Abstract
Botrocetin (bt)-facilitated binding of von Willebrand factor (VWF) to the platelet membrane glycoprotein (GP) Ib-IX-V complex on platelets in suspension initiates a signaling cascade that causes αIIbβ3 activation and platelet aggregation. Previous work has demonstrated that bt/VWF-mediated agglutination activates αIIbβ3 and elicits ATP secretion in a thromboxane A2 (TxA2)-dependent manner. The signaling that results in TxA2 production was shown to be initiated by Lyn, enhanced by Src, and propagated through Syk, SLP-76, PI3K, PLCγ2, and PKC. Here, we demonstrate that the signaling elicited by GPIb-mediated agglutination that results in TxA2 production is dependent on Bruton tyrosine kinase (Btk). The results demonstrate that Btk is downstream of Lyn, Syk, SLP-76, and PI3K; upstream of ERK1/2, PLCγ2, and PKC; and greatly enhances Akt phosphorylation. The relationship(s), if any, between ERK1/2, PLCγ2, and PKC were not elucidated. The requirement for Btk and TxA2 receptor function in GPIb-dependent arterial thrombosis was confirmed in vivo by characterizing blood flow in ferric chloride-treated mouse carotid arteries. These results demonstrate that the Btk family kinase, Tec, cannot provide the function(s) missing because of the absence of Btk and that Btk is essential for both bt/VWF-mediated agglutination-induced TxA2 production and GPIb-dependent stable arterial thrombus formation in vivo.
Introduction
The binding of von Willebrand factor (VWF) to the platelet membrane glycoprotein (GP) Ib-IX-V complex elicits αIIbβ3 activation by a variety of GPIb-dependent signaling mechanisms.1-5 This behavior demonstrates that GPIb is a versatile signaling molecule. For example, αIIbβ3 activation resulting from botrocetin (bt)-facilitated, GPIb-VWF-mediated agglutination is (thromboxane A2) TxA2 dependent, and the agglutination-elicited TxA2 production is not dependent on Ca2+ influx or mobilization of internal Ca2+ stores.5 In contrast, adhesion-independent shear stress-induced GPIb signaling (as in a cone and plate viscometer) also activates αIIbβ3 but requires Ca2+ influx and probably mobilization of internal Ca2+ stores, as well as ADP secretion, but not TxA2 production.3,4,6,7 In a different system, adhesion-dependent shear stress-induced GPIb signaling (flow) that activates αIIbβ3 does not require Ca2+ influx (although Ca2+ influx potentiates the process)8 but does require mobilization of internal Ca2+ stores2,8 and is not dependent on either ADP secretion or TxA2 production.2,8 Similarly, shear stress-independent, adhesion-dependent GPIb-induced activation of αIIbβ3 apparently requires mobilization of internal stores but not Ca2+ influx, ADP, or TxA2.8,9 Despite these differences, GPIb-mediated signaling has been found to be PI3K8,10-14 and PLCγ2 dependent14-16 in every system tested for these requirements. Because of the functional relationships between PI3K and Bruton tyrosine kinase (Btk),17-19 Btk and PLCγ2 20-23 activation, and the documented role of Btk in collagen-induced signaling in platelets,19,24 we investigated the role(s) of Btk in TxA2 production induced by signaling elicited by bt/VWF/GPIb-mediated agglutination. This aspect of GPIb signaling is important because agglutination-elicited TxA2 production is required for αIIbβ3 activation in this GPIb-dependent signaling system.5,25 The results of this investigation demonstrate that Btk is essential for both bt/VWF-induced TxA2 production and stable GPIb-dependent thrombus formation in FeCl3-treated, mouse carotid arteries, as well as clarify, at least in part, the role of Btk in this signaling system.
Materials and methods
Materials
Apyrase and PGE1 were from Sigma-Aldrich (St Louis, MO). Wortmannin was purchased from EMD Biosciences (San Diego, CA). Complete Mini Protease Inhibitor Cocktail Tablets were from Roche Diagnostics (Indianapolis, IN); 4G10 was a generous gift from Brian Druker (Department of Hematology/Oncology, Oregon Health Sciences University, Portland); and 1B5 was a generous gift from Barry S. Coller (Rockefeller University, New York, NY). Anti-phospho-Akt (Ser473) antibody, anti-phospho-ERK1/2 antibody, anti-ERK1/2 antibody, and anti-phospho-Btk (Tyr223) antibody were from Cell Signaling Technology (Beverly, MA). Protein A/G PLUS-Agarose, anti-PLCγ2 polyclonal antibody, and anti-Syk polyclonal antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). Antiactin antibody was from Boehringer Mannheim Biochemicals (BMB; Indianapolis, IN). Peroxidase-conjugated donkey antimouse and donkey antirabbit antibodies were purchased from Jackson ImmunoResearch Labs (West Grove, PA). PepTag assay kit for the nonradioactive detection of active PKC was purchased from Promega (Madison, WI). Human von Willebrand factor was from Haematologic Technologies (Essex Junction, VT). Botrocetin was prepared as previously described.26 VCL, a recombinant fragment of VWF,27,28 was a generous gift from Dr Steven Slack (University of Memphis).29
Animals
Btk mutant and control mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The Btk mutation is in the CBA/CaHN background and designated Btkxid. Because Btk is X-linked,30 the mutant and wild-type males are designated Btkxid/Y and Btk+/Y, respectively. The mutation conferring the X-linked immunodeficiency causes an Arg28 to Cys28 substitution located in the pleckstrin homology (PH) domain of Btk.31 All the Btk mice used in this study were males. The control mice were not littermate mice and were in the CBA/CaJ background. This strain has been used traditionally as the source of control animals for experiments using the Btkxid strain.31 All the mice used for the Btk experiments were sex and age matched. Mice deficient in Lyn,32 SLP76,33 or Tp 34 (thromboxane A2 receptors) were generated as described. Wild-type C57/BL6 mice were used as the Lyn+/+ and Tp+/+ controls. Syk-/- chimeric mice were produced by fetal liver cell transplantation as described.35 Unless otherwise stated, wild-type littermate siblings were used as controls.
Platelet aggregation
Blood was collected from the abdominal aorta of isofluorane-anesthetized mice into syringes containing 100 μL/mL White anticoagulant (2.94% sodium citrate, 136 mM glucose [pH 6.4]), 0.1 μg/mL PGE1, and 1 U/mL apyrase.36 Washed platelets were prepared from platelet-rich plasma (PRP) by differential centrifugation of the PRP containing 5 mM EDTA at 1100g for 10 minutes. Platelets were resuspended in modified Tyrode solution (12 mM NaHCO3, 138 mM NaCl, 5.5 mM glucose, 2.9 mM KCl, 2 mM MgCl2, 0.42 mM NaH2PO4, 10 mM HEPES), pH 7.4. Aggregation was measured in a lumiaggregometer (Chrono-Log, Havertown, PA) using washed platelets (300 μL) adjusted to approximately 106 platelets per microliter. Inhibitors were incubated with the platelets for 3 minutes prior to stimulation.
Measurement of ATP secretion
ATP secretion was measured using CHORONO-LUME reagent (Chrono-Log) according to the manufacturer's protocol. ATP secretion data were obtained from at least 3 tests. Bars in graph represent the means ± SEM.
Measurement of TxA2
After a 7-minute aggregation period, platelets were removed by centrifugation in the presence of 5 mM EDTA. The platelet-free supernatant fraction was diluted 1:50 with the assay buffer supplied in the TxB2 EIA kit (Assay Designs, Ann Arbor, MI). TxB2, a stable metabolite of TxA2, was measured using the manufacturer's protocol. TxB2 production data were obtained from at least 3 tests. The terms “TxA2” and “TxB2” are used interchangeably throughout the text. Bars in graph represent the means ± SEM.
Immunoprecipitation and Western blotting
For the detection of tyrosine-phosphorylated Syk and PLCγ, aggregated platelet samples were added to the same volume of lysis buffer (100 mM Tris-HCl [pH 7.4]; 2% NP-40; 300 mM NaCl; 2 mM EDTA; 2 mM PMSF; 2 μg/mL aprotinin, leupeptin, and pepstatin; 2 mM Na3VO4; 2 mM NaF; and a Complete Mini Protease Inhibitor Cocktail Tablet). Next, the samples were incubated on ice for 30 minutes, and then 4 μg/mL 4G10 was added and the samples incubated overnight at 4°C. Then, 50 μL Protein A/G PLUS-Agarose was added to each sample prior to incubatation for 2 hours at 4°C. The beads were harvested by centrifugation at 3000g for 2 minutes and washed 3 times with 500 μL lysis buffer and twice with PBS solution. Proteins were boiled in sample buffer and resolved on a 10% sodium dodecyl sulfate (SDS) polyacrylamide gel and transferred to nitrocellulose. Western blots were performed using anti-Syk or anti-PLCγ antibody at a 1:1000 dilution, followed by incubation with horseradish peroxidase-conjugated donkey antirabbit antibody at a 1:5000 dilution. Blots were developed using Supersignal chemiluminescent substrate (Pierce, Rockford, IL)
For detection of phospho-Btk, phospho-Akt, and phospho-ERK1/2, samples of aggregated platelets were washed and suspended in EHS buffer (10 mM HEPES, 150 mM NaCl, 1 mM EDTA [pH 7.4]) and solubilized in one-third volume of Laemmli reducing sample buffer (24.6 mg/mL DTT added immediately prior to use), boiled for 5 minutes, and loaded onto a 10% SDS polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane and treated with anti-phospho-Akt antibody, followed by a secondary peroxidase-conjugated antibody, and developed using chemiluminescence for phospho-Akt detection. After stripping, the membranes were incubated with anti-phospho-Btk, anti-phospho-ERK1/2, or antiactin and anti-ERK1/2 antibody to either detect phospho-Btk, phosphorERK1/2, or to confirm that a similar amount of protein was present in each lane.
Measurement of PKC activation
Samples of aggregated platelets were transferred to centrifuge tubes and centrifuged at 2000g for 2 minutes. Each pellet was dissolved with 150 μL lysis buffer (20 mM Tris-HCl [pH 7.4], 2 mM EDTA, 250 mM sucrose, 100 μM leupeptin, and 50 mg/mL PMSF). PKC activity was assayed using the PepTag nonradioactive detection kit. Reaction mixtures were prepared containing 5 μL PepTag PKC reaction 5 × buffer, 2 μg PepTag C1 peptide, 1 μL peptide protection solution, 5 μL protein sample, and 4 μL water. Four microliters of 2.5 μg/mL PKC supplied in the kit was substituted for the sample proteins as the positive control. For the negative control, protein samples were replaced by water. The reaction mixtures were incubated in a 30°C water bath for 1 hour. The reaction was stopped by placing the tubes in a boiling water bath for 10 minutes. Samples containing 1 μL 80% glycerol were loaded onto a 0.8% agarose gel using a solution of 50 mM Tris-HCl, pH 8.0, as the running buffer and then run at 100 V for 25 minutes to separate the phosphorylated and nonphosphorylated PepTag peptides. Visualization of results was accomplished by exposing the gel to ultraviolet (UV) radiation using a BIO-RAD (Hercules, CA) Gel Doc 2000.
Ferric chloride-induced carotid artery injury
Carotid artery was separated from other tissue in isofluorane-anesthetized mice, and injury was induced by 10% FeCl3 as described.37,38 Blood flow was measured in the exposed carotid artery using a laser Doppler system (Trimflo Fiber Optic Probe), which was held in place by a micromanipulator and connected to a BPM2 Blood Perfusion Monitor (Vasamedics, Eden Prairie, MN). The blood flow monitor was connected to a Macintosh computer via an A/D convertor (PowerLab System; ADInstruments, Castle Hill, Australia), and this computer-based system was used for data collecting. The monitoring of carotid artery blood flow was initiated at the time of FeCl3 treatment. This treatment consisted of the placement of a 4 × 10-mm strip of Whatman no. 1 filter paper (Brentford, Middlesex, United Kingdom) saturated with a 10% FeCl3 solution over the exposed carotid artery for 3 minutes. This was followed by rinsing 3 times with a physiologic saline solution while blood flow was continuously monitored for 45 minutes. Carotid artery blood flow of less than 0.2 blood flow units (BFUs) per minute was scored as occlusion, allowing the time to first occlusion time to be determined. Box-plot graphs were produced by using statistics software SPSS version 14.0 (SPSS, Chicago, IL); each box shows the median, quartiles, and extreme values of corresponding group mice carotid artery blood flow data.
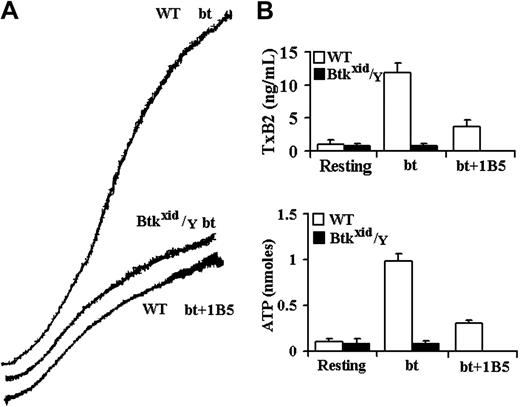
Btk is required for GPIb-elicited TxA2 production and ATP secretion by bt/VWF-stimulated washed platelets. (A) Aggregation and agglutination traces of wild-type (WT) and Btkxid/Y platelets treated with 0.5 μg/mL bt and 10 μg/mL human VWF. (B) Levels of TxA2 production and ATP secretion elicited by a combination of agglutination and aggregation. Btkxid/Y platelets did not produce TxA2 or secrete ATP. Therefore, Btk is required for agglutination-elicited TxA2 production. The error bars represent standard deviation; n = 3.
Btk is required for GPIb-elicited TxA2 production and ATP secretion by bt/VWF-stimulated washed platelets. (A) Aggregation and agglutination traces of wild-type (WT) and Btkxid/Y platelets treated with 0.5 μg/mL bt and 10 μg/mL human VWF. (B) Levels of TxA2 production and ATP secretion elicited by a combination of agglutination and aggregation. Btkxid/Y platelets did not produce TxA2 or secrete ATP. Therefore, Btk is required for agglutination-elicited TxA2 production. The error bars represent standard deviation; n = 3.
Results
Evaluation of the role(s) of Btk in TxA2 production elicited by bt/VWF-mediated agglutination
The role of the Tec family nonreceptor tyrosine kinases, Btk and Tec, in bt/VWF-induced signaling in platelets was investigated because of the close relationship between PI3K and Btk in signal transduction in B cells,17,18 neutrophils,39 and platelets19,40 and because previous work from this laboratory demonstrated that bt/VWF-induced TxA2 production is inhibited by the PI3K selective inhibitor wortmannin.14 Furthermore, Btk plays an important role in PLCγ activation,20-23 and PLCγ is required for bt/VWF-induced TxA2 production.14 Using platelets from Btkxid/Y mice,41 bt/VWF caused platelet agglutination but, in contrast to control platelets, the Btkxid platelets did not produce TxA2, secrete ATP, or aggregate (Figure 1). Btkxid platelets agglutinated to the same extent in the presence and absence of EDTA, demonstrating that there is no aggregation component in the response of those platelets to bt/VWF (not shown). These results demonstrate that Btk is required for bt/VWF-induced TxA2 production and ATP secretion by washed platelets in suspension.
The aggregation-like response of bt/VWF-stimulated wild-type platelets is biphasic. This biphasic response is composed of agglutination and agglutination-dependent aggregation.5 The hamster anti-mouse αIIbβ3 monoclonal antibody (mAb) 1B542 allows a clear distinction between the agglutination and aggregation phases of the biphasic aggregation-like response because it prevents fibrinogen binding to αIIbβ3 and thereby prevents aggregation without affecting agglutination. Similar results are obtained using EDTA to prevent aggregation.5,14 Agglutination-elicited signaling induces a characteristic level of TxA2 production (about 2.5 ng/mL) and ATP secretion. The level of TxA2 produced by the combination of agglutination and aggregation is about 4-fold to 5-fold greater (about 12 ng/mL) than that caused by agglutination in the absence of aggregation (Figure 1B).5 Likewise, aggregation greatly enhances ATP secretion. ATP secretion is dependent on TxA2 production in this system.5 The results in Figure 1 demonstrate that the absence of Btk eliminated bt/VWF-induced TxA2 production and ATP secretion and aggregation but had no effect on agglutination.
Btk is required for PLCγ2 phosphorylation
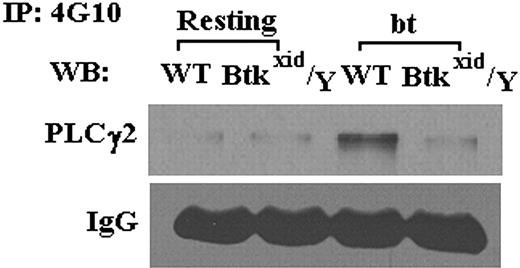
Part of the rationale for this study was that Btk may be required for the activation of PLCγ2 in bt/VWF-stimulated platelets. This relationship was tested by examining the Btk requirement for PLCγ2 phosphorylation in bt/VWF-stimulated platelets. Immunoprecipitation studies demonstrated that Btk is required for phosporylation of PLCγ2 (Figure 2). Previous work has demonstrated that PLCγ2 is required for TxA2 production.14 Therefore, Btk appears to be upstream of PLCγ2 in the signal transduction network that results in TxA2 production in response to bt/VWF stimulation of platelets.
Btk is downstream of Lyn, Syk, SLP-76, and PI3K
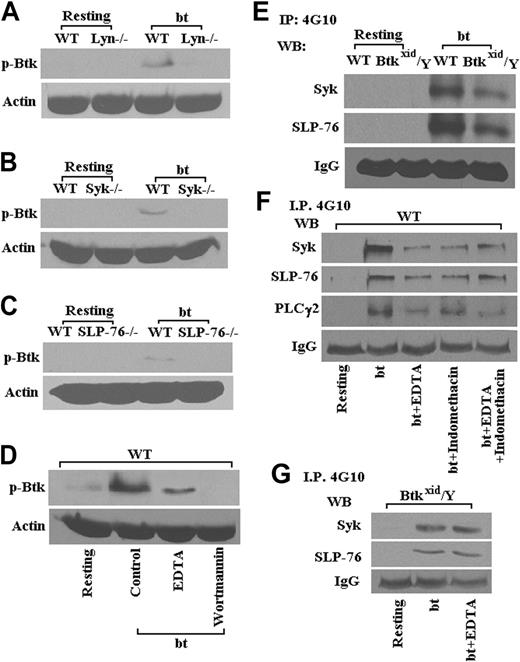
Previous work has demonstrated that bt/VWF-mediated, agglutination-induced TxA2 production is dependent on signaling apparently initiated by Lyn, enhanced by Src, and propagated through Syk, SLP-76, PI3K, PLCγ2, and PKC.14 Therefore, the following experiments were performed to identify the position of Btk in this signaling pathway. The data in Figure 3 show that Lyn (Figure 3A), Syk (Figure 3B), SLP-76 (Figure 3C), and PI3K (Figure 3D) are required for the phosphorylation of Btk. The interrelationship between Btk, Syk, and SLP-76 was confirmed by the data in Figure 3E, which show that Btk is downstream of Syk and SLP-76. Phosphorylation of Syk, SLP-76, and PLCγ2 was diminished in the presence of EDTA relative to the levels in the absence of EDTA, presumably demonstrating the enhancement of tyrosine phosphorylation resulting from aggregation (Figure 3F). Indomethacin treatment of the platelets caused agglutination in response to bt/VWF and had no greater effect on the phosphorylation of Syk, SLP-76, and PLCγ2 than EDTA alone, demonstrating that the effect of TxA2 on phosphorylation of those signaling molecules appears to be explicable in terms of its effect on aggregation (Figure 3F). Accordingly, phosphorylation of Syk and SLP-76 was diminished in the Btkxid background apparently because the Btk mutation resulted in normal agglutination, but it did not support aggregation because of the lack of TxA2 production (Figure 3E). This conclusion is supported by the observation that EDTA had no effect on the phosphorylation of Syk and SLP-76 in Btkxid platelets, demonstrating again that there is no aggregation component in the agglutination response of those platelets (Figure 3G).
Btk is upstream of Akt, PKC, and ERK1/2
As shown in Figure 3B, wortmannin prevented bt/VWF-induced Btk phosphorylation, revealing that PI3K is upstream of Btk. Nonetheless, Btk function greatly enhanced, or was required for, Akt phosphorylation (Figure 4A). Furthermore, PKC activation was dependent on Btk (Figure 4B). An earlier investigation demonstrated that PKC is required for bt/VWF-induced TxA2 production and that PLCγ2 is required for PKC activation.14 In combination, these data show that signaling appears to flow though PI3K via Btk to PLCγ2 and then to PKC. Others have shown that GPIb signaling activates ERK1/2.25,43 Our data demonstrate that bt/VWF-induced agglutination also activates ERK1/2 and that Btk is required for activation of ERK1/2 (Figure 4C).
Btk is required for bt/VWF-induced GPIb-dependent phosphorylation of PLCγ2. In contrast to results obtained using WT platelets, PLCγ2 was not tyrosine phosphorylated in Btkxid/Y platelets stimulated with bt/VWF. These results demonstrate that activation of PLCγ2 is dependent on Btk function.
Btk is required for bt/VWF-induced GPIb-dependent phosphorylation of PLCγ2. In contrast to results obtained using WT platelets, PLCγ2 was not tyrosine phosphorylated in Btkxid/Y platelets stimulated with bt/VWF. These results demonstrate that activation of PLCγ2 is dependent on Btk function.
Lyn, Syk, SLP-76, and PI3K are required for Btk phosphorylation (Tyr223) in platelets stimulated with bt/VWF. Btk (Tyr223) was not phosphorylated in Lyn-/- (A), Syk-/- (B), and SLP-76-/- (C) platelets in response to bt/VWF; the PI3K inhibitor wortmannin (100 nM) also blocked Btk (Tyr223) phosphorylation (D). Syk and SLP-76 were phosphorylated in Btkxid/Y platelets stimulated by bt/VWF (E). Phosphorylation of Syk, SLP-76, and PLCγ2 was diminished in the presence of EDTA (5 mM); indomethacin (75 μM) treatment had no greater effect on the phosphorylation of Syk, SLP-76, and PLCγ2 than EDTA alone (F). EDTA also had no effect on the phosphorylation of Syk and SLP-76 in Btkxid platelets (G). These results demonstrate that the activation of Btk in response to bt/VWF stimulation of platelets requires the functions of Lyn, Syk, SLP-76, and PI3K.
Lyn, Syk, SLP-76, and PI3K are required for Btk phosphorylation (Tyr223) in platelets stimulated with bt/VWF. Btk (Tyr223) was not phosphorylated in Lyn-/- (A), Syk-/- (B), and SLP-76-/- (C) platelets in response to bt/VWF; the PI3K inhibitor wortmannin (100 nM) also blocked Btk (Tyr223) phosphorylation (D). Syk and SLP-76 were phosphorylated in Btkxid/Y platelets stimulated by bt/VWF (E). Phosphorylation of Syk, SLP-76, and PLCγ2 was diminished in the presence of EDTA (5 mM); indomethacin (75 μM) treatment had no greater effect on the phosphorylation of Syk, SLP-76, and PLCγ2 than EDTA alone (F). EDTA also had no effect on the phosphorylation of Syk and SLP-76 in Btkxid platelets (G). These results demonstrate that the activation of Btk in response to bt/VWF stimulation of platelets requires the functions of Lyn, Syk, SLP-76, and PI3K.
GPIb-dependent FeCl3-induced stable occlusion of the carotid artery requires Btk
The FeCl3-induced arterial injury model37,38 was used to evaluate the in vivo requirement for Btk in GPIb-induced signaling. This system was appropriate for our purposes because the consequences of FeCl3 treatment of mouse mesentery are known. First, FeCl3 treatment causes denudation of the arterial endothelium and the coincident exposure of what appears to be VWF44 and collagen45 of the subendothelium, thereby generating a thrombogenic surface. Second, VWF is the dominant molecule involved in platelet deposition on the damaged endothelium of FeCl3-treated vessels.46 The latter fact supports the view that GPIb is the dominant receptor mediating platelet deposition on FeCl3-damaged arterial tissue. This was tested using VCL, a fragment of VWF spanning residues 504 to 728.27 This fragment contains the GPIbα binding site of VWF.28,47 VCL inhibits botrocetin- and ristocetin-facilitated binding of VWF to platelets5,27,48 and causes abolition of the cyclic flow variations in stenosed, endothelium-injured coronary arteries in nonhuman primates as well as inhibits platelet adhesion after balloon injury in rats.47
Btk is required for activation of Akt, PKC, and ERK1/2 in platelets stimulated by bt/VWF. In contrast to the results obtained using WT platelets, (A) phosphorylation of Akt was greatly diminished in Btkxid/Y platelets treated by bt/VWF, (B) PKC was not activated in Btkxid/Y platelets stimulated by bt/VWF (this assay demonstrates PKC activity by movement of a phosphorylated peptide substrate toward the positive electrode), and (C) ERK1/2 was not phosphorylated in Btkxid/Y platelets stimulated by bt/VWF.
Btk is required for activation of Akt, PKC, and ERK1/2 in platelets stimulated by bt/VWF. In contrast to the results obtained using WT platelets, (A) phosphorylation of Akt was greatly diminished in Btkxid/Y platelets treated by bt/VWF, (B) PKC was not activated in Btkxid/Y platelets stimulated by bt/VWF (this assay demonstrates PKC activity by movement of a phosphorylated peptide substrate toward the positive electrode), and (C) ERK1/2 was not phosphorylated in Btkxid/Y platelets stimulated by bt/VWF.
Wild-type C57BL/6 mice were used for the VCL experiments. The objective of these experiments was to evaluate whether or not stable thrombus formation is GPIbα dependent in FeCl3-injured carotid arteries.
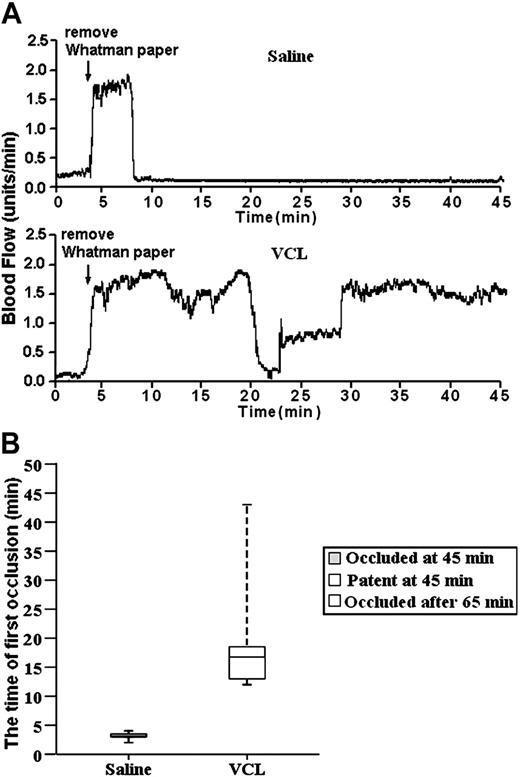
For these experiments, the tail veins of the mice were injected with either saline (saline is the traditional control in VCL infusion experiments28,47 ) or a saline solution of VCL. On the basis of the results from VCL infusion studies using baboons,47 VCL was infused at the level of 4.8 μg/g of body weight. Immediately after infusion, the carotid arteries were exposed and treated with FeCl3, and blood flow was monitored for 45 minutes using a laser Doppler system. The trace started at the time of the application of the FeCl3 and continued for 45 minutes. The results were unambiguous. The median time for thrombus formation in the carotid arteries of the 4 saline-treated mice was 3 minutes, with a range of 2 to 4 minutes. Importantly, embolism did not occur (Figure 5). So, the initial occlusion was the result of stable thrombus formation. In contrast, blood flow was not stably occluded during the 45-minute observation period in any of the 6 VCL/FeCl3-treated mice. However, stable thrombus formation did occur at about 65 minutes after FeCl3 treatment. The median time for formation of the first transient thrombus was 17 minutes, with a range of 12 to 45 minutes in the VCL-treated mice. So, VCL treatment delayed the onset of thrombus formation and prevented the stable thrombus formation during the 45-minute observation period. Therefore, it is clear that stable thrombus formation in the FeCl3-treated carotid arteries is GPIbα dependent. In view of these results, the FeCl3-induced injury model was ideal for evaluating the role of Btk in GPIbα-dependent thrombus formation in vivo. These results confirm those obtained by others using an anti-GPIb mAb.49
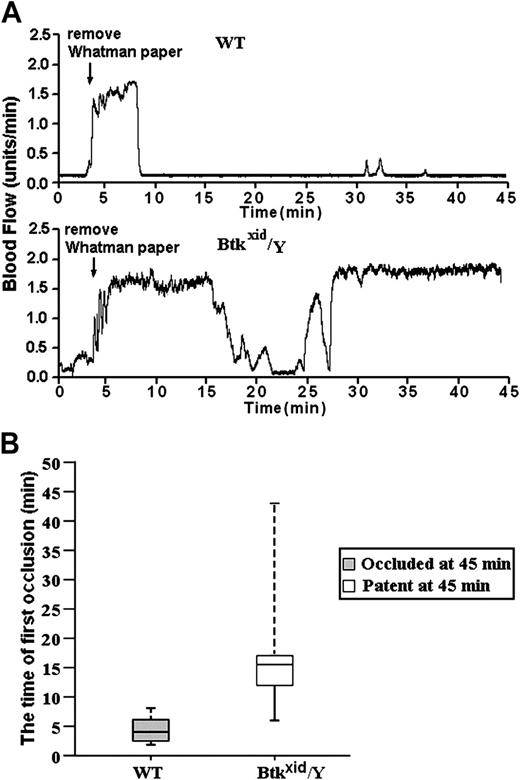
For these studies, blood flow was measured using a laser Doppler system in untreated and FeCl3-treated, exposed carotid arteries in wild-type and Btkxid/Y anesthetized mice. The data in Figure 6A are representative of the results obtained from 10 wild-type (Btk+/Y) mice and 6 Btkxid/Y mice. The trace started at the time of the application of the FeCl3 and continued for 45 minutes. The median time for the first thrombotic occlusion in the 10 Btk+/Y wild-type mice was 4.5 minutes, with a range of 2 to 8 minutes. Furthermore, blood flow remained occluded in all the wild-type arteries at the end of the monitoring period (Figure 6B). In contrast, the median time for the first thrombotic occlusion in 6 of the Btkxid arteries was 16.0 minutes, with a range of 6 to 45 minutes, and all of the treated Btkxid arteries were patent at the end of the monitoring period with blood flow returning to preocclusion levels (Figure 6B). One of the Btkxid arteries failed to show occlusion during the monitoring period. These results demonstrate that Btk plays a critical role in GPIbα-dependent thrombosis in vivo.
VCL treatment delayed the onset of unstable thrombus formation and prevented the stable thrombus formation. (A) The tracings are representative of blood flow in FeCl3-treated exposed carotid arteries of wild-type mice. The tail veins of these mice were injected with either saline (10 μL/g of body weight) or a saline solution of VCL (4.8 μg/g of body weight). Their blood flow was monitored for 45 minutes, including a 3-minute treatment starting at zero time with a strip of filter paper soaked in 10% FeCl3. The arrow indicates the time of removal of the FeCl3-containing strip of filter paper. (B) Box-plot graphs represent time of first occlusion of carotid arteries (blood flow less than 0.2 mL/min) from 6 WT mice given injections of saline and 6 WT mice given injections of VCL. The results show that the median time required for the first thrombotic occlusion in 6 WT mice treated with saline was 3 minutes, with a range of 2 to 4 minutes; the median time required for the first thrombotic occlusion in the WT mice treated with VCL was 17 minutes, with a range of 12 to 45 minutes. Arterial blood flow was occluded in all the WT mice treated with saline at the end of the monitoring period. Blood flow in all of WT mice treated with VCL was patent at the end of 45 minutes, but the stable thrombus formation occurred after 65 minutes. These results demonstrate VCL treatment delayed the onset of thrombus formation and prevented the stable thrombus formation during the 45-minute observation period. Therefore, it is clear that stable thrombus formation in the FeCl3-treated carotid arteries is GPIbα dependent. Bars represent extreme values for times of first occlusion.
VCL treatment delayed the onset of unstable thrombus formation and prevented the stable thrombus formation. (A) The tracings are representative of blood flow in FeCl3-treated exposed carotid arteries of wild-type mice. The tail veins of these mice were injected with either saline (10 μL/g of body weight) or a saline solution of VCL (4.8 μg/g of body weight). Their blood flow was monitored for 45 minutes, including a 3-minute treatment starting at zero time with a strip of filter paper soaked in 10% FeCl3. The arrow indicates the time of removal of the FeCl3-containing strip of filter paper. (B) Box-plot graphs represent time of first occlusion of carotid arteries (blood flow less than 0.2 mL/min) from 6 WT mice given injections of saline and 6 WT mice given injections of VCL. The results show that the median time required for the first thrombotic occlusion in 6 WT mice treated with saline was 3 minutes, with a range of 2 to 4 minutes; the median time required for the first thrombotic occlusion in the WT mice treated with VCL was 17 minutes, with a range of 12 to 45 minutes. Arterial blood flow was occluded in all the WT mice treated with saline at the end of the monitoring period. Blood flow in all of WT mice treated with VCL was patent at the end of 45 minutes, but the stable thrombus formation occurred after 65 minutes. These results demonstrate VCL treatment delayed the onset of thrombus formation and prevented the stable thrombus formation during the 45-minute observation period. Therefore, it is clear that stable thrombus formation in the FeCl3-treated carotid arteries is GPIbα dependent. Bars represent extreme values for times of first occlusion.
GPIb-dependent FeCl3-induced stable occlusion of the carotid artery requires TxA2 receptor-mediated signaling.
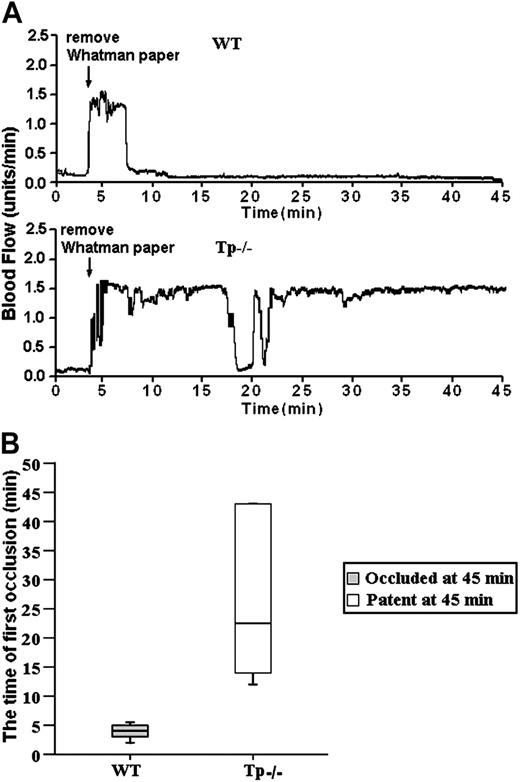
The results of our bt/VWF studies demonstrate that αIIbβ3 activation and platelet aggregation in this system are dependent on TxA2 production.5,14 The results presented here reveal that agglutination-induced TxA2 production and stable thrombus formation are Btk dependent (Figures 1 and 6). According to the rationale of this study, stable thrombus formation requires Btk because Btk is required for production of the TxA2 required for αIIbβ3 activation and platelet aggregation. Therefore, if this relationship is correct, stable thrombus formation should require signaling mediated by TxA2 receptors. The validity of this signaling relationship was tested by treating carotid arteries of mice that lack TxA2 receptors (Tp-/- mice) with FeCl3. As with the Btkxid mice, the results were unambiguous; FeCl3-induced stable thrombus formation did not occur in the Tp-/- mice (Figure 7). The data in Figure 7A are representative of the results obtained from 6 wild-type and 6 Tp-/- mice. The median time for the first thrombotic occlusion in the wild-type mice was 4.0 minutes, with a range of 2 to 6 minutes. Furthermore, blood flow remained occluded in all the wild-type arteries at the end of the monitoring period (Figure 7B). In contrast, the median time for the first thrombotic occlusion in 6 of the Tp-/- arteries was 22.5 minutes, with a range of 13 to 45 minutes, and all of the treated Tp-/- arteries were patent at the end of the monitoring period with blood flow returning to preocculsion levels (Figure 6A). Arteries in 2 of the Tp-/- mice failed to show occlusion during the monitoring period. These results demonstrate that TxA2 signaling plays a critical role in GPIbα-dependent thrombosis in vivo.
GPIb-dependent FeCl3-induced stable occlusion of the carotid artery requires Btk. (A) The tracings are representative of blood flow in FeCl3-treated exposed carotid arteries of wild-type and Btkxid/Y mice. Blood flow was monitored for 45 minutes, including a 3-minute treatment starting at zero time with a strip of filter paper soaked in 10% FeCl3. The arrow indicates the time of removal of the FeCl3-containing strip of filter paper. (B) Box-plot graphs represent time of first occlusion of carotid arteries (blood flow less than 0.2 mL/min) from 10 WT mice and 6 Btkxid/Y mice. The results show that the median time required for the first thrombotic occlusion in 10 Btk+/Y mice was 4.5 minutes, with a range of 2 to 8 minutes; the median time required for the first thrombotic occlusion in the Btkxid/Y arteries was 16 minutes, with a range of 6 to 45 minutes. Arterial blood flow was occluded in all the wild-type arteries at the end of the monitoring period. On the contrary, all of the treated Btkxid/Y arteries were patent at the end of the monitoring period. No occlusion occurred in the treated carotid artery of one Btkxid/Y mouse. These results demonstrate that Btk is required for GPIb-dependent stable thrombus formation in vivo. Bars represent extreme values for times of first occlusion.
GPIb-dependent FeCl3-induced stable occlusion of the carotid artery requires Btk. (A) The tracings are representative of blood flow in FeCl3-treated exposed carotid arteries of wild-type and Btkxid/Y mice. Blood flow was monitored for 45 minutes, including a 3-minute treatment starting at zero time with a strip of filter paper soaked in 10% FeCl3. The arrow indicates the time of removal of the FeCl3-containing strip of filter paper. (B) Box-plot graphs represent time of first occlusion of carotid arteries (blood flow less than 0.2 mL/min) from 10 WT mice and 6 Btkxid/Y mice. The results show that the median time required for the first thrombotic occlusion in 10 Btk+/Y mice was 4.5 minutes, with a range of 2 to 8 minutes; the median time required for the first thrombotic occlusion in the Btkxid/Y arteries was 16 minutes, with a range of 6 to 45 minutes. Arterial blood flow was occluded in all the wild-type arteries at the end of the monitoring period. On the contrary, all of the treated Btkxid/Y arteries were patent at the end of the monitoring period. No occlusion occurred in the treated carotid artery of one Btkxid/Y mouse. These results demonstrate that Btk is required for GPIb-dependent stable thrombus formation in vivo. Bars represent extreme values for times of first occlusion.
GPIb-dependent FeCl3-induced stable occlusion of the carotid artery requires Tp (thromboxane receptor). (A) The tracings are representative of blood flow in FeCl3-treated exposed carotid arteries of wild-type and Tp-/- mice. Blood flow was monitored for 45 minutes, including a 3-minute treatment starting at zero time with a strip of filter paper soaked in 10% Fe Cl3. The arrow indicates the time of removal of the FeCl3-containing strip of filter paper. (B) Box-plot graphs represent time of first occlusion of carotid arteries (blood flow less than 0.2 mL/min) from 6 WT mice and 6 Tp-/- mice. The results show that the median time required for the first thrombotic occlusion in 6 WT mice was 4 minutes, with a range of 2 to 6 minutes; the median time required for the first thrombotic occlusion in the Tp-/- arteries was 22.5 minutes, with a range of 13 to 45 minutes. Arterial blood flow was occluded in all the wild-type arteries at the end of the monitoring period. On the contrary, all of the treated Tp-/- arteries were patent at the end of the monitoring period. No occlusion occurred in the treated carotid artery of one Tp-/- mouse. These results demonstrate that Tp is required for GPIb-dependent stable thrombus formation in vivo. Bars represent extreme values for times of first occlusion.
GPIb-dependent FeCl3-induced stable occlusion of the carotid artery requires Tp (thromboxane receptor). (A) The tracings are representative of blood flow in FeCl3-treated exposed carotid arteries of wild-type and Tp-/- mice. Blood flow was monitored for 45 minutes, including a 3-minute treatment starting at zero time with a strip of filter paper soaked in 10% Fe Cl3. The arrow indicates the time of removal of the FeCl3-containing strip of filter paper. (B) Box-plot graphs represent time of first occlusion of carotid arteries (blood flow less than 0.2 mL/min) from 6 WT mice and 6 Tp-/- mice. The results show that the median time required for the first thrombotic occlusion in 6 WT mice was 4 minutes, with a range of 2 to 6 minutes; the median time required for the first thrombotic occlusion in the Tp-/- arteries was 22.5 minutes, with a range of 13 to 45 minutes. Arterial blood flow was occluded in all the wild-type arteries at the end of the monitoring period. On the contrary, all of the treated Tp-/- arteries were patent at the end of the monitoring period. No occlusion occurred in the treated carotid artery of one Tp-/- mouse. These results demonstrate that Tp is required for GPIb-dependent stable thrombus formation in vivo. Bars represent extreme values for times of first occlusion.
Discussion
Botrocetin/VWF stimulation of washed murine platelets under appropriate conditions causes a biphasic agglutination/aggregation response and platelet activation. The GPIbα-mediated agglutination phase of the biphasic response initiates a signaling cascade that elicits TxA2 production that is prerequisite for activation of αIIbβ3.5,25 The agglutination-elicited signaling that causes agglutination-dependent TxA2 production is initiated by Lyn, enhanced by Src, and propagated through Syk, SLP-76, PI3K, PLCγ2, and PKC.14 The work presented here extends our understanding of this agglutination-elicited signaling by demonstrating that the resultant TxA2 production requires Btk. Btk is a nonreceptor protein tyrosine kinase that is a member of the Tec kinase family that affects B-cell development and function as well as signaling in other white cells and platelets.17-24,38,40,41 Human19 and mouse platelets24 contain both Btk and Tec, members of the Tec kinase family. Both Tec and Btk are required for normal aggregation in response to a low level of collagen-related peptide (CRP) or collagen, but either Btk or Tec is sufficient for a normal aggregation response to a high level of either agonist.24 Thus, Tec compensates for the absence of Btk in Btk-/- platelets (the mutation present in the female mice used for that work contained a deletion of exons 13 and 14, which inactivated the Btk activity50 ) stimulated with collagen under certain conditions and in the platelets from a patient with X-linked agammaglobulinemia (XLA) caused by Btk deficiency.40 In the present study, Tec apparently could not substitute for Btk in response to bt/VWF stimulation or FeCl3-induced injury because, in contrast to wild-type platelets, Btkxid/Y platelets did not respond normally to stimulation by bt/VWF (Figure 1) or form stable thrombi in response to arterial injury (Figure 6). This conclusion is supported by the observation that, unlike the response to CRP-stimulated Btk-/- platelets,24 PLCγ2 was not phosphorylated in bt/VWF-stimulated Btkxid/Y platelets (Figure 2). This apparently absolute Btk requirement for PLCγ2 phosphorylation in response to bt/VWF (Figure 2) may reflect an essential difference between the roles of Tec in GPVI-versus GPIb-elicited signaling.24 Alternatively, Btkxid might have acted as a dominant negative allele preventing the function of Tec. Interestingly, at least 1 other striking difference exists between these 2 superficially similar signaling systems. That is, FcRγ-chain function is required for GPVI-initiated signaling that results in αIIbβ3 activation,51 whereas αIIbβ3 activation in response to signaling initiated by bt/VWF-facilitated, GPIb-mediated agglutination14 or by platelet adhesion to the purified A1 domain of dimeric VWF9 does not require FcRγ-chain function.
Our results show that Btk is downstream of Lyn, Syk, SLP-76, and PI3K (Figure 3) and is needed for TxA2 production, ATP secretion, as well as platelet aggregation (Figure 1). Further analysis revealed Btk is upstream of PLCγ2 (Figure 2), PKC (Figure 4), and ERK1/2 (Figure 4). The roles of PLCγ2 and PKC in bt/VWF-induced TxA2 production by mouse platelets have been extensively characterized.14 However, further work is required to evaluate the relationship between these signaling molecules and the documented role of ERK1/2 in ristocetin-induced TxA2 production by human platelets25 and the inhibition of both ERK2 phosphorylation and the activation of αIIbβ3 in reconstituted CHO cells by dominant negative forms of MEK1 and Raf-1.43 The observation that PKC can activate Raf in murine hematopoietic cells52 and NIH3T3 fibroblasts53 by phosphorylating it may provide a link between our data and the participation of Raf,43 MEK,43 and ERK25,43 in GPIb signaling. Furthermore, the recent demonstration that PKC can stimulate PKG in transfected HEK cells and vascular endothelial cells54 could provide a rationale for the requirement for PKC14 and ERK2 25 for TxA2 production as well as the controversial observation25,55 that PKG is required for ERK activation in platelets,43 if GPIb-initiated signaling in platelets enables PKC to activate PKG.
The roles of PI3K and Btk in PLCγ activation in platelets may be similar to their roles in this process in other systems. The pleckstrin homology (PH) domain of Btk may facilitate attachment of the kinase to the inner leaflet of the plasma membrane by binding to phosphatidylinositiol-3,4,5-P3 (PtdIns-3,4,5-P3).56,57 PtdIns-4,5-P2 is phosphorylated by PI3K, forming PtdIns-3,4,5-P3. According to one view of Btk activation, binding of Btk to the membrane apparently eliminates blockade or occlusion of the kinase and substrate binding sites, thereby “activating” the kinase.56 Phosphorylation of membrane-bound Btk may also enhance its kinase activity.56 Presumably, this binding places Btk in close proximity to PLCγ2 attached to PtdIns-3,4,5-P3 via its PH domain and thereby facilitates phosphorylation of 2 tyrosine residues in the SH2-SH3 linker region of PLCγ2.21,57 Furthermore, Btk acts as a shuttle to carry PIP5Ks to the plasma membrane in B cells, thereby enhancing PtdIns-4,5P2 synthesis.58 This Btk function may explain, at least in part, the role of Btk in Akt activation. PtdIns-4,5P2 is a substrate for PI3K for the production of PtdIns-3,4,5P3.58 Thus, Btk may play a central role in bt/VWF-induced signaling by facilitating PI3K, Akt, and PLCγ2 functions. While this hypothetical scheme explaining the role of PI3K and Btk in the activation of PLCγ2 is based on observations made in vitro in a variety of cell types, it may not accurately explain how Btk enables PLCγ2 phosphorylation in bt/VWF-stimulated mouse platelets. Likewise, the suggested mechanism for the Btk-dependent enhancement of Akt activation is hypothetical. Further work is required to clarify these issues. Although the PH and SH2 domains of Btk are required for PLCγ2 activation in response to B-cell receptor-initiated signaling in chicken DT40B cells,20 it is not known whether these Btk domains are also required for PLCγ2 activation in mouse platelets. It is clear, however, that Btk is required for bt/VWF-induced PLCγ2 activation (Figure 2), that PLCγ2 is required for PKC activation,14 and that PKC is required for TxA2 production.14
The in vivo significance of the data obtained here using bt/VWF-stimulated Btkxid/Y platelets was demonstrated using the FeCl3 arterial injury model. This was accomplished directly by using Btkxid/Y platelets in the FeCl3 injury model and confirmed by using Tp-/- platelets. Justification of the use of this model to evaluate the in vivo significance of Btk in GPIbα-dependent signaling is that thrombus formation in this system as reflected by occlusion of blood flow is GPIbα dependent (Figure 5) and that bt/VWF-induced TxA2 production is GPIb dependent5 and required for αIIbβ3 activation.5 Therefore, the results obtained in vivo with the Btkxid mice demonstrate that Btk is critically important for GPIbα-mediated stable thrombus formation in this model system.
The results obtained using this method of evaluating thrombus formation are in agreement with those obtained using mouse platelets lacking the extracellular domain of human GPIbα.59 As with the Btk-/- mice, all of the FeCl3-injured carotid arteries of mice lacking the extracellular domain of GPIbα were patent at the end of the monitoring period. These results contrast with those obtained using GPVI-/- mice, in which only 50% of the injured arteries were patent at the end of the monitoring period.59 Therefore, the results obtained in vivo with the Btk-/- mice demonstrate that Btk is critically important for GPIbα-mediated stable thrombus formation in this model system.
In summary, the data presented here demonstrate the previously undocumented and critical role of Btk in bt/VWF-induced signaling in vitro and GPIb-dependent stable thrombus formation in vivo. Additionally, it is clear that the Tec family kinase, Tec, cannot substitute for Btk either in vitro, in response to bt/VWF-induced signaling, or in vivo, in GPIb-initiated signaling. The dramatic protection against stable thrombus formation in the FeCl3 arterial injury model resulting from Btk deficiency strongly supports the results of preliminary studies60,61 that a Btk-selective inhibitor may prove to be a clinically useful antithrombotic agent during acute thrombotic episodes.
Prepublished online as Blood First Edition Paper, June 20, 2006; DOI 10.1182/blood-2006-01-011817.
Supported in part by HL079990 from the National Heart, Lung, and Blood Institute; NSF DUE-9850780, P30CA21765 Cancer Center Support Grant, and P01CA20180 from the National Cancer Institute; the National Health and Medical Research Council of Australia; the American Lebanese Syrian Associated Charities; and the W. Harry Feinstone Center for Genomic Research.
J.L. performed all of the experiments; M.E.F. provided the equipment and expertise for performing the blood flow experiments; M.C.B. provided the botrocetin; C.W.J. provided the Lyn-/- mice; and T.K.G. initiated and directed the project. All of the authors contributed to experimental design and helped with the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal