Abstract
JAK2 V617F mutation recently was identified as a pathogenic factor in typical chronic myeloproliferative diseases (CMPD). Some forms of myelodysplastic syndromes (MDS) show a significant overlap with CMPD (classified as MDS/MPD), but the diagnostic assignment may be challenging. We studied blood or bone marrow from 270 patients with MDS, MDS/MPD, and CMPD for the presence of JAK2 V617F mutation using polymerase chain reaction, sequencing, and melting curve analysis. The detection rate of JAK2 V617F mutants for polycythemia vera, chronic idiopathic myelofibrosis, and essential thrombocythemia (n = 103) was similar to the previously reported results. In typical forms of MDS (n = 89) JAK2 V617F mutation was very rare (n = 2). However, a higher prevalence of this mutation was found in patients with MDS/MPD-U (9 of 35). Within this group, most of the patients harboring JAK2 V617F mutation showed features consistent with the provisional MDS/MPD-U entity refractory anemia with ringed sideroblasts and thrombocytosis (RARS-T). Among 9 RARS-T patients, 6 showed the presence of JAK2 V617F mutation, and in 1 patient without mutation, aberrant, positive phospho-STAT5 staining was seen that is typically present in association with JAK2 V617F mutation. In summary, we found that RARS-T reveals a high frequency of JAK2 V617F mutation and likely constitutes another JAK2 mutation-associated form of CMPD.
Introduction
Although the distinction between myelodysplastic syndromes (MDSs) and chronic myeloproliferative diseases (CMPDs) is usually straightforward, the diagnosis can be challenging in certain cases that share clinical and/or morphologic features traditionally associated with either entity. Such “overlap” syndromes are recognized in the World Health Organization (WHO) classification of hematopoietic and lymphoid tumors as myelodysplastic/myeloproliferative diseases (MDS/MPD) and include chronic myelomonocytic leukemia (CMML), juvenile myelomonocytic leukemia (JMML), atypical chronic myeloid leukemia (aCML), and MDS/MPD-Unclassifiable (MDS/MPD-U). In view of the lack of objective molecular tests and subjectivity of morphologic diagnosis, the biologic relevance of these entities and their relationship to traditional MDS or CMPD requires further study.
Biomarkers offer the potential for determining whether the MDS/MPD entities are more appropriately classified separately or have a pathogenesis more closely related to either MDS or CMPD. Unfortunately, cytogenetic analysis does not resolve this issue, since many of the typical chromosome abnormalities associated with MDS, such as trisomy 8, del(13q), and del(20q) also can be found in CMPD.1 Recently, a point mutation in DNA coding for the Jak homology domain 2 (JH2) pseudokinase domain of Janus kinase 2 (JAK2) has been found in a significant proportion of patients with CMPD, including polycythemia vera (PV), essential thrombocythemia (ET), and chronic idiopathic myelofibrosis (CIMF).2-11 This G-to-T transversion at nucleotide 1849 results in substitution of phenylalanine for valine at codon 617 (JAK2 V617F; Gene Bank accession NM_004972), leading to constitutive activation of the JAK2 tyrosine kinase. As shown in transfection experiments using a murine bone marrow transplant model, expression of the JAK2 V617F kinase is responsible for the evolution of an erythrocytosis in recipient mice due to a defective hematopoietic clone.6 Interestingly, JAK2 V617F mutants have been found only rarely in patients with MDS, including refractory anemias and cytopenias.12-14
The relatively close association of the JAK2 V617F mutation with certain CMPD could provide insight into how the MDS/MPD entities are related to either MDS or CMPD. When a large cohort of patients with CMML was examined for the presence of the JAK2 V617F mutation, this defect was found only in a small proportion of patients,12,13,15 suggesting that CMML is less biologically related to CMPD.
Of particular interest for further study is the group of patients classified according to WHO criteria as MDS/MPD-U, one of the entities contained in the MDS/MPD category. These cases represent a significant challenge, and their morphologic assignment relies on the exclusion of many factors and lack of pathognomonic changes, allowing more precise diagnosis. Within this broader subgroup, a provisional entity has been described and called “refractory anemia with ringed sideroblasts associated with marked thrombocytosis (RARS-T).” Patients with this condition present with some signs of ET, including a marked increase in platelet count, hypercellular marrow, and increased megakaryocytes, but also have ringed sideroblasts, a feature usually associated with MDS.1,16-18 Clearly, the pathogenetic derivation of this condition is not well understood, and it remains unclear whether this disorder represents the chance association of 2 unrelated diseases or is more appropriately classified as a CMPD with ringed sideroblasts. The biologic classification of MDS/MPD or MDS/MPD-U entities may have even greater clinical and prognostic significance once specific inhibitors of JAK2 V617F are available.
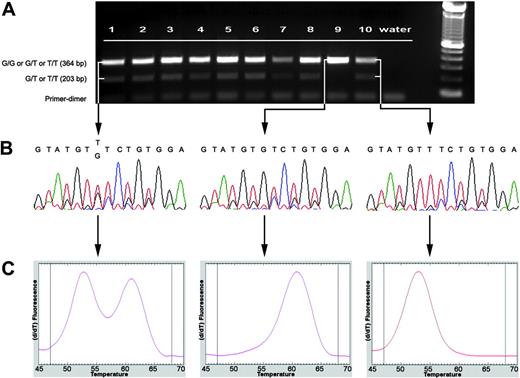
Molecular assay for rapid identification and characterization of JAK2 V617F mutation. (A) Electrophoretogram of AS-PCR for JAK2 V617F mutation in MDS/MPD-Unclassifiable disease. In a healthy patient with the wild-type G/G genotype, only a single 364-bp band is visible (lane 9). A point mutation V617F G→T (either G/T or T/T) is indicated by the presence of a second 203-bp band. Lanes 1-2, MDS/MPD-U; lanes 3-6, RARS-T; lane 7, AML; lane 8, CMML-1; lane 9, normal negative control; lane 10, CIMF (T/T genotype) positive control. (B) Partial sequence of JAK2 exon 14 showing wild-type (G/G) sequence in a healthy patient (middle) and mutated (G/T or T/T) sequences in patient with MDS/MPD-U (right) and CIMF (left). (C) LightTyper DNA melting curves: wild type (middle) is characterized by a single peak (higher melting temperature), G/T genotype (left) by 2 peaks, and T/T genotype (right) by one peak (lower melting temperature).
Molecular assay for rapid identification and characterization of JAK2 V617F mutation. (A) Electrophoretogram of AS-PCR for JAK2 V617F mutation in MDS/MPD-Unclassifiable disease. In a healthy patient with the wild-type G/G genotype, only a single 364-bp band is visible (lane 9). A point mutation V617F G→T (either G/T or T/T) is indicated by the presence of a second 203-bp band. Lanes 1-2, MDS/MPD-U; lanes 3-6, RARS-T; lane 7, AML; lane 8, CMML-1; lane 9, normal negative control; lane 10, CIMF (T/T genotype) positive control. (B) Partial sequence of JAK2 exon 14 showing wild-type (G/G) sequence in a healthy patient (middle) and mutated (G/T or T/T) sequences in patient with MDS/MPD-U (right) and CIMF (left). (C) LightTyper DNA melting curves: wild type (middle) is characterized by a single peak (higher melting temperature), G/T genotype (left) by 2 peaks, and T/T genotype (right) by one peak (lower melting temperature).
We have created a high throughput molecular assay allowing for rapid detection of JAK2 V617F mutants and studied patients with typical MDS and MDS/MPD to determine the frequency of JAK2 V617F mutations in these disorders. We focused on a cohort of patients with RARS-T to determine whether JAK2 V617F mutation status might suggest a pathogenesis more closely related to CMPD than to MDS.
Patients, materials, and methods
Patients
Informed consent was obtained in accordance with the Declaration of Helsinki for sample collection from patients and controls according to protocols approved by the Institutional Review Board of the Cleveland Clinic Foundation. The MDS patient characteristics are presented in Table 1. They were classified according to the WHO1,16,19 and were also assigned an International Prognosis Scoring System score.20 Hematologic controls included cohort of patients with CMPD (Table 1).
WHO diagnosis and results of JAK2 V617F mutation analysis in patients and controls
. | . | Positive for JAK2 mutation . | . | |
|---|---|---|---|---|
| WHO diagnosis by disease category . | N . | N . | % . | |
| MDS | ||||
| Refractory anemia/refractory anemia with ringed sideroblasts | 13 | 0 | 0 | |
| Refractory cytopenia with multilineage dysplasia/refractory cytopenia with multilineage dysplasia and ringed sideroblasts | 16 | 0 | 0 | |
| Refractory anemia with excess blasts 1/2 | 25 | 1 | 4 | |
| Myelodysplastic syndrome with isolated del(5q) | 4 | 0 | 0 | |
| Myelodysplastic syndrome-unclassifiable | 7 | 0 | 0 | |
| MDS/MPD | ||||
| Chronic myelomonocytic leukemia-1/2 | 22 | 2 | 9 | |
| Myelodysplastic/myeloproliferative disease-unclassifiable* | 26 | 3 | 12 | |
| Refractory anemia with ringed sideroblasts with thrombocytosis* | 9 | 6 | 67 | |
| Other | ||||
| Secondary AML† | 24 | 1 | 4 | |
| Hematologic controls | ||||
| Chronic myelogenous leukemia‡ | 21 | 0 | 0 | |
| Polycythemia vera | 40 | 36 | 90 | |
| Chronic idiopathic myelofibrosis | 33 | 18 | 55 | |
| Essential thrombocythemia | 30 | 17 | 57 | |
. | . | Positive for JAK2 mutation . | . | |
|---|---|---|---|---|
| WHO diagnosis by disease category . | N . | N . | % . | |
| MDS | ||||
| Refractory anemia/refractory anemia with ringed sideroblasts | 13 | 0 | 0 | |
| Refractory cytopenia with multilineage dysplasia/refractory cytopenia with multilineage dysplasia and ringed sideroblasts | 16 | 0 | 0 | |
| Refractory anemia with excess blasts 1/2 | 25 | 1 | 4 | |
| Myelodysplastic syndrome with isolated del(5q) | 4 | 0 | 0 | |
| Myelodysplastic syndrome-unclassifiable | 7 | 0 | 0 | |
| MDS/MPD | ||||
| Chronic myelomonocytic leukemia-1/2 | 22 | 2 | 9 | |
| Myelodysplastic/myeloproliferative disease-unclassifiable* | 26 | 3 | 12 | |
| Refractory anemia with ringed sideroblasts with thrombocytosis* | 9 | 6 | 67 | |
| Other | ||||
| Secondary AML† | 24 | 1 | 4 | |
| Hematologic controls | ||||
| Chronic myelogenous leukemia‡ | 21 | 0 | 0 | |
| Polycythemia vera | 40 | 36 | 90 | |
| Chronic idiopathic myelofibrosis | 33 | 18 | 55 | |
| Essential thrombocythemia | 30 | 17 | 57 | |
MDS indicates myelodysplastic syndrome; MDS/MPD, myelodysplastic/myeloproliferative disease; and AML, acute myeloid leukemia.
A total of 35 patients with the diagnosis of MDS/MPD-U were studied; 26 of them were not otherwise further classified, while 9 fulfilled the criteria for RARS-T provisional entity.
Secondary AML includes AML that transformed from MDS, but not therapy-related MDS.
Chronic myelogenous leukemia includes one patient with atypical chronic myeloid leukemia.
DNA extraction
DNA was extracted either from fresh bone marrow, peripheral blood, or paraffin-embedded bone marrow aspirate tissue. Total blood was subjected to density centrifugation with Lymphocyte Separation Medium (Mediatech, Manassas, VA). The granulocyte layer was collected, and residual erythrocytes were removed by osmotic lysis using 0.2% NaCl for 30 seconds.21 The Genomic DNA Purification Kit (Gentra Systems, Minneapolis, MN) was used for DNA isolation. When necessary, DNA was obtained from paraffin tissue blocks as follows: cells were lysed in buffer (50 mM Tris [tris(hydroxymethyl)aminomethane]*Cl, 1 mM EDTA [ethylenediaminetetraacetic acid], and 0.5% Tween-20, pH 8.6) following deparaffinization and proteinase K digestion. DNA was extracted with phenol/chloroform and precipitated with sodium acetate/ethanol.
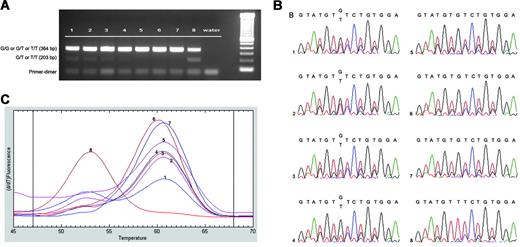
Sensitivity of molecular assay. Sensitivity of (A) AS-PCR, (B) sequencing, and (C) LightTyper melting curves. Genomic DNA isolated from granulocytes of both healthy (G/G) and JAK2 V617F mutated (T/T) patients were mixed at varying ratios respectively: lane 1, 1:1; lane 2, 1:2; lane 3, 1:5; lane 4, 1:10; lane 5, 1:100; and lane 6, 1:500. Lane 7, healthy patient with G/G genotype; and lane 8, CIMF patient with mutated T/T genotype. The 3 methods showed similar sensitivities in detecting a mutated population at a level of 10%.
Sensitivity of molecular assay. Sensitivity of (A) AS-PCR, (B) sequencing, and (C) LightTyper melting curves. Genomic DNA isolated from granulocytes of both healthy (G/G) and JAK2 V617F mutated (T/T) patients were mixed at varying ratios respectively: lane 1, 1:1; lane 2, 1:2; lane 3, 1:5; lane 4, 1:10; lane 5, 1:100; and lane 6, 1:500. Lane 7, healthy patient with G/G genotype; and lane 8, CIMF patient with mutated T/T genotype. The 3 methods showed similar sensitivities in detecting a mutated population at a level of 10%.
Allele-specific polymerase chain reaction (PCR)
40 ng of DNA was amplified using primers previously published.3 Amplicons were generated in 25 μL reaction volume with 120 nM forward primers and 120 nM reverse primer, 1 × PCR buffer without MgCl2, 0.8 mM dNTPs, 1.8 mM MgCl2, and Taq DNA polymerase (Invitrogen, Carlsbad, CA). PCR conditions were: initial denaturation at 94°C for 4 minutes, 30 cycles with denaturation at 94°C for 30 seconds, annealing at 58°C for 30 seconds, and elongation at 72°C for 40 seconds.
Sequencing
DNA samples were amplified using primers 5′-TGGACAACAGTCAAACAA-3′ (forward, JAK2 V617F intron 13) and 5′-TGTTTGGGCATTGTAACCTTC-3′ (reverse, JAK2 V617F intron 14) (accession number NM_004972). Amplicons were generated in 25 μL reaction volume with 120 nM forward primers and 120 nM reverse primer, 1 × PCR buffer without MgCl2, 0.8 mM dNTPs, 1.8 mM MgCl2, and Taq DNA polymerase (Invitrogen) using 40 ng genomic DNA. PCR conditions were: initial denaturation at 94°C for 4 minutes, 30 cycles with denaturation at 94°C for 30 seconds, annealing at 51°C for 30 seconds, and elongation at 72°C for 40 seconds. Amplicons were purified, as previously described,22 using the Montage PCR96 Cleanup Kit (Millipore, Billerica, MA). Purified products (5 μL) were sequenced using 1 μL of Big Dye Terminator v3.0 or v3.1 (Applied Biosystems, Foster City, CA), 1 μL of 3 μM JAK2 V617F-PCR forward primer, and 3 μL dH2O. Cycle sequencing was performed on an MJ thermocycler (MJ Research, Waltham, MA) as follows: an initial denaturation at 96°C for 40 seconds was followed by 31 cycles (96°C for 10 seconds, 51°C for 15 seconds, and 60°C for 4 minutes). Sequencing reactions were purified using the Montage SEQ96 Sequencing Reaction Cleanup Kit (Millipore), run on a 3100-Avant Genetic Analyzer (Applied Biosystems).
Genotyping of JAK2 V617F mutation by melt curve analysis
Genotyping involves PCR amplification of target sequence followed by melt analysis of amplicon-fluorescent probe duplex using the LightTyper.23 We have designed the primers and probes using the software provided with the LightTyper (Roche Applied Sciences, Indianapolis, IN) and procured them from TIB MolBiol (Freehold, NJ). PCR was carried out with LC FastStart DNA HP master mix from Roche Diagnostics (Indianapolis, IN). An amplicon of 177-bp length was generated using designed forward and reverse PCR primers. Melt curve analysis was performed using a designed sensor and an anchor probe. PCR was carried out in a 384-well plate (MJ Research) using the twin-block ABI GeneAmp PCR System 9700 in a total volume of 10 μL containing 12 ng of patient DNA, PCR master mix, primers, and probes. The final reaction contained 200 μM of dNTPs, 4 mM magnesium, 0.1 μM of forward primer, 0.5 μM of reverse primer, and 0.2 μM of probes. The following PCR program was used: initial denaturation at 94°C for 2 minutes; 40 amplification cycles at 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds; final cycle at 72°C for 7 minutes. Amplicon with probes was denatured at 94°C for 3 minutes, cooled to 25°C for 30 seconds, and subjected to melt analysis in the LightTyper at a ramp rate of 0.1°C per minute. A single peak corresponding to either wild-type (G/G; matched duplex, high Tm ∼63°C) or homozygous mutant (T/T; mismatched duplex, low Tm ∼55°C) and 2 peaks, corresponding to either heterozygote or mixed clonality with populations of normal and homozygous cells, were derived from the melt curve analysis.
Immunohistochemical detection of P-STAT5
Staining was performed on a Benchmark XT platform (Ventana Medical Systems, Tucson, AZ), according to the manufacturer's instructions, using mouse monoclonal anti–phospho-STAT5a/b (Y694/99; Advantex BioReagents LLP, Conroe, TX) at 1:500 dilution. All stains were scored without knowledge of the clinical diagnosis or the mutational status. Phospho-STAT5–positive staining (nMEG pSTAT5) was defined as megakaryocytic nuclear staining in more than 10% of the megakaryocytes examined. This cut-off was chosen to avoid false-positive results at the lower range of the spectrum, where weak cytoplasmic staining in occasional cells may be confused with nuclear staining.24
Digital imaging and preparation of photomicrographs
Images shown in Figures 4 and 5 were obtained via digital microscopy using an Olympus BX51 microscope (Olympus America, Melville, NY) equipped with either a UPlanFl 40×/0.75 numeric aperture (NA) or a UPlanFl 100×/1.30 NA objective. Images were captured using a Dage-MTI Model DC330E charge-coupled device (CCD) camera (Dage-MTI, Michigan City, IN) attached to the microscope with a U-TV1X-2 video adapter (Olympus America) and a 0.45× camera coupler (Diagnostic Instruments, Sterling Heights, MI). Imaging hardware included a Scion CG-7 RGB color frame grabber (Scion, Frederick, MD), and imaging software included Adobe Photoshop version 7.0 (Adobe Systems, San Jose, CA) and a Scion Series 7 TWAIN module version 2.0. Additional image processing was performed using Photoshop.
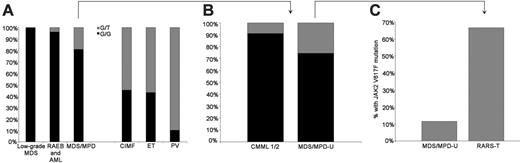
Frequency and type of JAK2 V617F mutation within diagnostic categories. (G/G: wild type; G/T or T/T: heterozygous or homozygous for mutation) (left panel). Within WHO MDS/MPD-U, 6 of 9 cases that were positive for JAK2 V617F mutation occurred in RARS-T. When separated from MDS/MPD-U, 67% of RARS-T cases showed a JAK2 V617F mutation, but only 12% of the remaining MDS-MPD-U were positive (right panel).
Frequency and type of JAK2 V617F mutation within diagnostic categories. (G/G: wild type; G/T or T/T: heterozygous or homozygous for mutation) (left panel). Within WHO MDS/MPD-U, 6 of 9 cases that were positive for JAK2 V617F mutation occurred in RARS-T. When separated from MDS/MPD-U, 67% of RARS-T cases showed a JAK2 V617F mutation, but only 12% of the remaining MDS-MPD-U were positive (right panel).
Results
Previous reports demonstrated that JAK2 V617F mutation is present in a significant proportion of patients with PV (65%-97%), ET (23%-57%), and CIMF (34%-50%).2-6 Subsequent studies in related diseases, including MDS, showed that in typical MDS and CMML,8,12-15 only a low prevalence of JAK2 V617F can be found. We have hypothesized that JAK2 V617F mutation may be present in patients who show features common to typical CMPD and MDS, such as those with MDS/MPD overlap subentities, and we focused our study on this group of patients. The experimental group included 57 patients with proliferative forms of MDS who under the WHO classification system were classified as MDS/MPD overlap syndromes.1,16,19 This group consisted of CMML, JMML, atypical CML, and MDS/MPD-U, which also includes the provisional entity RARS with thrombocytosis (RARS-T) (Table 1). Among these patients, 9 fulfilled the criteria for RARS-T, and 26 belonged to the MDS/MPD-U category. In addition, patients with traditional chronic myeloproliferative diseases and typical MDS patients served as hematologic control groups.
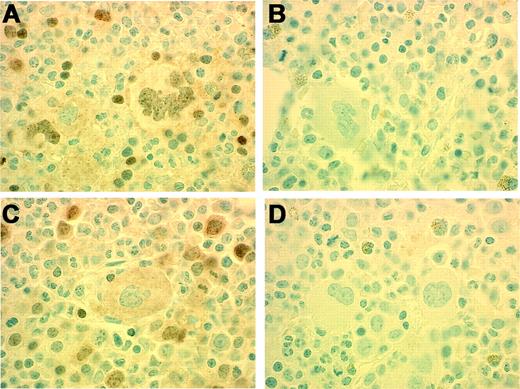
Immunohistochemical detection of p-STAT. Positive staining is present in nuclei of megakaryocytes and erythroid precursors (A) in a patient (Table 3, Patient no. 7) with RARS-T heterozygous for JAK2 V617F mutation (G/T). Negative antibody control (B). Positive staining is present in nuclei of erythroid precursors but absent in megakaryocytes (C) in a patient (Table 3, Patient no. 1) with RARS-T who lacks JAK2 V617F mutation. Negative antibody control (D). Original magnification, 100×.
Immunohistochemical detection of p-STAT. Positive staining is present in nuclei of megakaryocytes and erythroid precursors (A) in a patient (Table 3, Patient no. 7) with RARS-T heterozygous for JAK2 V617F mutation (G/T). Negative antibody control (B). Positive staining is present in nuclei of erythroid precursors but absent in megakaryocytes (C) in a patient (Table 3, Patient no. 1) with RARS-T who lacks JAK2 V617F mutation. Negative antibody control (D). Original magnification, 100×.
Molecular analysis of JAK2 V617F mutation
For the purpose of this study, we have devised and cross-validated molecular assays allowing for a sensitive and unambiguous detection of homozygous and heterozygous JAK2 V617F forms. As an initial test we carried out allele-specific PCR (AS-PCR). This method enabled us to distinguish between G/G wild-type and mutated G/T or T/T alleles (Figure 1A); positive result of AS-PCR (2 amplicons: 364 bp, control band; and 203 bp, carrying G/T or T/T mutation) was confirmed by either sequencing JAK2 V617F (exon 14) (Figure 1B) or Light Typer melt analysis (LTPMA; Figure 1C). DNA dilutions showed detection sensitivity of 10% (Figure 2). Both methods allowed for distinction between G/T and T/T JAK2 V617F mutants (Figure 1B-C).
Analysis of JAK2 V617F mutations in patients
Combining the AS-PCR, sequencing, and LTPMA allowed for sensitive detection of the JAK2 V617F mutation in DNA derived from the bone marrow of patients with CMPD (90%, 55%, and 57% of patients with PV, CIMF, and ET, respectively). JAK2 V617F mutation was not detected in CML patients, serving as negative controls, and among 33 patients with low-grade MDS. JAK2 V617F mutation was found in 4% patients with refractory anemia with excess blasts/acute myeloid leukemia (RAEB/AML) and 9% with CMML (2 of 49 and 2 of 22, respectively).
Analysis of patients within the MDS/MPD-U category revealed the presence of JAK2 V617F mutation in 6 of 9 patients (67%) with RARS-T and 3 of 26 (12%) with other forms of MDS/MPD-U (Figure 3). The JAK2 V617F mutation was associated with nuclear localization of phospho-STAT5 as shown by immunohistochemical staining and was absent in patients with wild-type JAK2 (Figure 4).
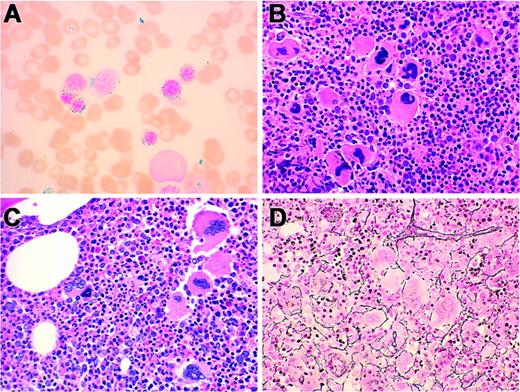
Pathologic features of RARS-T heterozygous for JAK2 V617F G/T mutation. (A) Bone marrow aspirate shows frequent ringed sideroblasts (Prussian blue stain, original magnification, × 100). (B, C) Bone marrow biopsies from different patients show hypercellularity with clustered megakaryocytes (H&E stain, original magnification × 40). (D) Bone marrow biopsy with increased stromal reticulin (2+/4+) (Gomori reticulum stain, original magnification, × 40). (Panels A, B, D: Table 3, Patient no. 5; Panel C: Table 3, Patient no. 3.)
Pathologic features of RARS-T heterozygous for JAK2 V617F G/T mutation. (A) Bone marrow aspirate shows frequent ringed sideroblasts (Prussian blue stain, original magnification, × 100). (B, C) Bone marrow biopsies from different patients show hypercellularity with clustered megakaryocytes (H&E stain, original magnification × 40). (D) Bone marrow biopsy with increased stromal reticulin (2+/4+) (Gomori reticulum stain, original magnification, × 40). (Panels A, B, D: Table 3, Patient no. 5; Panel C: Table 3, Patient no. 3.)
Clinical and phenotypic characteristics of patients with MDS and JAK2 V617F mutations
We have detected the JAK2 V617F mutation among a significant minority of patients with MDS/MPD overlap. The subanalysis of this group showed that patients with RARS-T had a remarkably high frequency of the JAK2 V617F mutation (67%) (Figure 3). Additionally in 1 of 3 JAK2 V617F mutation-negative RARS-T patients, an aberrant pattern of phospho-STAT5 staining was observed (Table 2). This is an unusual finding since in most instances, JAK2 V617F–negative cases are negative for phospho-STAT5 staining. When we analyzed clinical features of patients with RARS-T, there were no morphologic or biologic characteristics allowing distinction between those with JAK2 V617F and without this mutation: all patients exhibited typical features of RARS-T (Table 3, Figure 5), for instance, typical ringed sideroblasts, increased dysplastic megakaryocytes, and prominent reticulin fibrosis. Blasts were not increased. Of note is that 8 of 13 patients with the JAK2 V617F mutation had concomitant splenomegaly (Tables 3 and 4), consistent with CMPD.
WHO diagnosis versus JAK2 V617F mutation status and nMEG p-STAT5 staining
WHO diagnosis . | JAK2 V617F mutation . | nMEG p-STAT5 staining . |
|---|---|---|
| MDS/MPD-U* | G/T | Positive |
| MDS/MPD-U | G/T | NA |
| MDS/MPD-U | G/T | NA |
| RARS-T† | G/T | Positive |
| RARS-T | G/T | NA |
| RARS-T | G/T | NA |
| RARS-T‡ | G/G | Negative |
| RARS-T | G/T | NA |
| RARS-T§ | G/G | Positive |
| RARS-T | G/T | NA |
| RARS-T | G/G | NA |
| RARS-T∥ | T/T | Positive |
WHO diagnosis . | JAK2 V617F mutation . | nMEG p-STAT5 staining . |
|---|---|---|
| MDS/MPD-U* | G/T | Positive |
| MDS/MPD-U | G/T | NA |
| MDS/MPD-U | G/T | NA |
| RARS-T† | G/T | Positive |
| RARS-T | G/T | NA |
| RARS-T | G/T | NA |
| RARS-T‡ | G/G | Negative |
| RARS-T | G/T | NA |
| RARS-T§ | G/G | Positive |
| RARS-T | G/T | NA |
| RARS-T | G/G | NA |
| RARS-T∥ | T/T | Positive |
Clinical and pathologic features of patients with RARS-T
. | . | . | . | . | . | . | Bone marrow morphology . | . | . | . | . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO diagnosis . | Age . | Hgb, g/L . | Platelets, × 109/L . | MCV, fL . | ANC, × 109/L . | AMC, × 109/L . | Blasts, % . | Cellularity, % . | Megakaryocytes . | Megakaryocyte morphology . | Reticulin fibrosis . | RS, % . | Cytogenetics . | IPSS score . | Splenomegaly, Y/N . | JAK2 V617F mutation . | |||||
| RARS-T | 71 | 93 | 667 | 96.7 | 14.025 | 0.660 | 1 | 90 | Increased and clustered | Some hypolobate nuclei | 2+ | 77 | 47,XX,+8[4]/46,XX[16] | 0.5 | N | Wild type | |||||
| RARS-T | 78 | 89 | 277* | 103.0 | 3.195 | 0.405 | 1 | 70 | Increased and clustered | Large; complex lobulated nuclei | 2+ | 88 | 46,XY[20] | 0 | N | Wild type | |||||
| RARS-T | 73 | 90 | 664 | 86.1 | 5.103 | 0.252 | 1 | 65 | Increased and clustered | Large; complex lobulated nuclei | 2+ | 86 | 46,XY[20] | 0 | Y | Wild type | |||||
| RARS-T | 70 | 102 | 496∥ | 106.3 | 2.337 | 0.369 | 2 | 20 | Normal | Normal | 0 | 51 | 5q- | 0.5 | Y | G/T | |||||
| RARS-T | 61 | 86 | 329† | 86.6‡ | 5.384 | 0.269 | 1 | 95 | Increased and clustered | Large; complex lobulated nuclei | 2+ | 79 | 46,XY[20] | 0 | Y | G/T | |||||
| RARS-T | 85 | 114 | 598 | 104 | 9.690 | 0.800 | 2 | 85 | Increased and focally clustered | Normal | 1+ to 2+ | 42 | 46,XX[20] | 0 | Y | G/T | |||||
| RARS-T | 76 | 102 | 894 | 95.3 | 10.416 | 1.848 | 0 | 90 | Increased and clustered | Large; complex lobulated nuclei | 2+ | 72 | 46,XX[20] | 0 | N | G/T | |||||
| RARS-T | 72 | 94 | 512¶ | 110.8 | 3.490 | 0.820 | 1 | 90 | Increased | Normal | 0 | 55 | 45,X,-X,inv(10) (q21.2q24.3) [3]/46,XX,inv(10) (q21.2q24.3)[17]§ | 0.5 | N | G/T | |||||
| RARS-T | 77 | 124 | 587 | 103.0 | 17.108 | 1.226 | 1 | 60 | Increased and clustered | Large, complex lobulated nuclei | 1+ | 55 | 46,XY[20] | 0 | N | T/T | |||||
. | . | . | . | . | . | . | Bone marrow morphology . | . | . | . | . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO diagnosis . | Age . | Hgb, g/L . | Platelets, × 109/L . | MCV, fL . | ANC, × 109/L . | AMC, × 109/L . | Blasts, % . | Cellularity, % . | Megakaryocytes . | Megakaryocyte morphology . | Reticulin fibrosis . | RS, % . | Cytogenetics . | IPSS score . | Splenomegaly, Y/N . | JAK2 V617F mutation . | |||||
| RARS-T | 71 | 93 | 667 | 96.7 | 14.025 | 0.660 | 1 | 90 | Increased and clustered | Some hypolobate nuclei | 2+ | 77 | 47,XX,+8[4]/46,XX[16] | 0.5 | N | Wild type | |||||
| RARS-T | 78 | 89 | 277* | 103.0 | 3.195 | 0.405 | 1 | 70 | Increased and clustered | Large; complex lobulated nuclei | 2+ | 88 | 46,XY[20] | 0 | N | Wild type | |||||
| RARS-T | 73 | 90 | 664 | 86.1 | 5.103 | 0.252 | 1 | 65 | Increased and clustered | Large; complex lobulated nuclei | 2+ | 86 | 46,XY[20] | 0 | Y | Wild type | |||||
| RARS-T | 70 | 102 | 496∥ | 106.3 | 2.337 | 0.369 | 2 | 20 | Normal | Normal | 0 | 51 | 5q- | 0.5 | Y | G/T | |||||
| RARS-T | 61 | 86 | 329† | 86.6‡ | 5.384 | 0.269 | 1 | 95 | Increased and clustered | Large; complex lobulated nuclei | 2+ | 79 | 46,XY[20] | 0 | Y | G/T | |||||
| RARS-T | 85 | 114 | 598 | 104 | 9.690 | 0.800 | 2 | 85 | Increased and focally clustered | Normal | 1+ to 2+ | 42 | 46,XX[20] | 0 | Y | G/T | |||||
| RARS-T | 76 | 102 | 894 | 95.3 | 10.416 | 1.848 | 0 | 90 | Increased and clustered | Large; complex lobulated nuclei | 2+ | 72 | 46,XX[20] | 0 | N | G/T | |||||
| RARS-T | 72 | 94 | 512¶ | 110.8 | 3.490 | 0.820 | 1 | 90 | Increased | Normal | 0 | 55 | 45,X,-X,inv(10) (q21.2q24.3) [3]/46,XX,inv(10) (q21.2q24.3)[17]§ | 0.5 | N | G/T | |||||
| RARS-T | 77 | 124 | 587 | 103.0 | 17.108 | 1.226 | 1 | 60 | Increased and clustered | Large, complex lobulated nuclei | 1+ | 55 | 46,XY[20] | 0 | N | T/T | |||||
RARS-T indicates refractory anemia with ringed sideroblasts associated with marked thrombocytosis; MCV, mean cell volume; ANC, absolute neutrophil count; AMC, absolute monocyte count; BM, bone marrow; RS, ringed sideroblasts; and NA, not available.
Documented history of thrombocytosis over previous 6 months (to 1353 × 109/L).
Documented history of thrombocytosis over previous 6 months (546 to 1213 × 109/L).
On hydroxyurea at time of CBC.
Constitutional karyotype of peripheral-blood lymphocytes.
The highest recorded plateiet count was 691 × 109/L; at the time of biopsy patient was receiving Anagrelide.
A platelet count at the time of biopsy was 512 × 109/L blood. The true platelet count was estimated to be higher (per pathologist estimate “close to 1 000 × 109/L”).
Clinical and pathologic features of patients with MDS, MDS/MPD, or AML positive for JAK2 V617F mutation
. | . | . | . | . | . | . | Bone marrow morphology . | . | . | . | . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO diagnosis . | Age . | Hgb, g/L . | Platelets, × 109/L . | MCV, fL . | ANC, ×109/L . | AMC, ×109/L . | Blasts, % . | Cellularity, % . | Megakaryocytes . | Megakaryocyte morphology . | Reticulin fibrosis . | RS, % . | Cytogenetics . | IPSS score . | Splenomegaly, Y/N . | JAK2 V617F mutation . | |||||
| RAEB-2 | 82 | 114 | 7 | 86.3 | 1.278 | 0.711 | 9* | 10 | Decreased | Dysplastic, small, hyposegmented | 3+ | 2 | NA—no growth 43-45,XX,del(5)(q13q33), -6,-10,-13,add(17) (p11.2),-20,+r, +1-3mar[cp7]/46,XX[13] | 1 | Y | G/T | |||||
| AML | 75 | 108 | 18 | 94.3 | 2.369 | 0.115 | 24 | 70 | Decreased | Normal | 2+ | 4 | 2 | N | G/T | ||||||
| CMML-1 | 79 | 113 | 309 | 94.7 | 6.170 | 1.199 | 0 | 85 | Increased and clustered | Large, complex hyperlobate nuclei | 2+ | 2 | 47,XY,+8[7]/46,XY[13] | 0.5 | N | G/T | |||||
| CMML-2 | 44 | 89 | 499 | 86.1 | 24.078 | 7.223 | 9† | 100 | Increased and clustered | Dysplastic | 3+ | 1 | 46,XY,t(8;9)(q22;p24)[20] | 1 | Y | G/T | |||||
| MDS/MPD-U§ | 79 | 96 | 122 | 90.9 | 4.328 | 0.108 | 0 | 30‡ | Increased and clustered | Dysplastic hyperchromatic nuclei | 4+‡ | 65 | 46,XX[20] | 0.5 | Y | G/T | |||||
| MDS/MPD-U§ | 71 | 83 | 413 | 89.8 | 6.578 | 0 | 7 | 30‡ | Increased, focally clustered | Dysplastic hyperchromatic nuclei | 4+‡ | 0 | 47,XY,+8[14] | 1 | Y | G/T | |||||
| MDS/MPD-U§ | 63 | 85 | 8 | 95.3 | 5.852 | 0.228 | 2 | 10 | Decreased | Dysplastic, hyperchromatic hyposegmented nuclei | 3+ | 0 | NA-no growth | 0.5 | Y | G/T | |||||
. | . | . | . | . | . | . | Bone marrow morphology . | . | . | . | . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO diagnosis . | Age . | Hgb, g/L . | Platelets, × 109/L . | MCV, fL . | ANC, ×109/L . | AMC, ×109/L . | Blasts, % . | Cellularity, % . | Megakaryocytes . | Megakaryocyte morphology . | Reticulin fibrosis . | RS, % . | Cytogenetics . | IPSS score . | Splenomegaly, Y/N . | JAK2 V617F mutation . | |||||
| RAEB-2 | 82 | 114 | 7 | 86.3 | 1.278 | 0.711 | 9* | 10 | Decreased | Dysplastic, small, hyposegmented | 3+ | 2 | NA—no growth 43-45,XX,del(5)(q13q33), -6,-10,-13,add(17) (p11.2),-20,+r, +1-3mar[cp7]/46,XX[13] | 1 | Y | G/T | |||||
| AML | 75 | 108 | 18 | 94.3 | 2.369 | 0.115 | 24 | 70 | Decreased | Normal | 2+ | 4 | 2 | N | G/T | ||||||
| CMML-1 | 79 | 113 | 309 | 94.7 | 6.170 | 1.199 | 0 | 85 | Increased and clustered | Large, complex hyperlobate nuclei | 2+ | 2 | 47,XY,+8[7]/46,XY[13] | 0.5 | N | G/T | |||||
| CMML-2 | 44 | 89 | 499 | 86.1 | 24.078 | 7.223 | 9† | 100 | Increased and clustered | Dysplastic | 3+ | 1 | 46,XY,t(8;9)(q22;p24)[20] | 1 | Y | G/T | |||||
| MDS/MPD-U§ | 79 | 96 | 122 | 90.9 | 4.328 | 0.108 | 0 | 30‡ | Increased and clustered | Dysplastic hyperchromatic nuclei | 4+‡ | 65 | 46,XX[20] | 0.5 | Y | G/T | |||||
| MDS/MPD-U§ | 71 | 83 | 413 | 89.8 | 6.578 | 0 | 7 | 30‡ | Increased, focally clustered | Dysplastic hyperchromatic nuclei | 4+‡ | 0 | 47,XY,+8[14] | 1 | Y | G/T | |||||
| MDS/MPD-U§ | 63 | 85 | 8 | 95.3 | 5.852 | 0.228 | 2 | 10 | Decreased | Dysplastic, hyperchromatic hyposegmented nuclei | 3+ | 0 | NA-no growth | 0.5 | Y | G/T | |||||
MDS indicates myelodysplastic syndrome; MDS/MPD, myelodysplastic/myeloproliferative disease; AML, acute myeloid leukemia; RAEB, refractory anemia with excess blasts; CMML, chronic myelomonocytic leukemia; MCV, mean cell volume; ANC, absolute neutrophil count; AMC, absolute monocyte count; RS, ringed sideroblasts; and NA, not available.
10% blasts in peripheral blood.
12% blasts in peripheral blood.
Extensive reticulin and collagenous stromal fibrosis.
Cases of MDS/MPD-U classified as RARS-T were excluded and presented in a separate table (Table 3).
Among the 13 patients with various forms of MDS and the JAK2 V617F mutation, 3 belonged to the MDS/MPD-U category. When clinical and pathologic features of JAK2 V617F–positive and –negative cases were analyzed among various forms of MDS and MDS/MPD overlap syndromes, patients with the JAK2 V617F mutation, in analogy to those with RARS-T, were characterized by prominent thrombocytosis, splenomegaly, more extensive reticulin, fibrosis, and increased bone marrow cellularity (Tables 3 and 4).
As patients with RARS and RARS-T may belong to the same disease continuum, we have studied the similarities and differences within subgroups of patients with ringed sideroblasts. For the purpose of this study, patients with RARS and RCMD-RS were combined and compared to those with RARS-T; except for the low numbers of blasts, cytogenetics, and per definition ringed sideroblasts, it appears that RARS-T entity shows very distinct clinical features (Table 5).
Comparison of clinical features between patients with RARS and RARS-T
. | RARS . | RARS-T . | P value . |
|---|---|---|---|
| Platelet count greater than 500 × 109/L | 0 of 9 | 9 of 9 | .01 |
| ANC greater than 0.5 × 109/L | 9 of 9 | 9 of 9 | > .999 |
| Increased BM megakaryocytes | 3 of 9 | 8 of 9 | .27 |
| Abnormal megakaryocyte morphology | 1 of 6 | 6 of 9 | .35 |
| BM blasts fewer than 5% | 9 of 9 | 9 of 9 | > .999 |
| Increased BM cellularity | 7 of 9 | 9 of 9 | .74 |
| MCV greater than 100 fL | 3 of 9 | 5 of 9 | .68 |
| Splenomegaly | 0 of 9 | 4 of 9 | .11 |
| Reticulin fibrosis | 0 of 9 | 7 of 9 | .02 |
| Abnormal cytogenetics | 2 of 8 | 3 of 9 | > .999 |
| JAK2 V617F mutation | 0 of 9 | 6 of 9 | .05 |
. | RARS . | RARS-T . | P value . |
|---|---|---|---|
| Platelet count greater than 500 × 109/L | 0 of 9 | 9 of 9 | .01 |
| ANC greater than 0.5 × 109/L | 9 of 9 | 9 of 9 | > .999 |
| Increased BM megakaryocytes | 3 of 9 | 8 of 9 | .27 |
| Abnormal megakaryocyte morphology | 1 of 6 | 6 of 9 | .35 |
| BM blasts fewer than 5% | 9 of 9 | 9 of 9 | > .999 |
| Increased BM cellularity | 7 of 9 | 9 of 9 | .74 |
| MCV greater than 100 fL | 3 of 9 | 5 of 9 | .68 |
| Splenomegaly | 0 of 9 | 4 of 9 | .11 |
| Reticulin fibrosis | 0 of 9 | 7 of 9 | .02 |
| Abnormal cytogenetics | 2 of 8 | 3 of 9 | > .999 |
| JAK2 V617F mutation | 0 of 9 | 6 of 9 | .05 |
One of the patients classified as having RARS-T showed del(5q). However, the presence of JAK2 V617F mutation and thrombocytosis (and per definition ring sideroblasts) supports the diagnosis of RARS-T in this patient.
Discussion
The JAK2 V617F mutation is a key pathophysiologic element in a large proportion of patients with myeloproliferative diseases. The presence or absence of this mutation also has shed light on the possible pathogenetic links involved in clinically often and not easily distinguishable cases. Screening of patients with various hematologic diseases with myeloproliferative features, including atypical CML, CMML, and JMML, as well as typical MDS, did not reveal a major contribution of the JAK2 V617F mutation to the pathogenesis of these conditions.12-15,25,26 However, the JAK2 V617F mutation was found, albeit rarely, in AML, but not at all in lymphoid malignancies such as acute lymphoblastic leukemia or chronic lymphocytic leukemia.13,14,25 These studies indicate the specificity of the JAK2 V617F mutation for classic CMPD, and possibly some cases of AML (M6 or M7) derived from them.14,27 Here, we have studied a large cohort of patients with the histologic diagnosis of MDS/MPD overlap syndrome to determine which proportion of these often clinically and morphologically poorly defined conditions can be attributed to JAK2 V617F mutation, and thereby constitute an atypical variant of CMPD rather than MDS.
As expected, we have detected the JAK2 V617F mutation in the majority of patients with CMPD. The higher than previously reported detection rate in our study may be due to the use of bone marrow as a source of DNA and other technical factors. It is possible that the methods applied in our study allow for detection of JAK2 V617F mutation even when clonal cells constitute a relatively small proportion of the sample. In general, melting curve analysis may be a sensitive method, but it cannot distinguish between truly heterozygous cases or mixed clonality with populations of normal and homozygous mutant cells.
Within our cohort of 146 MDS patients (including typical MDS and MDS/MPD overlap), we identified 13 who had the JAK2 V617F mutation. Among those patients, 1 had RAEB1/2, 1 had AML that evolved from MDS, 2 had CMML, 3 had MDS/MPD-U, and 6 had the RARS-T variant of MDS/MPD-U. In the sole patient with secondary AML and JAK2 V617F mutation, there was no evidence in the history of a preceding CMPD. Clinically, the patient lacked thrombocytosis, splenomegaly, or other myeloproliferative features, and AML evolved from MDS after an extended period of time. Multiple chromosomal abnormalities were found (Table 4). The occasional presence of the JAK2 V617F mutation in AML has been noted in recent studies,13,14 but the index patient with AML usually evolved from antecedent CMPD.13,14
Similar to previous cohorts,12,13,15 the JAK2 V617F mutation was absent in typical forms of MDS, and only occasionally found in CMML. However, a significant minority of patients with MDS/MPD-U overlap syndrome harbored the JAK2 V617F mutation. The morphologic terms used to describe this entity also included synonyms such as mixed myeloproliferative/myelodysplastic syndrome, unclassifiable and “overlap” syndrome, unclassifiable, all reflecting lack of morphologic resolution and uncertainty in diagnosis. Our study suggests that a proportion of these patients harbor a typical JAK2 V617F mutation and may correspond to variants of myelofibrosis rather than MDS. Most significantly, our results indicate that the RARS-T variant is strongly associated with the JAK2 V617F mutation. Previously, we have described that JAK2 V617F mutation was strongly associated with the presence of phosphorylated STAT5, as detected by phospho-STAT5 staining of megakaryocytes in bone marrow biopsies.24 Such a pattern without the JAK2 V617F mutation was found only in CML or rare cases of ET. In addition to the expected correlation between JAK2 V617F and phospho-STAT5, one of the patients with RARS-T without the JAK2 V617F mutation also showed phospho-STAT5 staining in megakaryocytes (Table 5). As STAT5 may transduce signals from a variety of cytokines as well as corticosteroids,28-31 phospho-STAT5 staining may be due to exogenous stimulation. While such activation signals could operate in CMPD, we rather believe that STAT5 is aberrantly activated, for example, through other upstream elements.
RARS-T has been considered as a rare,”provisional” subentity of MDS/MPD-U, and our series is one of the largest cohorts of this class described in the literature.17,18 Theoretically, the morphologic picture in these patients may reflect coincidental RARS and ET, but such an association of relatively uncommon conditions is rather unlikely. Based on the comparison to typical RARS cases, it appears that RARS-T shows many distinct features. However, we cannot exclude that it may fall along a clinical continuum. Alternatively, RARS-T could be a separate CMPD entity that can be added to the list of myeloproliferative diseases associated with JAK2 V617F mutation.
Of significant interest is the clinical phenotype of MDS patients with the JAK2 V617F mutation, including a normal mean cell volume, high grade reticulin fibrosis in the bone marrow, increased BM megakaryocyte numbers, and splenomegaly. However, these features also can be present in patients with MDS/MPD overlap without the JAK2 V617F mutation. Similar clinical features suggest that other defects along the JAK2/STAT5 transduction pathway may exist. So far, the search for an associated mutation in JAK2 V617F–negative cases has been unsuccessful. Similarly, in our CMML cohort, the 2 cases with the JAK2 V617F mutation showed significant thrombocytosis and extensive reticulin fibrosis, but other features, including splenomegaly and the degree of monocytosis, were shared between patients with and without the JAK2 V617F mutation. Of note is that one of the CMML patients with the JAK2 V617F mutation exhibited abnormal cytogenetics showing an unusual translocation: t(8;9)(q22;p24). Since JAK2 has been mapped to 9p24, it is possible that the JAK2 gene was involved in the translocation, generating a novel fusion protein in addition to an activating JAK2 V617F mutant.
Another interesting patient classified in our study as having RARS-T showed a del(5q). While some patients with a typical 5q-syndrome may show similarities to RARS-T, RARS itself can present with various cytogenetic abnormalities. Detection of JAK2 V617 mutation in this case may help to resolve a diagnostic conundrum and justifies the RARS-T designation.
In summary, we describe here a new rare entity of MDS/MPD-U that is strongly associated with the presence of the JAK2 V617F mutation, RARS-T. In the future, detection and characterization of the JAK2 V617F mutation will prompt a search for specific inhibitors of JAK2 V617F kinase activity or other downstream elements. Clearly, in addition to PV, CIMF, and ET, these agents also may be effective in other disease entities, such as MDS/MPD overlap syndromes. Morphologic diagnosis of MDS/MPD overlap warrants the analysis of mutational status of the JAK2 gene and likely assignment of JAK2 V617F–positive cases to the classic myeloproliferative syndromes.
Prepublished online as Blood First Edition Paper, June 1, 2006; DOI 10.1182/blood-2006-02-005751.
Supported in part by a generous gift in memory of Robert Duggan; and National Institutes of Health grants U54 RR019391-01, R01 HL73429-01, and R01 HL082983-01 (J.P.M.).
H.S. designed and performed molecular assays and wrote the manuscript; R.T. performed classification of patients and database; G.M. performed Melt Curve Analysis; S.A. performed STAT5 staining; E.D.H. reviewed pathologic specimens, interpreted results, edited and corrected the manuscript; K.S.T. reviewed pathologic specimens, interpreted results, edited and corrected the manuscript; M.A.S. helped in classification and identification of patients, corrected the manuscript; and J.P.M. conceived the idea, designed the trial and the whole experiment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal