the thymus for representative mice of the indicated strain. (B) The bar chart shows total thymocyte (□) and CD8 SP thymocyte (▦) cell numbers of mice from panel A. (C) Dot plots show TCR versus CD8 expression by splenocytes from the corresponding mice in panel A. (D) The bar chart shows total spleen (□) and CD8+TCRhi (▦) cell numbers. (E) The histograms show IL-7R expression by CD4+CD8+ SP (broken line) and CD4–CD8+ DP (solid line) thymocytes from F5 TreIL-7R mice and CD4+CD8+ DP thymocytes from control F5 mice (gray fill) as negative control, because DP thymocytes are known not to express IL-7R. Data are representative of 3 or more independent experiments.
Reversible expression and function of the TreIL-7R transgene in F5 TreIL-7R mice. Groups of 6-week-old F5 TreIL-7R mice (n = 4) were taken off or maintained on dox food for 4 weeks, after which the phenotype of thymus and spleen was analyzed by FACS. Dot plots show CD4 versus CD8 expression in thymus (A) and TCR versus CD8 expression in spleen (B) of control F5, dox-fed, and dox-free F5 TreIL-7R mice. (C) Histograms show IL-7R expression by CD8+TCRhi splenocytes from F5 controls (broken line), dox-fed F5 TreIL-7R mice (solid line), and dox-free F5 TreIL-7R mice (gray fill). Data are representative of at least 2 independent experiments.
Reversible expression and function of the TreIL-7R transgene in F5 TreIL-7R mice. Groups of 6-week-old F5 TreIL-7R mice (n = 4) were taken off or maintained on dox food for 4 weeks, after which the phenotype of thymus and spleen was analyzed by FACS. Dot plots show CD4 versus CD8 expression in thymus (A) and TCR versus CD8 expression in spleen (B) of control F5, dox-fed, and dox-free F5 TreIL-7R mice. (C) Histograms show IL-7R expression by CD8+TCRhi splenocytes from F5 controls (broken line), dox-fed F5 TreIL-7R mice (solid line), and dox-free F5 TreIL-7R mice (gray fill). Data are representative of at least 2 independent experiments.
Normal activation, expansion, and effector cell generation in the absence of IL-7R expression
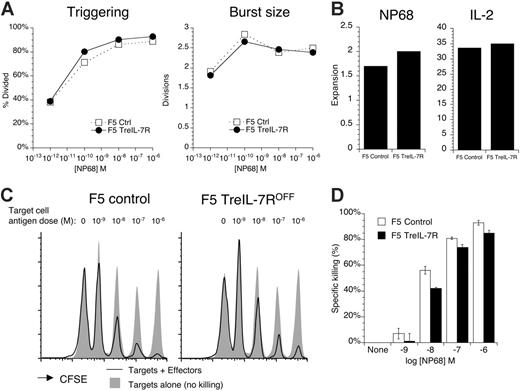
Next, we wanted to confirm that activation and generation of effector T cells from F5 TreIL-7ROFF mice was normal. T cells from control F5 mice and F5 TreIL-7R donors, taken off dox diet for at least 3 days, were labeled with CFSE and stimulated with a range of peptide doses in vitro for 72 hours. Both the proportion of F5 TreIL-7R T cells triggered into cell division and the size of their proliferative burst were similar to F5 cells (Figure 3A), as were cell recoveries in response to peptide antigen and further culture in IL-2 (Figure 3B). The functional capacity of cultured cells was tested in an in vivo killing assay (see “Materials and methods”). Splenocyte targets were pulsed with different concentrations of NP68 peptide and then labeled with distinct concentrations of CFSE. Targets were mixed in equal numbers and transferred with cultured F5 effectors into Rag1–/– recipients. Twenty-four hours later, spleens of recipient mice were analyzed for the presence of CFSE-positive Ly5.1-positive targets. The baseline of “no killing” was established by injecting the same mixture of target cells into either naive F5 Rag1–/– or Rag1–/– recipient mice that lack effector cells. Effector F5 TreIL-7ROFF T cells mediated similar levels of antigen dose–dependent killing as compared with control F5 effectors (Figure 3C-D). These data confirmed both the functional competence of effector cells generated from F5 TreIL-7ROFF mice in vivo and their ability to successfully engraft into new hosts.
F5 TreIL-7ROFF T cells activate, expand, and develop effector function similarly to F5 control T cells. (A) Splenocytes from F5 TreIL-7R donors taken off dox food 3 days previously were CFSE labeled and stimulated with different concentrations of NP68 peptide. After 72 hours, CFSE profiles were analyzed. Graphs show percent cells divided (triggering) and division index of dividing cells calculated (burst size) for cultures of control (□) and F5 TreIL-7ROFF T cells. (B) In similar cultures, T cells were stimulated with 10 nM NP68 for 72 hours. Viable cells were isolated by centrifugation over Ficoll gradient and then cultured for a further 4 days in IL-2. Cell recoveries were enumerated both after 3 days of peptide stimulation and after 4 days of further IL-2 culture. Bar charts show fold expansion over input cell number in NP68-stimulated and IL-2 expansion cultures. (C) Effector function of cultured T cells from panel B was tested in vivo. C57BL/6J Ly5.1 targets were pulsed in vitro with increasing concentrations of NP68 and then labeled with different levels of CFSE. The antigen dose for each individual CFSE peak is indicated above the histograms. Targets were mixed in equal numbers and 2 × 107 total cells cotransferred with 2 × 107 cultured F5 effectors to Rag1–/– recipients. Targets alone were injected into Rag1–/– or naive F5 Rag1–/– recipients as control for no killing. Twenty-four hours later, spleen from recipient mice was analyzed by FACS for the presence of Ly5.1-positive target cells. Histograms show CFSE labeling of Ly5.1-positive targets cotransferred with effectors (solid line) and targets transferred alone as control (gray fill) for the F5 effector donor indicated. Target cell recoveries from naive F5 Rag1–/– were identical to those recovered from Rag1–/– hosts (data not shown). (D) Bar chart indicates the percent killing of targets pulsed with the indicated concentrations of NP68 by F5 control (open) and F5 TreIL-7ROFF effectors (filled). Data are representative of at least 2 independent experiments.
F5 TreIL-7ROFF T cells activate, expand, and develop effector function similarly to F5 control T cells. (A) Splenocytes from F5 TreIL-7R donors taken off dox food 3 days previously were CFSE labeled and stimulated with different concentrations of NP68 peptide. After 72 hours, CFSE profiles were analyzed. Graphs show percent cells divided (triggering) and division index of dividing cells calculated (burst size) for cultures of control (□) and F5 TreIL-7ROFF T cells. (B) In similar cultures, T cells were stimulated with 10 nM NP68 for 72 hours. Viable cells were isolated by centrifugation over Ficoll gradient and then cultured for a further 4 days in IL-2. Cell recoveries were enumerated both after 3 days of peptide stimulation and after 4 days of further IL-2 culture. Bar charts show fold expansion over input cell number in NP68-stimulated and IL-2 expansion cultures. (C) Effector function of cultured T cells from panel B was tested in vivo. C57BL/6J Ly5.1 targets were pulsed in vitro with increasing concentrations of NP68 and then labeled with different levels of CFSE. The antigen dose for each individual CFSE peak is indicated above the histograms. Targets were mixed in equal numbers and 2 × 107 total cells cotransferred with 2 × 107 cultured F5 effectors to Rag1–/– recipients. Targets alone were injected into Rag1–/– or naive F5 Rag1–/– recipients as control for no killing. Twenty-four hours later, spleen from recipient mice was analyzed by FACS for the presence of Ly5.1-positive target cells. Histograms show CFSE labeling of Ly5.1-positive targets cotransferred with effectors (solid line) and targets transferred alone as control (gray fill) for the F5 effector donor indicated. Target cell recoveries from naive F5 Rag1–/– were identical to those recovered from Rag1–/– hosts (data not shown). (D) Bar chart indicates the percent killing of targets pulsed with the indicated concentrations of NP68 by F5 control (open) and F5 TreIL-7ROFF effectors (filled). Data are representative of at least 2 independent experiments.
IL-7R–deficient effectors give rise to resting memory cells in vivo
To examine the role of IL-7R in the formation of memory, we initially tested the ability of F5 TreIL-7ROFF T cells to respond to flu virus in vivo. These experiments revealed a partial defect in memory generation from IL-7R–/– cells (Figure S2) similar to that described previously.10 However, the relative reduction in F5 TreIL-7ROFF T cells appeared to be established even in the effector phase of the response (day 6), and it was unclear which point of the response IL-7R deficiency was affecting: initial priming and effector generation, effector-to-memory transition, survival of newly formed memory cells, or a combination of all 3. Furthermore, it is also not possible to control the presence of viral antigen that affects both the persistence and recruitment of new effector cells during a response, making it difficult to observe the effector-to-memory transition. Therefore, we adopted a system in which effectors were generated in vitro by stimulation with specific antigen and then engrafted into naive hosts, similar to systems used in other studies.22,23 Using this system we were able to observe the differentiation of a synchronized, homogenous population of effector cells into resting memory in the absence of any further antigenic stimulation in a way that is not possible using in vivo infectious disease models, which exhibit significant heterogeneity at all stages of a response.
Having established that IL-7R–deficient F5 T cells activate, expand, and develop effector function in a manner indistinguishable from that of control F5 T cells, we next asked whether these IL-7R–deficient effector cells could give rise to resting memory in vivo. T cells from F5 TreIL-7ROFF and control F5 mice were stimulated with peptide and expanded in IL-2. Cultured F5 TreIL-7ROFF and F5 control T cells were mixed in equal numbers and injected into Rag1–/– recipients. Analysis of CD8 T cells in peripheral blood of recipient mice over time revealed an initial contraction in all F5 T-cell populations followed by a stabilization in their levels (Figure 4A). Interestingly, control F5 T-cell numbers stabilized more quickly and at a higher level than F5 TreIL-7ROFF T cells and even started increasing in number after some weeks. Administration of dox to recipients of F5 TreIL-7R T cells did not have any detectable effect on their behavior compared with F5 TreIL-7ROFF T cells in dox-free hosts (Figure S3A-B). Expression of the IL-7R transgene by peripheral T cells in dox-fed F5 TreIL-7R mice was much lower than that of endogenous expression by F5 control T cells (Figures 2C and S2C) and appears not to be at a level sufficient to elicit any detectable function in these experiments. Therefore, to be absolutely sure that the comparison of F5 TreIL-7ROFF T cells with control F5 T cells was fair and that the difference in engraftment observed (Figure 4A) was IL-7 dependent and not a result of a developmental defect intrinsic to T cells from F5 TreIL-7R mice, mixtures of cultured F5 TreIL-7ROFF and F5 control T cells were injected into Rag1–/– and Il7–/–Rag1–/– recipients. Figure 4B shows the representation of F5 TreIL-7ROFF in these hosts. Although F5 TreIL-7ROFF T cells were initially present in Rag1–/– mice in equal numbers to control F5 T cells, their proportion steadily decreased with time (Figure 4B). In contrast, in Il7–/–Rag1–/– hosts, the proportion of F5 TreIL-7ROFF and control F5 T cells remained equal, demonstrating that their different behavior in Rag1–/– hosts was indeed IL-7 dependent.
F5 effector cells develop into functional long-term resting memory in the absence of IL-7R expression in Rag1–/– hosts. Splenocytes from F5 control mice and F5 TreIL-7R donors taken off dox food 3 days previously were stimulated with NP68 peptide for 72 hours followed by a further 96 hours of culture in IL-2. Blasts from cultures of F5 TreIL-7ROFF and control F5 T cells were mixed in equal numbers and 2 × 107 total cells transferred into Rag1–/– and Il7–/–Rag1–/– recipients (n = 6). Recipient mice were bled at different times after transfer and analyzed for CD8, TCR, and Ly5.1 expression. (A) The graph shows the frequency of Ly5.1-negative Ly5.2-positive F5 TreIL-7ROFF T cells (○) and Ly5.1-positive F5 control cells (•) in PBLs of Rag1–/– hosts. (B) The graph shows the proportion of donor CD8+ T cells that were of Ly5.1-negative Ly5.2-positive F5 TreIL-7ROFF origin in Rag1–/– (•) and Il7–/–Rag1–/– (○) hosts. (C) Antigen-specific in vivo killing function was determined in Rag1–/– recipients of either 2 × 107 F5 TreIL-7ROFF or 4 × 106 control F5 cultured effector cells 7 weeks after transfer. Targets were prepared and injected into hosts as described in Figure 3C and killing determined by comparison with targets transferred to empty Rag1–/– hosts 24 hours later. (D) Dot plots show FSc versus SSc profiles of the indicated F5 blasts after 7 days of culture in vitro and 1 day after transfer in vivo. (E) Histograms show CD122 expression by F5 TreIL-7ROFF (solid line) and F5 control T cells (gray fill) ex vivo and at day 7 culture in vitro. Reference histogram gates are identical in width and position with respect to CD122 staining as circular gates shown in panel F set by gating CD122hiCD44hiCD8+ memory phenotype cells in WT C57BL/6J mice. (F) Dot plots show CD44 versus CD122 expression by F5 TreIL-7ROFF blasts and F5 control blasts in peripheral blood 1 day after transfer and plots of peripheral blood from WT C57BL/6J and naive F5 Rag1–/– controls. Data are representative of 4 or more experiments.
F5 effector cells develop into functional long-term resting memory in the absence of IL-7R expression in Rag1–/– hosts. Splenocytes from F5 control mice and F5 TreIL-7R donors taken off dox food 3 days previously were stimulated with NP68 peptide for 72 hours followed by a further 96 hours of culture in IL-2. Blasts from cultures of F5 TreIL-7ROFF and control F5 T cells were mixed in equal numbers and 2 × 107 total cells transferred into Rag1–/– and Il7–/–Rag1–/– recipients (n = 6). Recipient mice were bled at different times after transfer and analyzed for CD8, TCR, and Ly5.1 expression. (A) The graph shows the frequency of Ly5.1-negative Ly5.2-positive F5 TreIL-7ROFF T cells (○) and Ly5.1-positive F5 control cells (•) in PBLs of Rag1–/– hosts. (B) The graph shows the proportion of donor CD8+ T cells that were of Ly5.1-negative Ly5.2-positive F5 TreIL-7ROFF origin in Rag1–/– (•) and Il7–/–Rag1–/– (○) hosts. (C) Antigen-specific in vivo killing function was determined in Rag1–/– recipients of either 2 × 107 F5 TreIL-7ROFF or 4 × 106 control F5 cultured effector cells 7 weeks after transfer. Targets were prepared and injected into hosts as described in Figure 3C and killing determined by comparison with targets transferred to empty Rag1–/– hosts 24 hours later. (D) Dot plots show FSc versus SSc profiles of the indicated F5 blasts after 7 days of culture in vitro and 1 day after transfer in vivo. (E) Histograms show CD122 expression by F5 TreIL-7ROFF (solid line) and F5 control T cells (gray fill) ex vivo and at day 7 culture in vitro. Reference histogram gates are identical in width and position with respect to CD122 staining as circular gates shown in panel F set by gating CD122hiCD44hiCD8+ memory phenotype cells in WT C57BL/6J mice. (F) Dot plots show CD44 versus CD122 expression by F5 TreIL-7ROFF blasts and F5 control blasts in peripheral blood 1 day after transfer and plots of peripheral blood from WT C57BL/6J and naive F5 Rag1–/– controls. Data are representative of 4 or more experiments.
We next asked whether the F5 TreIL-7ROFF effectors that survive long term in Rag1–/– hosts were functional and could therefore be considered memory cells. Functionality of the cells was tested by in vivo killing assay, as described earlier (Figure 3C). Because transfer of control F5 effectors engrafted at a higher level than F5 TreIL-7ROFF effectors (Figure 4A), 5-fold fewer F5 control than F5 TreIL-7ROFF effectors were transferred to Rag1–/– hosts so that in vivo killing could be compared in hosts containing similar numbers of cells. After 7 weeks, the frequency of F5 T cells in peripheral blood was similar between recipients of control F5 (1.5% ± 0.3%) and F5 TreIL-7ROFF (2.1% ± 1.3%) effector cells. At this time point, control F5 and F5 TreIL-7ROFF cells killed targets in a similar manner (Figure 4C). Therefore, not only were F5 TreIL-7ROFF effectors able to survive long term in the absence of continued IL-7R expression, but they exhibited a functional potency comparable to that of control F5 T cells.
To further examine the conversion of blasts generated in vitro to resting cells in vivo, we analyzed cell size and scatter profiles of the different populations. As expected, cultured F5 T cells had a scatter profile characteristic of blasting cells (ie, high forward scatter [FSc] and side scatter [SSc] signals). As soon as 1 day after adoptive transfer in vivo, however, these cells acquired a small resting phenotype (Figure 4D) that they retained thereafter and was accompanied by their successful engraftment into the host. Similarly, F5 TreIL-7ROFF blasts were able to undergo a similar conversion to a small resting quiescent state soon after their transfer (Figure 4D), suggesting that acquisition of this phenotype did not depend on IL-7R. Because IL-15 is known to play a crucial role in CD8 memory survival and homeostasis, we also examined expression of CD122 to see whether F5 TreIL-7ROFF T cells were normally responsive to IL-15. CD122 is the IL-2Rβ chain that is essential for stimulation by IL-15 transpresented by IL-15Rα 24,25 and is essentially the functional IL-15 receptor on T cells. CD122 expression was up-regulated to identical levels on F5 TreIL-7ROFF and control F5 T cells during 7-day culture in vitro (Figure 4E) and remained unchanged following transfer into Rag1–/– recipients (Figure 4F). Furthermore, levels of CD122 and CD44 on activated F5 T cells following their adoptive transfer were similar to those of memory phenotype cells in normal mice (Figure 4F).
Reexpression of IL-7R by F5 effectors
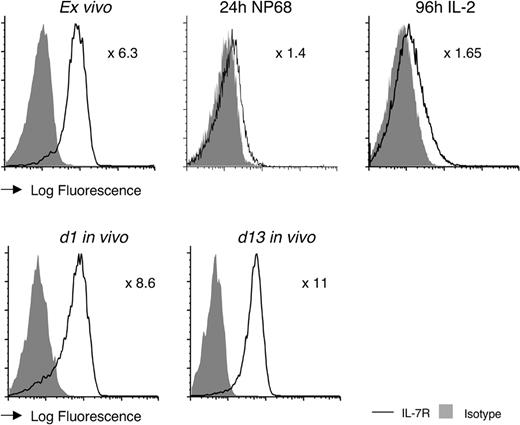
T cells rapidly down-regulate IL-7R immediately following their stimulation by antigen.10 To see whether the long-term differences in cellular engraftment between F5 TreIL-7ROFF and F5 control T cells (Figure 4A) directly correlated with expression of IL-7R, we examined receptor expression by F5 control T cells following activation in vitro and in vivo. As expected, F5 T cells almost completely down-regulated expression of IL-7R by 24 hours following initial stimulation with specific peptide (Figure 5). Although the T cells underwent substantial proliferation and expansion in IL-2 (Figure 3B), cells did start to reexpress IL-7R by the end of this culture period, albeit at a lower level than observed on naive F5 T cells (Figure 5). Remarkably, expression rapidly increased following transfer into Rag1–/– recipients (Figure 5) and had reached its maximal level as soon as day 1 and did not significantly change thereafter.
IL-7R expression is essential for survival of newly formed memory cells in replete hosts
Although F5 TreIL-7ROFF effector cells were able to revert to a resting state and persist in Rag1–/– hosts in the absence of continued IL-7R expression, the level of long-term engraftment was consistently less than IL-7R–expressing F5 control T cells (Figure 4A). Because Rag1–/– hosts are T-cell deficient, we next examined the behavior of effector cells in replete hosts to determine whether the differences we observed were a consequence of the lymphopenic environment. Previous studies have shown that IL-7 is necessary for homeostatic proliferation of CD8 memory cells in lymphopenic hosts.7 However, because F5 TreIL-7R mice are not backcrossed to a pure C57BL6/J background, as replete hosts we used both naive F5 Rag1–/– recipient mice, which have a peripheral T-cell compartment that is “full” with respect to naive F5 T cells, and bone marrow chimeras, in which F5 TreIL-7R mice had been reconstituted with bone marrow from Ly5.1 C57BL6/J and therefore had a normal WT T-cell compartment but were fully tolerant with respect to their F5 TreIL-7R hosts (henceforth, “WT bm chimeras”). Additionally, blasts were labeled with CFSE so we could follow cell division.
F5 effectors reexpress IL-7R in vitro and in vivo. Histograms show IL-7R expression by control F5 T cells (solid line) compared with isotype control (gray fill) prior to stimulation ex vivo, after 24 hours of stimulation with NP68 peptide, following 72-hour peptide and further 96-hour culture with IL-2, and on day 1 and day 13 following adoptive transfer in vivo into Rag1–/– hosts. Numbers indicate fold increase in mean fluorescence intensity (MFI) of IL-7R expression of F5 control T cells over the background of the negative control stain. Data are representative of at least 3 independent experiments.
F5 effectors reexpress IL-7R in vitro and in vivo. Histograms show IL-7R expression by control F5 T cells (solid line) compared with isotype control (gray fill) prior to stimulation ex vivo, after 24 hours of stimulation with NP68 peptide, following 72-hour peptide and further 96-hour culture with IL-2, and on day 1 and day 13 following adoptive transfer in vivo into Rag1–/– hosts. Numbers indicate fold increase in mean fluorescence intensity (MFI) of IL-7R expression of F5 control T cells over the background of the negative control stain. Data are representative of at least 3 independent experiments.
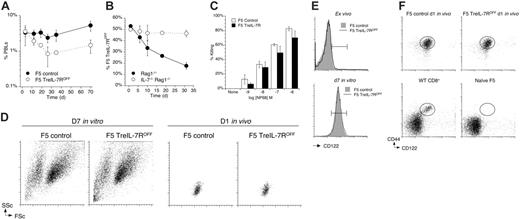
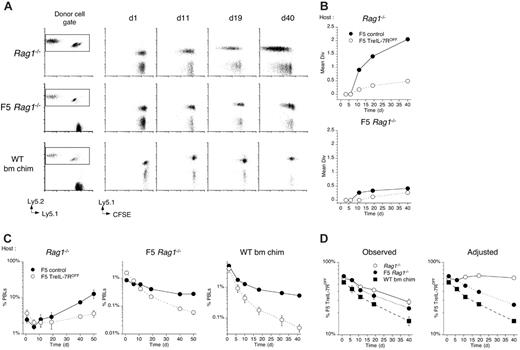
Following transfer to Rag1–/– hosts, control F5 effectors underwent extensive cell division compared with only limited cell divisions by F5 TreIL-7ROFF cells (Figure 6A), confirming the role of IL-7R in facilitating their homeostatic proliferation in these hosts. In contrast, the same cells underwent very limited divisions in naive F5 Rag1–/– hosts and no detectable divisions in replete WT bm chimeras (Figure 6A-B). Examining cell frequencies in PBLs revealed that, in replete hosts, control F5 T cells underwent a steady contraction before cell numbers leveled out, in contrast to their behavior in Rag1–/– hosts where the contraction was far shorter and was followed by a gradual increase in frequency. In contrast, whereas cell frequencies of F5 TreIL-7ROFF cells did stabilize in Rag1–/– hosts, the same effectors transferred to replete hosts underwent a more rapid and protracted contraction and their levels never stabilized (Figure 6C).
The differences in the behavior of F5 T cells in the different hosts were also reflected in the chimerism observed between control F5 and F5 TreIL-7R T cells in these hosts. Although the representation of F5 TreIL-7ROFF T cells appeared to decrease in all the hosts, the cause of the decrease was not the same for all cases. In the empty Rag1–/– hosts, control F5 T cells underwent extensive proliferation not observed in “full” hosts (Figure 6A). Therefore, to control for the effects of expansion due to cell division alone, we calculated cell frequenciesof F5 T cells that excluded the expansion predicted by their CFSE profile (Figure 6A) and then recalculated the chimerism (see “Materials and methods”). This analysis revealed that in Rag1–/– hosts, when differences in cell division are taken into account, the ratio of F5 TreIL-7R T cells to F5 control T cells was maintained at a constant level, suggesting that the survival of control and F5 TreIL-7ROFF cells was approximately similar even if cell division was not. In contrast, in naive F5 Rag1–/– and lymphoreplete WT bm chimera hosts where neither F5 T-cell population underwent much cell division, the proportion of F5 TreIL-7ROFF T cells underwent a clear and progressive reduction (Figure 6D) reflecting their failure to survive and become memory.
IL-15 maintains CD8 effectors in the absence of IL-7R
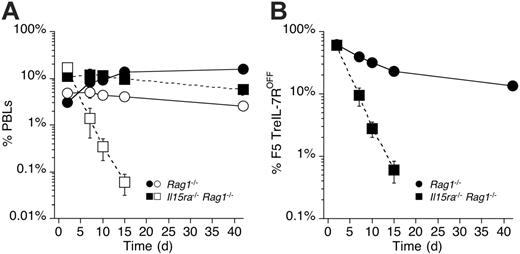
The ability of IL-7R–deficient F5 effectors to survive in empty but not replete hosts suggested that survival was determined by a factor that was limiting in “full” hosts. The role of IL-15 in the maintenance of CD8 memory is well recognized and more recently has been implicated in the generation of resting memory from effectors.26 As described earlier (Figure 4E), F5 effectors up-regulated and maintained expression of CD122 shortly after activation. Therefore, we examined whether survival of F5 TreIL-7ROFF in Rag1–/– hosts was IL-15 dependent. In vitro–generated F5 TreIL-7ROFF and control F5 effectors were mixed in equal numbers and transferred into either Rag1–/– or Il15ra–/–Rag1–/– hosts. Because IL-15Rα expression is required for the transpresentation of IL-15 by IL-15–producing cells to T cells, Il15ra–/–Rag1–/– hosts are therefore functionally IL-15 deficient. F5 T cells transferred to Rag1–/– hosts (Figure 7A) behaved essentially as described earlier (Figures 4A and 6C), surviving for many weeks. In contrast, while control F5 T effectors transferred to Il15ra–/–Rag1–/– hosts survived, F5 TreIL-7ROFF effectors in the same hosts underwent a remarkably rapid contraction such that they were no longer detectable 14 days after transfer. Their rapid decline was also reflected in their chimerism with control F5 T cells in Rag1–/– versus Il15ra–/–Rag1–/– hosts (Figure 7B). While the representation of F5 TreIL-7ROFF cells underwent a gradual decline in Rag1–/– hosts, it collapsed dramatically in Il15ra–/–Rag1–/– hosts. Thus, in the absence of IL-7, IL-15, and antigenic stimulation, CD8 effectors have a half-life of less than 40 hours in vivo.
Discussion
The role of IL-7R in the formation of CD8 memory has been a subject of much investigation in recent years but remains controversial. While some studies report a correlation between reexpression of IL-7R by effector cells and formation of long-term memory,11,12 others do not.15 Furthermore, maintenance of established memory cells appears to depend more on IL-15 than IL-7, which seems only to affect CD8 memory cell homeostasis when it is not limiting, such as in lymphopenic hosts,6,7 or when overexpressed by transgenesis.8 Here we show that whether or not expression of IL-7R is required for formation of CD8 memory cells depends on the environment in which they reside. Most strikingly, we found that IL-7R expression was essential for generation of long-term memory in mice with a “full” T-cell compartment. Control F5 T cells that could express IL-7R normally underwent a short contraction following cell transfer but soon stabilized. In contrast, our IL-7R–deficient F5 cells underwent a profound and unremitting collapse. Thus, in our system, reexpression of IL-7R was essential for stable formation of memory in “full” mice.
Surprisingly, IL-7R expression was not essential for formation and subsequent survival of memory cells transferred into lymphopenic Rag1–/– hosts. The observed differences in T-cell recovery between F5 TreIL-7R and control F5 T cells were largely accounted for by differences in lymphopenia-induced homeostatic proliferation, which is known to be IL-7 dependent for CD8 memory cells.6,7 Interestingly, our data show that in the absence of IL-7R expression, IL-15 was able to promote survival of and therefore formation of memory from F5 effectors. However, this was possible only in lymphopenic hosts where there were no host T cells and therefore little competition for IL-15. Thus, both IL-7 and IL-15 appear to have the capacity to independently promote survival of effectors to form memory, but only in circumstances that result in enhanced signaling through their respective receptors. Under normal physiological conditions, where both IL-7 and IL-15 are subject to host cell competition and therefore likely to be limited in availability, we found that formation of CD8 memory from effector cells was strictly dependent on expression of IL-7R. This was despite the fact that IL-7R–deficient effector cells expressed high levels of CD122 that, for established memory cells, are sufficient to promote both survival and proliferation in the context of normal host competition for available IL-15. Therefore, our data reveal an essential synergy between IL-7 and IL-15 for the generation of memory cells from effectors. Importantly, this synergy appears to be regulated at the level of IL-7R expression, because effector cells rapidly acquire and maintain CD122 expression after activation but only up-regulate IL-7R later, and it is at this point that generation of memory cells from effectors is controlled.
IL-7R–deficient F5 T cells fail to proliferate or survive in replete hosts. Splenocytes from F5 TreIL-7R donors taken off dox food 3 days previously and F5 controls were stimulated with NP68 peptide for 72 hours followed by a further 4-day culture in IL-2. Blasts from cultures of F5 TreIL-7ROFF and control F5 T cells were labeled with CFSE, mixed in equal numbers, and 2 × 107 total cells transferred into either Rag1–/– (n = 3), F5 Rag1–/– Ly5.1 recipients (n = 3), or fully lymphoreplete tolerant bone marrow chimeras (see “Materials and methods”) (C57BL6/J bm chim, n = 3). Recipient mice were bled at different times after transfer and analyzed for CFSE profile, CD8, TCR, and Ly5.1 expression. (A) Dot plots are of Ly5.2 versus Ly5.1 staining by CD8+TCRhi cells, and the square indicates the gate used to identify Ly5.2-positive donor cells. Plots of Ly5.1 versus CFSE are gated on CD8+TCRhi Ly5.2-positive cells for days 1, 11, 19, and 40 after transfer. (B) Mean divisions were calculated from CFSE profiles of F5 control (•) and F5 TreIL-7ROFF cells (○) transferred into Rag1–/– or F5 Rag1–/– Ly5.1 recipients. (C) Graphs show the frequency of F5 control (•) and F5 TreIL-7ROFF cells (○) in PBLs of Rag1–/– or F5 Rag1–/– Ly5.1 and C57BL6/J bone marrow chimera recipients. (D) Graphs show unmanipulated chimerism between F5 control and F5 TreIL-7ROFF cells in Rag1–/– (•), naive F5 Rag1–/– Ly5.1 hosts (○), and C57BL6/J bone marrow chimera recipients (▪) and adjusted chimerisms that exclude the expansive effects of proliferation as predicted by a population CFSE profile (see “Materials and methods”).
IL-7R–deficient F5 T cells fail to proliferate or survive in replete hosts. Splenocytes from F5 TreIL-7R donors taken off dox food 3 days previously and F5 controls were stimulated with NP68 peptide for 72 hours followed by a further 4-day culture in IL-2. Blasts from cultures of F5 TreIL-7ROFF and control F5 T cells were labeled with CFSE, mixed in equal numbers, and 2 × 107 total cells transferred into either Rag1–/– (n = 3), F5 Rag1–/– Ly5.1 recipients (n = 3), or fully lymphoreplete tolerant bone marrow chimeras (see “Materials and methods”) (C57BL6/J bm chim, n = 3). Recipient mice were bled at different times after transfer and analyzed for CFSE profile, CD8, TCR, and Ly5.1 expression. (A) Dot plots are of Ly5.2 versus Ly5.1 staining by CD8+TCRhi cells, and the square indicates the gate used to identify Ly5.2-positive donor cells. Plots of Ly5.1 versus CFSE are gated on CD8+TCRhi Ly5.2-positive cells for days 1, 11, 19, and 40 after transfer. (B) Mean divisions were calculated from CFSE profiles of F5 control (•) and F5 TreIL-7ROFF cells (○) transferred into Rag1–/– or F5 Rag1–/– Ly5.1 recipients. (C) Graphs show the frequency of F5 control (•) and F5 TreIL-7ROFF cells (○) in PBLs of Rag1–/– or F5 Rag1–/– Ly5.1 and C57BL6/J bone marrow chimera recipients. (D) Graphs show unmanipulated chimerism between F5 control and F5 TreIL-7ROFF cells in Rag1–/– (•), naive F5 Rag1–/– Ly5.1 hosts (○), and C57BL6/J bone marrow chimera recipients (▪) and adjusted chimerisms that exclude the expansive effects of proliferation as predicted by a population CFSE profile (see “Materials and methods”).
CD8 memory fails to form in the absence of both IL-7R and IL-15. F5 TreIL-7ROFF and control F5 effector cells were generated in vitro, mixed in equal numbers, and 2 × 107 total cells injected into either Rag1–/– or Il15ra–/–Rag1–/– recipients. Recipients were bled at various times after transfer and frequencies of F5 T cells in PBLs determined by staining cells for CD8, TCR, and Ly5.1 expression and analyzed by FACS. (A) The graph shows the frequency of F5 TreIL-7ROFF (open symbols) and control F5 T cells (filled symbols) in either Rag1–/– (circles) or Il15ra–/–Rag1–/– (squares) hosts. F5 TreIL-7ROFF T cells in Il15ra–/–Rag1–/– were undetectable more than 14 days after transfer. (B) The graph shows the chimerism between F5 TreIL-7ROFF and control F5 T cells in either Rag1–/– (•) or Il15ra–/–Rag1–/– hosts (▪). Data are from 2 independent experiments.
CD8 memory fails to form in the absence of both IL-7R and IL-15. F5 TreIL-7ROFF and control F5 effector cells were generated in vitro, mixed in equal numbers, and 2 × 107 total cells injected into either Rag1–/– or Il15ra–/–Rag1–/– recipients. Recipients were bled at various times after transfer and frequencies of F5 T cells in PBLs determined by staining cells for CD8, TCR, and Ly5.1 expression and analyzed by FACS. (A) The graph shows the frequency of F5 TreIL-7ROFF (open symbols) and control F5 T cells (filled symbols) in either Rag1–/– (circles) or Il15ra–/–Rag1–/– (squares) hosts. F5 TreIL-7ROFF T cells in Il15ra–/–Rag1–/– were undetectable more than 14 days after transfer. (B) The graph shows the chimerism between F5 TreIL-7ROFF and control F5 T cells in either Rag1–/– (•) or Il15ra–/–Rag1–/– hosts (▪). Data are from 2 independent experiments.
The question of whether IL-7R expression by CD8 effectors is a good predictor of memory formation, as suggested by some,11 may ultimately depend on the nature of the antigenic stimulus that occurs during the course of an infection. TCR signals10 and IL-227 both negatively regulate IL-7R expression. The extent and duration of IL-7R down-regulation may therefore depend on the strength and duration of antigenic challenge. In acute infections28 and in models employing antigen-pulsed dendritic cell immunization,15 antigen and therefore IL-2 are strictly limited and IL-7R down-regulation is only transient and reexpression relatively synchronous. In this situation, memory cells are in direct competition with each other for IL-7, and survival then becomes stochastic and, paradoxically, not directly related to IL-7R expression levels. In contrast, in chronic infection models such as LCMV,11 persistent antigen results in more prolonged down-regulation of IL-7R leading to far greater heterogeneity of expression among effectors. In the latter case, a relationship between IL-7R expression and eventual formation of memory may exist, because effectors already expressing receptor will have a competitive advantage when antigen does become limiting.
Our data also revealed the crucial role of both IL-7 and IL-15 in controlling and limiting the contraction that occurs at the end of an immune response. In empty Rag1–/– hosts in which neither cytokine is limiting, control F5 T cells underwent minimal contraction. In the same hosts, but in the absence of IL-7R, the contraction was more noticeable. In replete mice where both IL-7 and IL-15 are subject to host competition, control F5 effectors underwent a more sustained contraction before levels stabilized, while F5 TreIL-7ROFF effectors underwent a protracted contraction. However, the contraction of F5 TreIL-7ROFF cells in these hosts (half-life approximately 7 to 10 days) was still not as short as observed in the complete absence of both IL-7R and IL-15 in Il15ra–/–Rag1–/– hosts (half-life less than 40 hours), suggesting that IL-15 alone does have a limited capacity to prolong the life of F5 cells in the absence of IL-7R. Thus, the contraction of F5 effectors was directly proportional to the availability of IL-7 and IL-15 to T cells in these hosts. It also appears that effector cells can generate memory cells shortly after removal of TCR and IL-2 signaling. We showed that effector cells acquired a small resting phenotype within 24 hours of transfer in vivo, and this occurred independently of IL-7R expression. Similarly, control F5 T cells up-regulated IL-7R to maximal levels within the same period. Whether these cells were able to survive long term, and thus become long-lived memory cells, was crucially dependent on the availability of IL-7 and IL-15. Our observations that memory formation is directly related to availability of these key cytokines would suggest that generation of memory could be augmented by cytokine therapy. Interestingly, recent studies using both IL-7 and IL-15 suggest that this is indeed the case.29
Prepublished online as Blood First Edition Paper, May 16, 2006; DOI 10.1182/blood-2006-04-016857.
Supported by the Medical Research Council, United Kingdom.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank R. Murphy, T. Matambanadzo, T. Norton, K. Williams, H. Boyes, S. Kelly, and Biological Services staff for assistance with mouse breeding and typing, and Odile Richard-Le Goff for technical assistance. We also thank George Kassiotis and Roslyn Kemp for critical reading of the manuscript, together with other members of the Division of Immune Cell Biology for their interest and discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal