Abstract
Type 1 von Willebrand disease (VWD) is characterized by a personal and family history of bleeding coincident with reduced levels of normal plasma von Willebrand factor (VWF). The molecular basis of the disorder is poorly understood. The aims of this study were to determine phenotype and genotype and their relationship in patients historically diagnosed with type 1 VWD. Families were recruited in 9 European countries based on previous type 1 VWD diagnosis. Bleeding symptoms were recorded, plasma phenotype analyzed, and VWF mutation analysis performed in all index cases (ICs). Phenotypic and molecular analysis stratified patients into those with or without phenotypes suggestive of qualitative VWF defects (abnormal multimers) and with or without mutations. A total of 105 of 150 ICs (70%) had mutations identified. A subgroup with abnormal multimers (38% of ICs, 57 of 150) showed a high prevalence of VWF gene mutations (95% of ICs, 54 of 57), whereas in those with qualitatively normal VWF, fewer mutations were identified (55% of ICs, 51 of 93). About one third of the type 1 VWD cases recruited could be reconsidered as type 2. The remaining group could be considered “true” type 1 VWD, although mutations were found in only 55%.
Introduction
von Willebrand disease (VWD) is still considered the most common inherited bleeding disorder and is described as being caused by genetic defects in the von Willebrand factor (VWF) gene.1-3 Diagnosis of VWD is based on the presence of autosomal inheritance of mucocutaneous bleeding symptoms and reduced VWF levels. A reduced concentration of structurally normal VWF is classified as type 1 VWD (50% to 75% of VWD), whereas qualitatively abnormal variants of VWF are classified as type 2 VWD.4 Provisional diagnostic criteria for type 1 VWD were published recently on behalf of the International Society on Thrombosis and Haemostasis Scientific and Standardization Committee VWF Subcommittee (ISTH-SSC on VWF).5
Diagnosis of type 1 VWD can be difficult, especially in mild cases, because patients with reduced, structurally normal VWF due to VWF gene mutations cannot be distinguished phenotypically (apart from their bleeding symptoms) from healthy individuals with VWF levels at the lower end of the normal distribution. Diagnosis is further complicated by incomplete penetrance of the disease and the influence of several genetic and environmental factors on VWF levels. There is no single assay to definitely diagnose type 1 VWD. Mutation analysis of the VWF gene could potentially contribute to the diagnosis. However, because of the size and complexity of the VWF gene (52 exons covering 178 kb,6 with a partial pseudogene7 ), complete gene analysis has until recently been conducted in only limited numbers of patients.8
A more reliable diagnostic pathway for type 1 VWD is required to prevent false positive diagnoses, which may lead to stigmatization and unnecessary treatment with potentially dangerous plasma-derived factor concentrates, and to prevent false negative diagnoses with the risk of unnecessary bleeding complications.9 To systematically study the value of clinical, phenotypic, and molecular markers for the diagnosis of type 1 VWD, a multicenter European study was initiated entitled Molecular and Clinical Markers for the Diagnosis and Management of Type 1 von Willebrand Disease (MCMDM-1VWD).10 Previous reports from this study have focused on bleeding symptoms in type 1 VWD11 and on linkage with the VWF gene.12 It was shown that a quantitative analysis of bleeding symptoms using a standardized bleeding score could be useful for more reliable diagnosis of type 1 VWD.11 Linkage analysis revealed that more severe phenotypes and phenotypes suggestive of qualitative VWF defects were more likely to completely cosegregate with the VWF gene.12
In the present study, the phenotype-genotype relationship in patients previously diagnosed with type 1 VWD within the MCMDM-1VWD cohort was analyzed through detailed phenotypic analysis and mutation analysis of the entire coding sequence and approximately 2 kb of 5′ noncoding sequence of the VWF gene.
Patients, materials, and methods
Study design
The MCMDM-1VWD study is a European Union (EU)–funded survey on type 1 VWD.10-12 Fourteen VWD treatment centers in 9 European countries participated in the study. Partner (P) numbers were as follows; P1, Sheffield, United Kingdom; P2, Vicenza, Italy; P3, Milano, Italy; P4, La Coruna, Spain; P5, Paris and Lille, France; P6, Leiden, The Netherlands; P7, Hamburg, Germany; P8, Aarhus, Denmark; P9, Prague, Czech Republic; P10, Malmö and Lund, Sweden; P11, Leicester, United Kingdom; and P12, Birmingham, United Kingdom. The study aimed at recruiting the full spectrum of type 1 VWD families, including milder cases to mimic the clinical situation as much as possible and not to have bias toward the more severe, highly penetrant type 1 VWD. We also aimed to include as many affected and unaffected family members as possible and to include families that consisted of at least 2 generations.
VWD families and controls
A total of 154 families historically diagnosed to have type 1 VWD by their treatment center based on ISTH-SSC on VWF guidelines4 were recruited into the study. However, samples from some individuals taken at recruitment did not meet these criteria. Each family comprised an index case (IC) with VWD, at least 1 further affected family member (AFM), plus unaffected family members (UFMs). Additionally, each center recruited approximately 100 healthy controls (HCs) approximately age and sex matched with family members. (These individuals were referred to previously11,12 as “normal controls” [NCs] rather than “healthy controls” [HCs].) HCs had never sought medical attention for a bleeding symptom prior to enrollment. Local ethics review committees at each center approved the study protocol. In accordance with the Declaration of Helsinki, informed consent was obtained from all individuals at recruitment by signing a consent form in the individual's own language. A pedigree was obtained for each family, and venous-blood samples were taken from all participants at enrollment into the study. Venous blood anticoagulated with 0.109 M sodium citrate (1:9 vol/vol) was centrifuged at 2500g for 15 minutes. Citrated plasma was separated from the cells, aliquoted, and stored at −70°C. Packed cells were used for DNA extraction.11,12
Bleeding questionnaire and score
A physician who was aware of a patient's diagnosis but unaware of his or her previous bleeding history administered the questionnaire to all enrolled family members at recruitment. A bleeding history was derived from detailed questions on 12 bleeding symptoms, and a score was compiled.11 Each was graded for hemorrhagic severity from −1 (did not bleed when challenged by 2 surgeries, tooth extractions, or deliveries) to +4 (required transfusion or replacement therapy). The summed score of all symptoms resulted in a quantitative measure of bleeding severity. Total possible scores were −3 to +45.11,13A
Laboratory phenotype
Coagulation studies were undertaken as previously described.11,12 All recruited individuals' plasma was assessed for VWF antigen (VWF:Ag), VWF ristocetin cofactor activity (VWF:RCo) (determined on the same samples both locally and centrally by P2, correlation r2 0.86 and 0.74, respectively11 ), and factor VIII activity (FVIII:C) (determined locally). For the present analysis, VWF:Ag and VWF:RCo values measured centrally by P2 were considered together with FVIII:C measured by each enrolling center. VWF:RCo was determined aggregometrically using normal formalin-fixed platelets and 1.0 mg/mL ristocetin. Samples with VWF:RCo too low to detect reliably were set at 3 IU/dL, the lower limit of detection. All IC and selected AFM, UFM, and HC plasma samples were assessed for VWF collagen binding (VWF:CB) at P3.13B All FVIII/VWF activities were expressed as international units per deciliter with reference to a normal plasma (NP) pool calibrated against the 4th World Health Organization (WHO) international plasma standard for FVIII/VWF (97/586) (National Institute for Biological Standards and Control ([NIBSC], Potters Bar, United Kingdom). Ristocetin-induced platelet agglutination (RIPA) using 2 ristocetin final concentrations (0.5 mg/mL and 1.25 mg/mL) was measured in platelet-rich plasma obtained at enrollment from each IC to exclude type 2B VWD.
Multimeric pattern was determined for all 744 family members and 70 healthy controls centrally at P7 by comparison with that of normal plasma (NP) as described.14 Family samples were analyzed together on a gel. Absolute and relative deviations from a normal distribution (loss of multimers or supranormal multimers) were detected using low-resolution 1.2% agarose gels and designated as abnormal. Structural multimer defects were detected by altered migration of individual oligomers using medium resolution (1.6% agarose) gels and were also designated abnormal. Normal multimers (NMs) lacked these alterations. Densitometric analysis ensured reproducibility and comparability of multimer analysis. Evaluation of all multimer patterns was performed by a single individual without knowledge of mutations upon completion of all analyses. Binding of FVIII to VWF (VWF:FVIIIB) was assessed in ICs and selected family members as percentages compared with NP (100%).15
Genotype analysis
The VWF gene of each IC was analyzed for candidate mutations. DNA samples were distributed among 7 laboratories. In 11 ICs, insufficient DNA was available, and an AFM was substituted. Seven of these AFMs shared 2 alleles with the IC, and 4 shared 1 allele. Mutations identified in AFMs were confirmed in ICs. One family (P8F6) had insufficient DNA and was not screened. Two kilobases of 5′ sequence (from nucleotide 1/1 of the sequence described by Mancuso et al6 ), exons 1 to 52, and intron/exon boundaries were analyzed, for a total of up to 21 kb, using 5 different primer sets (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). P1 and P12 used conformation-sensitive gel electrophoresis (CSGE),16 P3 used single-strand conformation polymorphism (SSCP) analysis,17 and P9 used denaturing high-pressure liquid chromatography (DHPLC) where amplicons were separated on a Helix DVB 50 × 3 mm column (Varian, Palo Alto, CA) at temperatures suggested by the Melt Program.18A Sequence alterations were characterized in each case by DNA sequencing. P2, P7, and P10 used only direct sequencing as described.18B Mutation-negative cases were reviewed by the steering committee following phenotypic and linkage analysis. When it was considered likely that a mutation would be identified, a second laboratory undertook a second screen using a different analysis method. A few individuals were screened for a third time by DNA sequencing. Mutations identified in ICs were sought in all family members. The ISTH-SSC on VWF website was referred to for previously reported mutations and polymorphisms.8 A number of novel sequence changes were sought in 100 HCs from the same partner to exclude single-nucleotide polymorphisms (SNPs.)
Linkage analysis was based on cosegregation of short tandem repeat polymorphisms in the VWF gene and diagnosis of VWD as described.12 The following definitions were used: complete cosegregation: pedigrees with no phenocopies and fully penetrant, where a phenocopy is an individual diagnosed with VWD but who does not share the same VWF allele as other diagnosed individuals; incomplete cosegregation: pedigrees with either phenocopies or nonpenetrances; and noninformative: usually due to small pedigree size.
All study participants were genotyped by P1 for A2, B, and O alleles to determine A1/A2/B/O genotype using allelic discrimination, as described.12
Exon 21 skipping resulting from splicing mutations was demonstrated by in vitro splicing of a 2.5-kb minigene comprising the 3′ end of intron 20, exon and intron 21, exon 22, and the 5′ part of intron 22 cloned into an exon-trapping vector pSPL3 (exon trapping kit, Invitrogen, Karlsruhe, Germany) and transfection of HEK 293 EBNA cells in comparison with the wild-type (wt) minigene, according to the manufacturer's instructions.
Statistical analysis
Data analysis (median values, Mann-Whitney test) was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA). SPSS for Windows version 12.0.1 (SPSS, Chicago, IL) was used for calculating odds ratios.
Results
VWD cohort
A total of 154 ICs, 278 AFMs, and 312 UFMs belonging to 154 families were enrolled into the study. In addition, 1166 healthy controls (approximately 100 per center; range, 74-105) were also recruited. The phenotypic data for the cohort are shown in Table 1. Although the cohort represents a heterogeneous group of patients, VWF:Ag, VWF:RCo, and FVIII:C levels were similar when comparing ICs with AFMs and UFMs with HCs. This was less apparent for VWF:CB measurements, although an incomplete cohort was available for analysis in this case.
Phenotypic data for the cohort
| . | FVIII:C . | VWF:RCo . | VWF:Ag . | VWF:CB . | VWF:RCo/VWF:Ag . | Bleeding score . |
|---|---|---|---|---|---|---|
| IC | ||||||
| Arithmetic median, IU/dL (25th to 75th percentiles) | 58 (31-80) | 35 (13-53) | 38 (21-51) | 39 (20-60) | 0.92 (0.64-1.12) | 9.0 (4.5-13.0) |
| No. of individuals | 150 | 149 | 150 | 149 | 149 | 150 |
| AFM | ||||||
| Arithmetic median, IU/dL (25th to 75th percentiles) | 65 (34-89) | 36 (13-55) | 37 (22-56) | 52 (24-80) | 0.93 (0.61-1.14) | 5.0 (2.0-8.0) |
| No. of individuals | 273 | 270 | 271 | 87 | 270 | 274 |
| UFM | ||||||
| Arithmetic median, IU/dL (25th to 75th percentiles) | 103 (78-135) | 88 (65-113) | 93 (71-119) | 100 (78-121) | 0.95 (0.80-1.16) | 0.0 (−1.0-1.0) |
| No. of individuals | 300 | 295 | 295 | 111 | 295 | 303 |
| HC | ||||||
| Arithmetic median, IU/dL (25th to 75th percentiles) | 108 (88-133) | 94 (75-117) | 97 (76-121) | 130 (103-150) | 1.00 (0.85-1.17) | −1.0 (−1.0-0.0) |
| 2.5th to 97.5th percentiles | 57-198 | 47-194 | 48-167 | 66-226 | 0.59-1.62 | −3.0-3.0 |
| No. of individuals | 1160 | 1156 | 1156 | 99 | 1156 | 195 |
| . | FVIII:C . | VWF:RCo . | VWF:Ag . | VWF:CB . | VWF:RCo/VWF:Ag . | Bleeding score . |
|---|---|---|---|---|---|---|
| IC | ||||||
| Arithmetic median, IU/dL (25th to 75th percentiles) | 58 (31-80) | 35 (13-53) | 38 (21-51) | 39 (20-60) | 0.92 (0.64-1.12) | 9.0 (4.5-13.0) |
| No. of individuals | 150 | 149 | 150 | 149 | 149 | 150 |
| AFM | ||||||
| Arithmetic median, IU/dL (25th to 75th percentiles) | 65 (34-89) | 36 (13-55) | 37 (22-56) | 52 (24-80) | 0.93 (0.61-1.14) | 5.0 (2.0-8.0) |
| No. of individuals | 273 | 270 | 271 | 87 | 270 | 274 |
| UFM | ||||||
| Arithmetic median, IU/dL (25th to 75th percentiles) | 103 (78-135) | 88 (65-113) | 93 (71-119) | 100 (78-121) | 0.95 (0.80-1.16) | 0.0 (−1.0-1.0) |
| No. of individuals | 300 | 295 | 295 | 111 | 295 | 303 |
| HC | ||||||
| Arithmetic median, IU/dL (25th to 75th percentiles) | 108 (88-133) | 94 (75-117) | 97 (76-121) | 130 (103-150) | 1.00 (0.85-1.17) | −1.0 (−1.0-0.0) |
| 2.5th to 97.5th percentiles | 57-198 | 47-194 | 48-167 | 66-226 | 0.59-1.62 | −3.0-3.0 |
| No. of individuals | 1160 | 1156 | 1156 | 99 | 1156 | 195 |
P values using the Mann-Whitney test are as follows:
FVIII:C IC versus AFM, P = nonsignificant (NS); versus UFM and HC, P < .001. UFM versus HC, P = .027.
VWF:RCo IC versus AFM, P = NS; versus UFM and HC, P < .001. UFM versus HC, P = .001.
VWF:Ag IC versus AFM, P = NS; versus UFM and HC, P < .001. UFM versus HC, P = NS.
VWF:CB IC versus AFM, P = .009; versus UFM and HC, P < .001. UFM versus HC, P < .001.
VWF:RCo/VWF:Ag IC versus AFM, P = NS; versus UFM, P = .015; versus HC, P < .001. UFM versus HC, P = .01.
Bleeding score (BS) IC versus AFM, UFM, and HC, P < .001. UFM versus HC, P < .001.
IC indicates index case; AFM, affected family member; UFM, unaffected family member; HC, healthy control.
Phenotype determination by multimer profile and VWF:FVIIIB
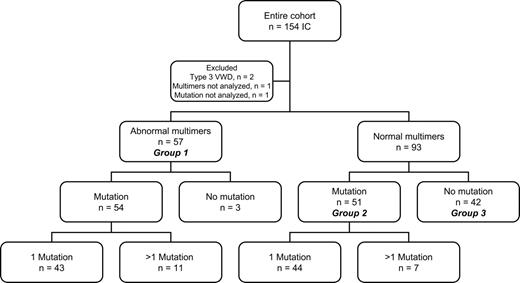
An abnormal multimer (AbM) profile was defined as either one in which there was a reduction in high molecular weight multimers (HMWMs) or a variation in the overall multimer profile when compared with normal pooled plasma. Of the 154 ICs, 3 were excluded, 2 because of very low levels of VWF (no multimers seen) and the third because of an insufficient sample. Additionally, 1 IC was excluded because of insufficient DNA for mutation analysis. All family members of these 4 ICs were also excluded from this analysis. Of the remaining 150 ICs, 93 showed normal multimers (NMs) using both low- and medium-resolution gels and 57 demonstrated abnormal multimers (AbMs). All further analyses are based on these 150 ICs. Details of specific types of AbM patterns will be reported in the future. Two of 150 cases had a classical type 2A VWD pattern (1.3%) and were shown to have the mutation R1374H, previously reported in type 1 platelet-discordant (type 2 according to the 1994 classification)4 and 2M VWD.19,20 Absence of increased sensitivity to ristocetin in the RIPA assay at initial IC screening excluded any phenotypic type 2B VWD. Multimer analysis showed that a substantial part of the cohort (38% of ICs, 57 of 150) had an AbM pattern that formally does not fit into the accepted criteria for type 1 VWD. Multimer profile was subsequently used as the major discriminator for analysis of the cohort. Analyses of VWF:Ag, VWF:RCo, FVIII:C, and VWF:CB levels in the 2 multimer groups (NM and AbM) showed significant differences between levels (Table 2), with all parameters significantly lower in the AbM group than the NM group (P < .001 for each pairwise comparison). Additionally, the median ratio of VWF:RCo to VWF:Ag was significantly lower (P < .001) in the AbM ICs (0.53) than in the NM ICs (0.96).
Number of mutations and median phenotype in 150 index cases with type 1 VWD
| Multimer pattern . | No. of ICs per group . | No. of mutations per IC . | No. of mutations per group . | FVIII:C,† IU/dL . | VWF:RCo,† IU/dL . | VWF:Ag,† IU/dL . | VWF:CB†, IU/dL . | VWF:RCo/VWF:Ag,† . | % blood group O . | Bleeding score† . |
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1* | ||||||||||
| AbM | 57 | 0, 1, or > 1 | 65 | 34 | 10 | 19 | 19 | 0.53 | 60 | 10.0 |
| AbM | 54 | 1 or > 1 | 65 | 33 | 10 | 19 | 17 | 0.53 | 61 | 10.0 |
| AbM | 43 | 1 | 43 | 33 | 10 | 20 | 19 | 0.50 | 61 | 10.0 |
| AbM | 11 | > 1 | 22 | 34 | 11 | 13 | 15 | 0.85 | 64 | 12.0 |
| AbM | 3 | 0 | 0 | 39 | 28 | 36 | 20 | 0.78 | 33 | 6.0 |
| Groups 2+3 | ||||||||||
| NM | 93 | 0, 1, or > 1 | 59 | 70 | 45 | 47 | 54 | 0.96 | 67 | 8.0 |
| Group 2 | ||||||||||
| NM | 51 | 1 or > 1 | 59 | 66 | 42 | 45 | 48 | 0.93 | 60 | 8.0 |
| NM | 44 | 1 | 44 | 67 | 43 | 45 | 49 | 0.96 | 61 | 7.5 |
| NM | 7 | > 1 | 15 | 30 | 38 | 47 | 38 | 0.81 | 71 | 10.0 |
| Group 3 | ||||||||||
| NM | 42 | 0 | 0 | 77 | 51 | 49 | 57 | 1.04 | 76 | 8.0 |
| All | ||||||||||
| AbM or NM | 150 | 0, 1, or > 1 | 124 | 58 | 35 | 38 | 39 | 0.92 | 65 | 9.0 |
| Multimer pattern . | No. of ICs per group . | No. of mutations per IC . | No. of mutations per group . | FVIII:C,† IU/dL . | VWF:RCo,† IU/dL . | VWF:Ag,† IU/dL . | VWF:CB†, IU/dL . | VWF:RCo/VWF:Ag,† . | % blood group O . | Bleeding score† . |
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1* | ||||||||||
| AbM | 57 | 0, 1, or > 1 | 65 | 34 | 10 | 19 | 19 | 0.53 | 60 | 10.0 |
| AbM | 54 | 1 or > 1 | 65 | 33 | 10 | 19 | 17 | 0.53 | 61 | 10.0 |
| AbM | 43 | 1 | 43 | 33 | 10 | 20 | 19 | 0.50 | 61 | 10.0 |
| AbM | 11 | > 1 | 22 | 34 | 11 | 13 | 15 | 0.85 | 64 | 12.0 |
| AbM | 3 | 0 | 0 | 39 | 28 | 36 | 20 | 0.78 | 33 | 6.0 |
| Groups 2+3 | ||||||||||
| NM | 93 | 0, 1, or > 1 | 59 | 70 | 45 | 47 | 54 | 0.96 | 67 | 8.0 |
| Group 2 | ||||||||||
| NM | 51 | 1 or > 1 | 59 | 66 | 42 | 45 | 48 | 0.93 | 60 | 8.0 |
| NM | 44 | 1 | 44 | 67 | 43 | 45 | 49 | 0.96 | 61 | 7.5 |
| NM | 7 | > 1 | 15 | 30 | 38 | 47 | 38 | 0.81 | 71 | 10.0 |
| Group 3 | ||||||||||
| NM | 42 | 0 | 0 | 77 | 51 | 49 | 57 | 1.04 | 76 | 8.0 |
| All | ||||||||||
| AbM or NM | 150 | 0, 1, or > 1 | 124 | 58 | 35 | 38 | 39 | 0.92 | 65 | 9.0 |
P values using the Mann-Whitney test are as follows:
VWF:RCo group 1 versus 2 and group 1 versus 3, P < .001; group 2 versus 3, P = .033.
VWF:Ag group 1 versus 2 and group 1 versus 3, P < .001; group 2 versus 3, P = .127.
BS group 1 versus 2, P = .252; group 1 versus 3, P = .086; group 2 versus 3, P = .586.
The groups represent categories of ICs: 1, abnormal multimers (AbM); 2, normal multimers (NM) with a mutation; 3, normal multimers (NM) without a mutation.
Phenotypic data are indicated as the median.
Nine of 150 ICs had reduced binding of FVIII to VWF; 6 showed intermediate VWF:FVIIIB (mean binding, 55%); while 3, representing 2% of ICs, had markedly reduced binding (less than 10%), the phenotypic hallmark of type 2N VWD.
Mutation analysis
Candidate mutations were sought in each IC and were identified in 105 (70%). Eighty-seven had 1 mutation, 17 had 2, and 1 had 3. A total of 124 candidate mutations were identified, of which 75 were different and 54 were novel. Patients were divided into 3 groups based on multimers and mutations (Figure 1). Group 1 comprised 57 ICs with abnormal multimers, group 2 comprised 51 ICs with NMs and at least 1 candidate mutation detected, while group 3 comprised 42 ICs with NMs and no mutation detected.
Group 1
Mutations were identified in all but 3 ICs with AbMs. Because repeated sequence analysis using different primer sets failed to identify a candidate mutation in these ICs, a missed heterozygous mutation in the region analyzed was unlikely. A single haplotype was associated with low VWF levels and AbMs in each of the families, and affected members showed a consistent multimer abnormality in each pedigree. Exon skipping resulting from undetected intronic mutations could explain their multimer pattern. However, analysis of platelet RNA of 1 IC and their AFMs did not reveal any abnormality. Heterozygous complete gene deletions were excluded by heterozygosity for at least 1 short tandem repeat (STR) in each of these 3 ICs. Partial deletions were not excluded.
Candidate mutations identified in group 1 are summarized in Table 3(43 ICs with AbMs and a single mutation) and Table 4(11 ICs with 2 mutations). Missense mutations were present in 39 of 43 ICs with a single mutation. Among these 43 ICs, 24 different missense mutations affecting 20 codons were identified, of which 10 predicted either loss or gain of a cysteine residue. One IC had an in-frame deletion, and 3 had different splice mutations each leading to skipping of exon 21, demonstrated by in vitro splicing using an exon-trapping kit. Mutations seen in this group, with the exception of a lone occurrence of R854Q, were all located carboxyl to the D′ domain in mature VWF; none were in the propeptide (Figure 2).
VWF mutations and phenotype in 43 group 1 index cases with abnormal multimers and a single mutation
| IC* . | Type of mutation . | Exon/intron . | Nucleotide change† . | Amino acid change . | No. of ICs with this mutation . | FVIII:C, IU/dL . | VWF:RCo, IU/dL . | VWF:Ag, IU/dL . | VWF:CB, IU/dL . | VWF:RCo/VWF:Ag . | Cosegregation‡ (no. of families) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P6F1II1§ | Missense | 20 | 2561G>A40 | R854Q | 1 | 25 | 23 | 32 | 15 | 0.72 | 1 |
| P7F9I1 | Splice | Int20 | 2686−2A>G†‖ | Exon 21 skip | 1 | 85 | 36 | 44 | 27 | 0.82 | 1 |
| P7F13II1 | Splice | Int21 | 2820+1G>C†‖ | Exon 21 skip | 1 | 68 | 7 | 21 | 15 | 0.33 | 1 |
| P5F8I3 | Splice | Int21 | 2820+1G>A†‖ | Exon 21 skip | 1 | 115 | 38 | 32 | 33 | 1.19 | 1 |
| Range¶ | Missense | 26 | 3388T>C† | C1130R | 3 | 13-15 | 7-13 | 12-22 | 6-8 | 0.58-0.59 | 1 (1), 2 (1), 3 (1) |
| P9F5II2 | Missense | 26 | 3388T>G† | C1130G | 1 | 8 | 10 | 10 | 26 | 1.00 | 1 |
| Range | Missense | 26 | 3389C>T30 | C1130F | 2 | 10-22 | 3-4 | 8 | 2-6 | 0.38-0.50 | 1 (1), 3 (1) |
| P9F1I2 | Missense | 26 | 3430T>G† | W1144G | 1 | 24 | 12 | 31 | 12 | 0.39 | 1 |
| Range | Missense | 27 | 3614G>A26 | R1205H | 5 | 7-19# | 3-10 | 5-10 | 4-6 | 0.40-1.43 | 1 (2), 2 (2), 3 (1) |
| P3F1II1 | Deletion | 28 | 3831-3833delCCT† | D1277−E78delinsL | 1 | 22 | 3 | 18 | 20 | 0.17 | 1 |
| P6F3II1 | Missense | 28 | 3853T>C† | S1285P | 1 | 27 | 3 | 16 | 8 | 0.19 | 1 |
| P6F13II1 | Missense | 28 | 3920T>C†‖ | L1307P | 1 | 33 | 7 | 16 | 6 | 0.44 | 1 |
| Range | Missense | 28 | 3943C>T39 | R1315C | 4 | 20-62 | 3-10 | 7-25 | 6-25 | 0.40-0.45 | 1 (2), 2 (2) |
| P5F4II1 | Missense | 28 | 4024C>T† | R1342C | 1 | 43 | 3 | 20 | 28 | 0.15 | 1 |
| Range | Missense | 28 | 4120C>T20 | R1374C | 3 | 30-34 | 3-7 | 10-45 | 10-19 | 0.07-0.70 | 1 (3) |
| Range | Missense | 28 | 4121G>A20 | R1374H | 2 | 48-79 | 10-16 | 29-74 | 22-23 | 0.22-0.34 | 2 (1), 3 (1) |
| Range | Missense | 28 | 4244G>A† | G1415D | 2 | 13-18 | 3-14 | 14 | 10-15 | 0.21-1.00 | 1 (1), 2 (1) |
| P12F10II1 | Missense | 28 | 4247T>A† | I1416N | 1 | 43 | 3 | 19 | 20 | 0.16 | 1 |
| P2F22I2 | Missense | 32 | 5465T>G† | V1822G | 1 | 35 | 26 | 19 | 45 | 1.37 | 3 |
| P5F9II2 | Missense | 38 | 6620T>C8 | L2207P | 1 | 71 | 19 | 26 | 35 | 0.73 | 2 |
| P12F12II2 | Missense | 38 | 6769T>A† | C2257S | 1 | 70 | 42 | 29 | 30 | 1.45 | 2 |
| P5F2II2 | Missense | 40 | 6911G>A† | C2304Y | 1 | 42 | 19 | 20 | 37 | 0.95 | 3 |
| P2F16II2 | Missense | 42 | 7239C>T8.42 | C2362F | 1 | 88 | 34 | 35 | 39 | 0.97 | 1 |
| P5F10II1 | Missense | 43 | 7321G>T† | G2441C | 1 | 61 | 20 | 22 | 26 | 0.91 | 1 |
| Range | Missense | 43 | 7390C>T† | R2464C | 2 | 32-52 | 25-30 | 25-33 | 37-50 | 0.91-1.00 | 1 (1), 3 (1) |
| P7F22I2 | Missense | 43 | 7430G>A† | C2477Y | 1 | 68 | 33 | 38 | 43 | 0.87 | 2 |
| P10F2I1 | Missense | 43 | 7430G>C† | C2477S | 1 | 58 | 40 | 28 | 31 | 1.43 | 1 |
| P3F11II1 | Missense | 45 | 7559A>C† | Q2520P | 1 | 48 | 27 | 19 | 19 | 1.42 | 2 |
| IC* . | Type of mutation . | Exon/intron . | Nucleotide change† . | Amino acid change . | No. of ICs with this mutation . | FVIII:C, IU/dL . | VWF:RCo, IU/dL . | VWF:Ag, IU/dL . | VWF:CB, IU/dL . | VWF:RCo/VWF:Ag . | Cosegregation‡ (no. of families) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P6F1II1§ | Missense | 20 | 2561G>A40 | R854Q | 1 | 25 | 23 | 32 | 15 | 0.72 | 1 |
| P7F9I1 | Splice | Int20 | 2686−2A>G†‖ | Exon 21 skip | 1 | 85 | 36 | 44 | 27 | 0.82 | 1 |
| P7F13II1 | Splice | Int21 | 2820+1G>C†‖ | Exon 21 skip | 1 | 68 | 7 | 21 | 15 | 0.33 | 1 |
| P5F8I3 | Splice | Int21 | 2820+1G>A†‖ | Exon 21 skip | 1 | 115 | 38 | 32 | 33 | 1.19 | 1 |
| Range¶ | Missense | 26 | 3388T>C† | C1130R | 3 | 13-15 | 7-13 | 12-22 | 6-8 | 0.58-0.59 | 1 (1), 2 (1), 3 (1) |
| P9F5II2 | Missense | 26 | 3388T>G† | C1130G | 1 | 8 | 10 | 10 | 26 | 1.00 | 1 |
| Range | Missense | 26 | 3389C>T30 | C1130F | 2 | 10-22 | 3-4 | 8 | 2-6 | 0.38-0.50 | 1 (1), 3 (1) |
| P9F1I2 | Missense | 26 | 3430T>G† | W1144G | 1 | 24 | 12 | 31 | 12 | 0.39 | 1 |
| Range | Missense | 27 | 3614G>A26 | R1205H | 5 | 7-19# | 3-10 | 5-10 | 4-6 | 0.40-1.43 | 1 (2), 2 (2), 3 (1) |
| P3F1II1 | Deletion | 28 | 3831-3833delCCT† | D1277−E78delinsL | 1 | 22 | 3 | 18 | 20 | 0.17 | 1 |
| P6F3II1 | Missense | 28 | 3853T>C† | S1285P | 1 | 27 | 3 | 16 | 8 | 0.19 | 1 |
| P6F13II1 | Missense | 28 | 3920T>C†‖ | L1307P | 1 | 33 | 7 | 16 | 6 | 0.44 | 1 |
| Range | Missense | 28 | 3943C>T39 | R1315C | 4 | 20-62 | 3-10 | 7-25 | 6-25 | 0.40-0.45 | 1 (2), 2 (2) |
| P5F4II1 | Missense | 28 | 4024C>T† | R1342C | 1 | 43 | 3 | 20 | 28 | 0.15 | 1 |
| Range | Missense | 28 | 4120C>T20 | R1374C | 3 | 30-34 | 3-7 | 10-45 | 10-19 | 0.07-0.70 | 1 (3) |
| Range | Missense | 28 | 4121G>A20 | R1374H | 2 | 48-79 | 10-16 | 29-74 | 22-23 | 0.22-0.34 | 2 (1), 3 (1) |
| Range | Missense | 28 | 4244G>A† | G1415D | 2 | 13-18 | 3-14 | 14 | 10-15 | 0.21-1.00 | 1 (1), 2 (1) |
| P12F10II1 | Missense | 28 | 4247T>A† | I1416N | 1 | 43 | 3 | 19 | 20 | 0.16 | 1 |
| P2F22I2 | Missense | 32 | 5465T>G† | V1822G | 1 | 35 | 26 | 19 | 45 | 1.37 | 3 |
| P5F9II2 | Missense | 38 | 6620T>C8 | L2207P | 1 | 71 | 19 | 26 | 35 | 0.73 | 2 |
| P12F12II2 | Missense | 38 | 6769T>A† | C2257S | 1 | 70 | 42 | 29 | 30 | 1.45 | 2 |
| P5F2II2 | Missense | 40 | 6911G>A† | C2304Y | 1 | 42 | 19 | 20 | 37 | 0.95 | 3 |
| P2F16II2 | Missense | 42 | 7239C>T8.42 | C2362F | 1 | 88 | 34 | 35 | 39 | 0.97 | 1 |
| P5F10II1 | Missense | 43 | 7321G>T† | G2441C | 1 | 61 | 20 | 22 | 26 | 0.91 | 1 |
| Range | Missense | 43 | 7390C>T† | R2464C | 2 | 32-52 | 25-30 | 25-33 | 37-50 | 0.91-1.00 | 1 (1), 3 (1) |
| P7F22I2 | Missense | 43 | 7430G>A† | C2477Y | 1 | 68 | 33 | 38 | 43 | 0.87 | 2 |
| P10F2I1 | Missense | 43 | 7430G>C† | C2477S | 1 | 58 | 40 | 28 | 31 | 1.43 | 1 |
| P3F11II1 | Missense | 45 | 7559A>C† | Q2520P | 1 | 48 | 27 | 19 | 19 | 1.42 | 2 |
ICs are indicated by their study ID; when a mutation is present in more than 1 IC, the phenotypic data are indicated as “range.”
Novel mutations not previously reported on the ISTH VWF database.
Cosegregation of VWD with the VWF gene12 ; 1 indicates complete cosegregation; 2, incomplete cosegregation; 3, not informative.
Reduced VWF:FVIIIB.
Excluded as an SNP by absence from 100 healthy controls.
Reduced VWF:FVIIIB in only 1 of the 3 ICs.
One high outlier was excluded from the range of values shown but was included in all analyses.
VWF mutations and phenotype in 11 group 1 index cases with abnormal multimers and 2 mutations
| Type of mutation by IC* . | Exon . | Nucleotide change† . | Amino acid change . | No. of ICs with this mutation . | FVIII:C, IU/dL . | VWF:RCo, IU/dL . | VWF:Ag, IU/dL . | VWF:CB, IU/dL . | VWF:RCo/VWF:Ag . | Cosegregation‡ (no. of families) . |
|---|---|---|---|---|---|---|---|---|---|---|
| P1F4II1§ | 1 | 34 | 25 | 19 | 15 | 1.32 | 1 | |||
| Missense | 28 | 4751A>G21 | Y1584C | |||||||
| Missense | 43 | 7390C>T† | R2464C | |||||||
| P2F1II1§ | 1 | 7 | 3 | 9 | 17 | 0.33 | 3 | |||
| Deletion | 28 | 4449delG† | L1481fs | |||||||
| Missense | 28 | 4751A>G21 | Y1584C | |||||||
| Range‖ | 3 | 3-14 | 3-6 | 6-14 | 5-18 | 0.43-0.75 | 1 (2), 3 (1) | |||
| Missense | 17 | 2220G>A43 | M740I | |||||||
| Missense | 27 | 3614G>A43 | R1205H | |||||||
| P2F6II1§¶ | 1 | 55 | 11 | 13 | ND | 0.85 | 1 | |||
| Missense | 26 | 3389G>T30 | C1130F | |||||||
| Missense | 49 | 7988G>C† | R2663P | |||||||
| P3F7II1§ | 1 | 12 | 3 | 3 | 6 | 1.00 | 2 | |||
| Nonsense | 31 | 5335C>T† | R1779X | |||||||
| Missense | 43 | 7405T>C† | S2469P | |||||||
| P3F13I1‖ | 1 | 78 | 13 | 42 | 24 | 0.31 | 1 | |||
| Missense | 21 | 2771G>A44 | R924Q | |||||||
| Missense | 28 | 3944G>T† | R1315L | |||||||
| P4F4I1§ | 1 | 34 | 3 | 10 | 14 | 0.30 | 1 | |||
| Missense | 28 | 4120C>T20 | R1374C | |||||||
| Missense | 37 | 6433C>T† | P2145S | |||||||
| P5F3II2‖# | 1 | 65 | 11 | 27 | 42 | 0.41 | 1 | |||
| Missense | 28 | 3797C>T22 | P1266L | |||||||
| Missense | 28 | 3944G>A† | R1315H | |||||||
| P10F10II1‖ | 1 | 25 | 13 | 14 | 7 | 0.93 | 3 | |||
| Missense | 26 | 3437A>G† | Y1146C | |||||||
| Missense | 28 | 4133C>T† | S1378F |
| Type of mutation by IC* . | Exon . | Nucleotide change† . | Amino acid change . | No. of ICs with this mutation . | FVIII:C, IU/dL . | VWF:RCo, IU/dL . | VWF:Ag, IU/dL . | VWF:CB, IU/dL . | VWF:RCo/VWF:Ag . | Cosegregation‡ (no. of families) . |
|---|---|---|---|---|---|---|---|---|---|---|
| P1F4II1§ | 1 | 34 | 25 | 19 | 15 | 1.32 | 1 | |||
| Missense | 28 | 4751A>G21 | Y1584C | |||||||
| Missense | 43 | 7390C>T† | R2464C | |||||||
| P2F1II1§ | 1 | 7 | 3 | 9 | 17 | 0.33 | 3 | |||
| Deletion | 28 | 4449delG† | L1481fs | |||||||
| Missense | 28 | 4751A>G21 | Y1584C | |||||||
| Range‖ | 3 | 3-14 | 3-6 | 6-14 | 5-18 | 0.43-0.75 | 1 (2), 3 (1) | |||
| Missense | 17 | 2220G>A43 | M740I | |||||||
| Missense | 27 | 3614G>A43 | R1205H | |||||||
| P2F6II1§¶ | 1 | 55 | 11 | 13 | ND | 0.85 | 1 | |||
| Missense | 26 | 3389G>T30 | C1130F | |||||||
| Missense | 49 | 7988G>C† | R2663P | |||||||
| P3F7II1§ | 1 | 12 | 3 | 3 | 6 | 1.00 | 2 | |||
| Nonsense | 31 | 5335C>T† | R1779X | |||||||
| Missense | 43 | 7405T>C† | S2469P | |||||||
| P3F13I1‖ | 1 | 78 | 13 | 42 | 24 | 0.31 | 1 | |||
| Missense | 21 | 2771G>A44 | R924Q | |||||||
| Missense | 28 | 3944G>T† | R1315L | |||||||
| P4F4I1§ | 1 | 34 | 3 | 10 | 14 | 0.30 | 1 | |||
| Missense | 28 | 4120C>T20 | R1374C | |||||||
| Missense | 37 | 6433C>T† | P2145S | |||||||
| P5F3II2‖# | 1 | 65 | 11 | 27 | 42 | 0.41 | 1 | |||
| Missense | 28 | 3797C>T22 | P1266L | |||||||
| Missense | 28 | 3944G>A† | R1315H | |||||||
| P10F10II1‖ | 1 | 25 | 13 | 14 | 7 | 0.93 | 3 | |||
| Missense | 26 | 3437A>G† | Y1146C | |||||||
| Missense | 28 | 4133C>T† | S1378F |
ND indicates not done.
ICs are indicated by their study ID; when a mutation is present in more than 1 IC, the phenotypic data are indicated as “range.”
Novel mutations.
Cosegregation of VWD with the VWF gene12 ; 1 indicates complete cosegregation; 2, incomplete cosegregation; 3, not informative.
Compound heterozygous inheritance of the 2 mutations.
Allelic inheritance of the 2 mutations.
Reduced VWF:FVIIIB.
P1266L is the result of gene conversion with the VWF pseudogene VWFP. The IC also has 3789G>A; S1269.
Missense mutations in index cases with normal and abnormal multimers. Pre-pro VWF, showing exons and protein domains encoded. The upper panel shows singly occurring mutations resulting in an abnormal multimer profile. The lower panel shows singly occurring mutations resulting in a normal multimer profile. Three substitutions in bold displayed both normal and abnormal multimers in different index cases.
Missense mutations in index cases with normal and abnormal multimers. Pre-pro VWF, showing exons and protein domains encoded. The upper panel shows singly occurring mutations resulting in an abnormal multimer profile. The lower panel shows singly occurring mutations resulting in a normal multimer profile. Three substitutions in bold displayed both normal and abnormal multimers in different index cases.
Eleven ICs with AbMs had 2 mutations each, 10 had 2 missense changes, while 1 had a missense plus a single nucleotide deletion. Pedigree analysis showed that 5 ICs were compound heterozygotes while 6 had 2 allelic mutations. Overall median VWF levels in group 1 ICs demonstrated very low VWF:RCo (10 IU/dL) with higher VWF:Ag (19 IU/dL). The presence of a second mutation reduced VWF:Ag to 13 IU/dL (Table 2).
Linkage analysis, reported separately,12 identified families that had dominantly inherited VWD linked to a single VWF allele. Thirty-four group 1 ICs (60%) showed complete cosegregation of VWD to VWF, whereas in families of 12 ICs, incomplete cosegregation was observed (not all family members possessing the familial candidate mutation were affected, and/or not all affected members possessed the familial mutation). Families of the remaining 11 ICs were not informative. Median VWF:RCo and BS were not significantly different in the ICs from families with VWD completely cosegregating with VWF compared with those incompletely cosegregating (VWF:RCo, 7 IU/dL versus 10 IU/dL and BS, 10 versus 11, respectively).
Group 2
Of 51 ICs with NMs and a mutation, 44 ICs had 1 candidate mutation identified (Table 5), while 7 had more than 1 mutation (Table 6). Six ICs had promoter region changes: 5 had a single sequence alteration, while 1 had 3. Twenty-two different single missense changes were identified; Y1584C21 alone was seen repeatedly, in 10 ICs. Only 8 ICs had a single mutation predicted to lead to a nonexpressed (null) allele, comprising 3 splice site changes, 2 deletions and 1 insertion leading to frameshifts, plus 2 nonsense mutations. VWF:RCo and VWF:Ag in these 8 ICs ranged from 29 to 53 and 26 to 55, respectively; median values were 44 IU/dL for VWF:RCo and 39 IU/dL for VWF:Ag. Missense changes were seen throughout VWF, from G19R close to the signal peptide cleavage site to P2722A in the CK domain. One third of the group 2 missense changes were between the D4 and CK VWF domains, where few mutations have been reported previously. Among these, only 4 mutations affected a cysteine residue: Y1584C, R1379C, C2304Y, and C2693Y. Four group 2 ICs had a VWF:RCo of at least 60 IU/dL at recruitment. Bleeding scores were between 3 and 20 for these individuals, and bleeding symptoms may have influenced diagnosis. Six group 2 ICs had 2 mutations: 5 had 2 missense changes, while the sixth had a missense plus a splice site change. Four of the 6 were compound heterozygotes, while 2 had allelic changes (Table 6). A diagrammatic comparison of singly occurring missense mutations in groups 1 and 2 is shown in Figure 2. Only 3 mutations were observed to occur in each group (R854Q, R1205H, and C2304Y).
VWF mutations and phenotype in 44 group 2 index cases with normal multimers and a single mutation
| IC* . | Type of mutation . | Exon/intron . | Nucleotide change† . | Amino acid change . | No. of ICs with this mutation . | FVIII:C, IU/dL . | VWF:Rco, IU/dL . | VWF:Ag, IU/dL . | VWF:CB, IU/dL . | VWF:RCo/VWF:Ag . | Cosegregation‡ (no. of families) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Promoter | Promoter | −2520C>T† | — | 4 | 74 | 57 | 65 | 61 | 0.88 | 1 (1), 2 (1), 3 (2) |
| P12F2II1 | Promoter | Promoter | −1896C>T†‖ | — | 1 | 75 | 48 | 43 | 69 | 1.12 | 3 |
| P6F7II3 | Missense | 2 | 55G>A† | G19R | 1 | 80 | 20 | 30 | 20 | 0.67 | 2 |
| P9F17II2 | Missense | 5 | 478G>T† | G160W | 1 | 50 | 52 | 53 | 38 | 0.98 | 2 |
| P9F12II2 | Missense | 5 | 497A>T† | N166I | 1 | 55 | 29 | 38 | 22 | 0.76 | 1 |
| P2F15II1 | Splice | Int13 | 1534-3C>A‖8 | — | 1 | 132 | 53 | 55 | 62 | 0.96 | 3 |
| P7F20II1 | Missense | 15 | 1922C>T† | A641V | 1 | 56 | 60 | 97 | 77 | 0.62 | 2 |
| P9F16IV3 | Missense | 18 | 2313G>T† | M771I | 1 | 20 | 59 | 38 | 49 | 1.55 | 2 |
| P7F2II1 | Deletion | 18 | 2435delC45 | P812fs | 1 | 56 | 29 | 28 | 31 | 1.04 | 3 |
| P10F8II1¶ | Missense | 19 | 2446C>T40 | R816W | 1 | 14 | 52 | 49 | 57 | 1.06 | 3 |
| P12F6II2 | Splice | Int20 | 2686-1G>C† | Exon 21 skip | 1 | 159 | 42 | 51 | 60 | 0.82 | 1 |
| P5F1II3§ | Missense | 20 | 2561G>A40 | R854Q | 1 | 28 | 32 | 37 | 43 | 0.86 | 1 |
| P6F11II1 | Missense | 21 | 2771G>A44 | R924Q | 1 | 52 | 34 | 40 | 49 | 0.85 | 3 |
| P3F10II1 | Deletion | 23 | 3072delC† | S1024fs | 1 | 76 | 38 | 31 | 35 | 1.23 | 1 |
| P7F1I2 | Missense | 25 | 3281T>C† | I1094T | 1 | 148 | 74 | 71 | 76 | 1.04 | 3 |
| P2F4I2 | Missense | 27 | 3614G>A26 | R1205H | 1 | 11 | 3 | 3 | 4 | 1.00 | 1 |
| P12F4II12 | Insertion | 28 | 3839-3845ins7† | F1280fs | 1 | 66 | 35 | 29 | 59 | 1.21 | 1 |
| P12F7II2 | Missense | 28 | 4082T>C†‖ | L1361S | 1 | 86 | 14 | 90 | 35 | 0.16 | 1 |
| P10F3II1 | Missense | 28 | 4135C>T46 | R1379C | 1 | 107 | 60 | 59 | 62 | 1.02 | 3 |
| P10F1II2 | Missense | 28 | 4238C>T† | P1413L | 1 | 58 | 39 | 44 | 37 | 0.89 | 1 |
| P2F12II1 | Nonsense | 28 | 4423C>T† | Q1475X | 1 | 36 | 38 | 27 | 48 | 1.41 | 1 |
| P7F4II1 | Missense | 28 | 4747C>T8 | R1583W | 1 | 56 | 54 | 52 | 57 | 1.04 | 3 |
| Range | Missense | 28 | 4751A>G21 | Y1584C | 10 | 21-182 | 36-93 | 21-80 | 20-115 | 0.75-1.24 | 1 (5), 2 (2), 3 (3) |
| P7F11II1 | Splice | Int29 | 5170+10C>T† | — | 1 | 67 | 45 | 39 | 44 | 1.15 | 2 |
| P12F13II1 | Missense | 31 | 5321T>C†‖ | L1774S | 1 | 107 | 97 | 64 | 45 | 1.52 | 2 |
| P9F10II1 | Missense | 31 | 5380A>G† | K1794E | 1 | 70 | 26 | 34 | 30 | 0.76 | 1 |
| P10F6II1 | Missense | 40 | 6911G>A† | C2304Y | 1 | 150 | 61 | 68 | 71 | 0.90 | 1 |
| P3F91II1 | Missense | 40 | 6938G>A†‖ | R2313H | 1 | 70 | 52 | 61 | 60 | 0.85 | 2 |
| P5F6II3 | Missense | 45 | 7551G>A† | G2518S | 1 | 114 | 54 | 55 | 65 | 0.98 | 1 |
| P8F2II2 | Nonsense | 45 | 7630C>T47 | Q2544X | 1 | 75 | 40 | 26 | 43 | 1.54 | 3 |
| P6F16II1 | Missense | 49 | 8078G>A† | C2693Y | 1 | 69 | 64 | 46 | 42 | 1.39 | 1 |
| P9F11II1 | Missense | 51 | 8164C>G† | P2722A | 1 | 5 | 20 | 31 | 31 | 0.65 | 1 |
| IC* . | Type of mutation . | Exon/intron . | Nucleotide change† . | Amino acid change . | No. of ICs with this mutation . | FVIII:C, IU/dL . | VWF:Rco, IU/dL . | VWF:Ag, IU/dL . | VWF:CB, IU/dL . | VWF:RCo/VWF:Ag . | Cosegregation‡ (no. of families) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Promoter | Promoter | −2520C>T† | — | 4 | 74 | 57 | 65 | 61 | 0.88 | 1 (1), 2 (1), 3 (2) |
| P12F2II1 | Promoter | Promoter | −1896C>T†‖ | — | 1 | 75 | 48 | 43 | 69 | 1.12 | 3 |
| P6F7II3 | Missense | 2 | 55G>A† | G19R | 1 | 80 | 20 | 30 | 20 | 0.67 | 2 |
| P9F17II2 | Missense | 5 | 478G>T† | G160W | 1 | 50 | 52 | 53 | 38 | 0.98 | 2 |
| P9F12II2 | Missense | 5 | 497A>T† | N166I | 1 | 55 | 29 | 38 | 22 | 0.76 | 1 |
| P2F15II1 | Splice | Int13 | 1534-3C>A‖8 | — | 1 | 132 | 53 | 55 | 62 | 0.96 | 3 |
| P7F20II1 | Missense | 15 | 1922C>T† | A641V | 1 | 56 | 60 | 97 | 77 | 0.62 | 2 |
| P9F16IV3 | Missense | 18 | 2313G>T† | M771I | 1 | 20 | 59 | 38 | 49 | 1.55 | 2 |
| P7F2II1 | Deletion | 18 | 2435delC45 | P812fs | 1 | 56 | 29 | 28 | 31 | 1.04 | 3 |
| P10F8II1¶ | Missense | 19 | 2446C>T40 | R816W | 1 | 14 | 52 | 49 | 57 | 1.06 | 3 |
| P12F6II2 | Splice | Int20 | 2686-1G>C† | Exon 21 skip | 1 | 159 | 42 | 51 | 60 | 0.82 | 1 |
| P5F1II3§ | Missense | 20 | 2561G>A40 | R854Q | 1 | 28 | 32 | 37 | 43 | 0.86 | 1 |
| P6F11II1 | Missense | 21 | 2771G>A44 | R924Q | 1 | 52 | 34 | 40 | 49 | 0.85 | 3 |
| P3F10II1 | Deletion | 23 | 3072delC† | S1024fs | 1 | 76 | 38 | 31 | 35 | 1.23 | 1 |
| P7F1I2 | Missense | 25 | 3281T>C† | I1094T | 1 | 148 | 74 | 71 | 76 | 1.04 | 3 |
| P2F4I2 | Missense | 27 | 3614G>A26 | R1205H | 1 | 11 | 3 | 3 | 4 | 1.00 | 1 |
| P12F4II12 | Insertion | 28 | 3839-3845ins7† | F1280fs | 1 | 66 | 35 | 29 | 59 | 1.21 | 1 |
| P12F7II2 | Missense | 28 | 4082T>C†‖ | L1361S | 1 | 86 | 14 | 90 | 35 | 0.16 | 1 |
| P10F3II1 | Missense | 28 | 4135C>T46 | R1379C | 1 | 107 | 60 | 59 | 62 | 1.02 | 3 |
| P10F1II2 | Missense | 28 | 4238C>T† | P1413L | 1 | 58 | 39 | 44 | 37 | 0.89 | 1 |
| P2F12II1 | Nonsense | 28 | 4423C>T† | Q1475X | 1 | 36 | 38 | 27 | 48 | 1.41 | 1 |
| P7F4II1 | Missense | 28 | 4747C>T8 | R1583W | 1 | 56 | 54 | 52 | 57 | 1.04 | 3 |
| Range | Missense | 28 | 4751A>G21 | Y1584C | 10 | 21-182 | 36-93 | 21-80 | 20-115 | 0.75-1.24 | 1 (5), 2 (2), 3 (3) |
| P7F11II1 | Splice | Int29 | 5170+10C>T† | — | 1 | 67 | 45 | 39 | 44 | 1.15 | 2 |
| P12F13II1 | Missense | 31 | 5321T>C†‖ | L1774S | 1 | 107 | 97 | 64 | 45 | 1.52 | 2 |
| P9F10II1 | Missense | 31 | 5380A>G† | K1794E | 1 | 70 | 26 | 34 | 30 | 0.76 | 1 |
| P10F6II1 | Missense | 40 | 6911G>A† | C2304Y | 1 | 150 | 61 | 68 | 71 | 0.90 | 1 |
| P3F91II1 | Missense | 40 | 6938G>A†‖ | R2313H | 1 | 70 | 52 | 61 | 60 | 0.85 | 2 |
| P5F6II3 | Missense | 45 | 7551G>A† | G2518S | 1 | 114 | 54 | 55 | 65 | 0.98 | 1 |
| P8F2II2 | Nonsense | 45 | 7630C>T47 | Q2544X | 1 | 75 | 40 | 26 | 43 | 1.54 | 3 |
| P6F16II1 | Missense | 49 | 8078G>A† | C2693Y | 1 | 69 | 64 | 46 | 42 | 1.39 | 1 |
| P9F11II1 | Missense | 51 | 8164C>G† | P2722A | 1 | 5 | 20 | 31 | 31 | 0.65 | 1 |
— indicates not applicable.
ICs are indicated by their study ID; when a mutation is present in more than 1 IC, the phenotypic data are indicated as “range.‘
Novel mutations.
Cosegregation of VWD with the VWF gene12 ; 1 indicates complete cosegregation; 2, incomplete cosegregation; 3, not informative.
Reduced VWF:FVIIIB.
Excluded as an SNP by absence from 100 healthy controls.
Absent VWF:FVIIIB.
VWF mutations and phenotype in 7 group 2 index cases with normal multimers and more than 1 mutation
| Type of mutation by IC* . | Exon/intron . | Nucleotide change† . | Amino acid change . | FVIII:C, IU/dL . | VWF:Rco, IU/dL . | VWF:Ag, IU/dL . | VWF:CB, IU/dL . | VWF:RCo/VWF:Ag . | Cosegregation‡ . |
|---|---|---|---|---|---|---|---|---|---|
| P7F14I1§ | 93 | 60 | 58 | 95 | 1.03 | 2 | |||
| Promoter | Promoter | −3266G>C† | — | ||||||
| Promoter | Promoter | −2730C>T† | — | ||||||
| Promoter | Promoter | −2326T>G† | — | ||||||
| P2F3II1‖¶ | 9 | 44 | 59 | 38 | 0.75 | 3 | |||
| Splice | Int9 | 1109+2T>C† | — | ||||||
| Missense | 20 | 2561G>A40 | R854Q | ||||||
| P5F11II1#¶ | 40 | 38 | 47 | 59 | 0.81 | 2 | |||
| Missense | 20 | 2561G>A40 | R854Q | ||||||
| Missense | 28 | 4751A>G21 | Y1584C | ||||||
| P7F3II1‖¶ | 15 | 38 | 25 | 38 | 1.52 | 3 | |||
| Missense | 20 | 2561G>A40 | R854Q | ||||||
| Missense | 21 | 2771G>A44 | R924Q | ||||||
| P10F4I2§ | 30 | 3 | 27 | 22 | 0.11 | 3 | |||
| Missense | 20 | 2642T>G† | L881R | ||||||
| Missense | 28 | 4263C>G8 | N1421K | ||||||
| P6F5II1¶ | 18 | 7 | 11 | 6 | 0.64 | 1 | |||
| Missense | 21 | 2771G>A44 | R924Q | ||||||
| Missense | 27 | 3614G>A26 | R1205H | ||||||
| P7F16I1§ | 119 | 49 | 47 | 81 | 1.04 | 3 | |||
| Missense | 36 | 6187C>T8** | P2063S | ||||||
| Missense | 39 | 6859C>T† | R2287W |
| Type of mutation by IC* . | Exon/intron . | Nucleotide change† . | Amino acid change . | FVIII:C, IU/dL . | VWF:Rco, IU/dL . | VWF:Ag, IU/dL . | VWF:CB, IU/dL . | VWF:RCo/VWF:Ag . | Cosegregation‡ . |
|---|---|---|---|---|---|---|---|---|---|
| P7F14I1§ | 93 | 60 | 58 | 95 | 1.03 | 2 | |||
| Promoter | Promoter | −3266G>C† | — | ||||||
| Promoter | Promoter | −2730C>T† | — | ||||||
| Promoter | Promoter | −2326T>G† | — | ||||||
| P2F3II1‖¶ | 9 | 44 | 59 | 38 | 0.75 | 3 | |||
| Splice | Int9 | 1109+2T>C† | — | ||||||
| Missense | 20 | 2561G>A40 | R854Q | ||||||
| P5F11II1#¶ | 40 | 38 | 47 | 59 | 0.81 | 2 | |||
| Missense | 20 | 2561G>A40 | R854Q | ||||||
| Missense | 28 | 4751A>G21 | Y1584C | ||||||
| P7F3II1‖¶ | 15 | 38 | 25 | 38 | 1.52 | 3 | |||
| Missense | 20 | 2561G>A40 | R854Q | ||||||
| Missense | 21 | 2771G>A44 | R924Q | ||||||
| P10F4I2§ | 30 | 3 | 27 | 22 | 0.11 | 3 | |||
| Missense | 20 | 2642T>G† | L881R | ||||||
| Missense | 28 | 4263C>G8 | N1421K | ||||||
| P6F5II1¶ | 18 | 7 | 11 | 6 | 0.64 | 1 | |||
| Missense | 21 | 2771G>A44 | R924Q | ||||||
| Missense | 27 | 3614G>A26 | R1205H | ||||||
| P7F16I1§ | 119 | 49 | 47 | 81 | 1.04 | 3 | |||
| Missense | 36 | 6187C>T8** | P2063S | ||||||
| Missense | 39 | 6859C>T† | R2287W |
— indicates not applicable.
ICs are indicated by their study ID.
Novel mutations.
Cosegregation of VWD with the VWF gene12 ; 1 indicates complete cosegregation; 2, incomplete cosegregation; 3, not informative.
Allelic inheritance of 2 mutations.
Absent VWF:FVIIIB.
Compound heterozygous inheritance of 2 mutations.
Reduced VWF:FVIIIB.
Frequency checked in matched local population; allele frequency, 0.025.
Linkage analysis demonstrated that 21 (41%) of group 2 ICs were from families in which VWD completely cosegregated with VWF. Families of 12 ICs demonstrated incomplete cosegregation, while families of the remaining 18 ICs were not informative. Median VWF:RCo but not BS was significantly lower in ICs from families in which VWD completely cosegregated with VWF compared with those incompletely cosegregating (VWF:RCo, 37 IU/dL versus 52 IU/dL, P = .049; and BS, 9 versus 7, respectively).
Group 3
No VWF gene mutation was detected in 42 ICs with NMs constituting group 3. Median VWF:RCo of 51 IU/dL and VWF:Ag of 49 IU/dL were significantly higher than levels in group 1 (10 IU/dL and 19 IU/dL, respectively, P < .001 for both), and VWF:RCo (P = .033), but not VWF:Ag, was significantly higher than group 2 (42 IU/dL and 45 IU/dL, respectively; Table 2). Linkage analysis in group 3 demonstrated that 8 (19%) ICs were from families in which VWD completely cosegregated with VWF, 19 (45%) had incomplete cosegregation, and 15 (36%) were not informative. Median VWF:RCo but not BS was significantly lower in 8 ICs from families with VWD cosegregating with VWF than in 19 incompletely cosegregating cases (VWF:RCo, 36.5 IU/dL versus 53.0 IU/dL, P = .034; and BS, 8.5 versus 9.0, respectively). Overall, 32 of 42 (76%) group 3 ICs had blood group O. Whereas 63% of the 8 completely cosegregating ICs had blood group O, 89% of the 19 incompletely cosegregating ICs had blood group O. As discussed later, non-VWF influences on the diagnosis of VWD in group 3 should be considered.
Phenotype and genotype association
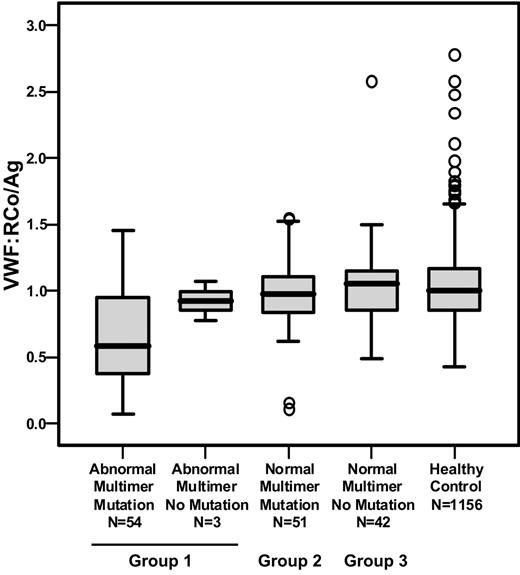
Group 3 had the highest levels of VWF parameters (Table 2) but, surprisingly, BS was substantially similar to those in the 2 other groups (BS was 10 in group 1 ICs and 8 in each of groups 2 and 3, P = NS). Analysis of the VWF:RCo/VWF:Ag ratio in groups 2 and 3 confirmed the overall association of normal multimer profile with a ratio close to 1.0 and few ratios below 0.7. There was, however, a wide overlapping distribution of VWF:RCo/VWF:Ag ratio within the groups (Figure 3), and several group 1 ICs had ratios close to 1.0. Much lower VWF:Ag and VWF:RCo levels in group 1 ICs may contribute to the observation that small changes in levels can dramatically alter VWF:Ag/VWF:RCo ratios. The presence or absence of a candidate mutation was significantly associated with VWF:Ag and VWF:RCo levels (Table 7). ICs with VWF levels of 0 to 15 IU/dL were more than 20 times as likely to have a candidate VWF mutation than those with levels above 45 IU/dL.
Distribution of the VWF:RCo/VWF:Ag ratio in the 3 groups of index cases and healthy controls. The box plots indicate the median, interquartile range (box), extreme values (T bars), and outliers (○).
Distribution of the VWF:RCo/VWF:Ag ratio in the 3 groups of index cases and healthy controls. The box plots indicate the median, interquartile range (box), extreme values (T bars), and outliers (○).
Association between the presence of mutations and VWF level in index cases
| VWF level in IC . | Mutation . | No mutation . | OR (95% CI) . | |||
|---|---|---|---|---|---|---|
| VWF:Ag, IU/dL | ||||||
| More than 45 | 27 | 27 | 1* | |||
| 31 to 45 | 24 | 11 | 2.2 (0.90-5.3) | |||
| 16 to 30 | 30 | 6 | 5.0 (1.8-14.0) | |||
| 0 to 15 | 23 | 1 | 23.0 (2.9-182.6) | |||
| VWF:RCo, IU/dL | ||||||
| More than 45 | 23 | 25 | 1* | |||
| 31 to 45 | 24 | 12 | 2.2 (0.89-5.3) | |||
| 16 to 30 | 17 | 6 | 3.1 (1.04-9.2) | |||
| 0 to 15 | 40 | 2 | 21.7 (4.7-100.3) | |||
| VWF level in IC . | Mutation . | No mutation . | OR (95% CI) . | |||
|---|---|---|---|---|---|---|
| VWF:Ag, IU/dL | ||||||
| More than 45 | 27 | 27 | 1* | |||
| 31 to 45 | 24 | 11 | 2.2 (0.90-5.3) | |||
| 16 to 30 | 30 | 6 | 5.0 (1.8-14.0) | |||
| 0 to 15 | 23 | 1 | 23.0 (2.9-182.6) | |||
| VWF:RCo, IU/dL | ||||||
| More than 45 | 23 | 25 | 1* | |||
| 31 to 45 | 24 | 12 | 2.2 (0.89-5.3) | |||
| 16 to 30 | 17 | 6 | 3.1 (1.04-9.2) | |||
| 0 to 15 | 40 | 2 | 21.7 (4.7-100.3) | |||
OR indicates odds ratio; CI, confidence interval.
Reference category.
Discussion
Type 1 VWD has been considered the most common of the 3 VWD disease types, affecting 50% to 75% of all patients with the disorder. Until now, this type has remained enigmatic. The MCMDM-1VWD study aimed to characterize 154 index cases and their affected and unaffected family members with the disorder, and this paper summarizes phenotypic and molecular analysis. All families recruited into the study were previously registered at a participating center and had historic low VWF levels and a personal and family history of bleeding. The study aimed to characterize the phenotype and genotype of patients diagnosed with type 1 VWD, with a view to making future recommendations for more correct type 1 VWD diagnosis. A range of disease severities from “severe” type 1 to cases with only mildly reduced VWF levels were recruited to illustrate the full range of diagnostic difficulty. Although at recruitment some ICs displayed decreased VWF:RCo/VWF:Ag ratios based on historic data, all were registered at their treatment centers as having type 1 VWD.
Three groups of patients were defined segregated by multimer profile and mutation analysis. VWF multimers were analyzed to determine whether there was an absence of HMWM, indicating type 2A or 2B VWD. Low-resolution gels are considered sufficient for this assessment and are commonly used in laboratories worldwide. In this study, more detailed analysis was undertaken using 2 different gel resolutions, enabling both reductions in HMWM to be characterized and a number of subtle multimer abnormalities to be detected. All ICs with these alterations were classed as having AbMs, and these patients constituted group 1. Only 2 ICs in this group were subsequently classed as having classical type 2A VWD (both had R1374H, which has also been classed as 2M)19,20 and none as 2B. One IC had a P1266L missense mutation previously reported in type IB VWD.22 However, a second mutation (R1315H) on the same allele may have ameliorated the phenotype, because platelet-rich plasma did not demonstrate increased ristocetin sensitivity.
Use of the VWF:RCo/VWF:Ag ratio as a discriminator between type 1 and type 2A, 2B, or 2M VWD has been suggested, and a cutoff of 0.7 has been proposed.23-25 This cutoff correlates in the current study when comparing the median ratio in groups 1 with 2 + 3 with abnormal or normal multimers (0.53 versus 0.96), but there was considerable overlap in individual data (Figure 3), and we therefore preferred to rely on the multimer pattern to indicate structural normality of plasma VWF. The knowledge that the coefficient of variation (CV) of the VWF:RCo assay is often large (48% where the mean VWF:RCo level of a sample was 12.5 IU/dL in a recent United Kingdom National External Quality Assessment Scheme [UKNEQAS] study; S. Kitchen, personal communication, May 2006) persuaded us not to use VWF:RCo/VWF:Ag to subdivide the IC cohort. Using this information, for a sample in which both VWF:RCo and VWF:Ag were 12.5 IU/dL, ratios could range from 0.67 to 1.48.
VWF:FVIIIB analysis identified 3 of 150 ICs with markedly reduced binding (less than 10%) of VWF to FVIII, consistent with recessively inherited type 2N VWD. Each of the 3 was a compound heterozygote for 1 of the commonly described type 2N mutations plus a nonexpressed VWF allele (not identified in 1 case).
The large number of different mutations identified in this study was a surprise, given the low number of previously reported mutations in type 1 VWD. Expression studies are being performed on a number of these mutations to assess their role in VWD. The observation that, in ICs in which a single mutation was found, only 3 mutations were seen in both the normal and abnormal multimer groups supports the subdivision of the full cohort by multimer analysis (Figure 2) and clearly shows a relationship between abnormal multimers and a specific group of VWF mutations. The 3 mutations identified in both groups 1 and 2 in different patients were R854Q, R1205H, and C2304Y. Multimer abnormalities were very subtle for both the R1205H and C2304Y mutations. Other VWF sequence variation or possibly preanalytical variability may have influenced the multimer profile in these cases. The patient with R854Q was assumed to have a second, undetermined sequence alteration giving rise to the multimer abnormality, because this mutation alone does not affect multimer profile. Patients analyzed by others with mutations such as R1205H, R1315C, and R1374C have previously been described as having Vicenza subtype or type 2M VWD.26-28
The 95% mutation detection sensitivity in group 1 (at least 1 mutation identified in 54 of 57 ICs) indicated that the combination of mutation detection techniques used in the study detected most exonic and closely flanking intronic mutations. Because sequence alterations should be no more difficult to detect among group 3 ICs, only a few heterozygous mutations were likely to have been missed. Large deletions from 1 allele would be difficult to detect due to the presence of the second normal allele. However, complete gene deletions were excluded, as all 42 ICs from group 3 were heterozygous for at least 1 STR.
Prior to this study, few mutations had been described in type 1 VWD. We are now able to relate a series of mutations to VWD phenotype. ICs with the recurrent mutations affecting C1130, and R1205, plus a number of less common mutations demonstrated low levels of VWF, with VWF:RCo being at least 10 IU/dL in virtually all cases. A dominant negative effect of mutant VWF has already been demonstrated for some of these mutations.29,30 Mechanisms resulting in low plasma VWF, including increased clearance rate from plasma, have been demonstrated.29 In contrast, ICs with Y1584C showed a broad range of VWF:RCo values (26-93 IU/dL; median, 37 IU/dL), believed to be mediated mainly through proteolysis of VWF by ADAMTS13.31 Additionally, reduced expression of homozygous and heterozygous rVWFY1584C in comparison with rVWFwt was demonstrated by in vitro analysis (data not shown). Y1584C demonstrated incomplete penetrance in at least 1 reported family.32 In common with the Canadian21 and United Kingdom Haemophilia Centre Doctors' Organization (UKHCDO)33 studies on type 1 VWD, Y1584C was the most common alteration in MCMDM-1VWD, being seen in 8% of ICs. Mutations affecting R1315 and R1374 have been described as responsible for type 2 VWD (2M or unclassified).20,27 Binding of VWF to platelet GPIb in the presence of ristocetin is strikingly decreased in these latter mutations.
The pattern of mutation observed in this cohort differs from that observed previously in type 3 VWD (reviewed by Eikenboom34 ), where approximately 80% of mutations are predicted to lead to null alleles. Only a few such mutations were identified in this cohort (14 of 124 mutations, 11%). Thus, heterozygosity for a “type 3” VWD allele is not the main cause of disease in patients diagnosed with type 1 VWD.35 Null VWF mutations must therefore be prevalent in the population but, in the heterozygous state, rarely cause VWD. Similarly to type 2 VWD, most mutant alleles identified in our patients diagnosed with type 1 VWD are missense mutations. Therefore, a broad range of different mutant VWF allele types, including missense, nonsense, small insertion and deletion, large deletion, and splice mutations, are present in the population and can be inherited alone or in combination to result in the complex array of VWD types.
Group 1 ICs had median VWF:RCo and VWF:Ag values of 10 IU/dL and 19 IU/dL, whereas those in groups 2 and 3 were higher at 42 IU/dL and 45 IU/dL and at 51 IU/dL and 49 IU/dL, respectively. Unexpectedly, patients belonging to the 3 groups had similar bleeding scores, although their VWF levels were significantly different. Initial diagnosis of some group 2 and 3 ICs may have been based more on clinical manifestations than on particularly low VWF levels. Importantly, bleeding score refers to symptoms at historical diagnosis, often several years before enrollment, while VWF levels were measured at enrollment into the MCMDM-1VWD study.
In group 3 ICs with no VWF mutation identified and normal multimers, other causes of low VWF levels and bleeding can now be sought. Misdiagnosis of VWD itself was possible in this group but was not readily identifiable. Blood group O, known to contribute to variation in VWF plasma level,36 was particularly prevalent in this group at 76%, compared with 58% (group 1), 63% (group 2), and 38% (HCs). Eight group 3 ICs had VWD completely cosegregating with VWF, and ongoing investigations may identify VWF sequence alteration(s) associated with their VWD. Nineteen group 3 ICs were from families that demonstrated incomplete cosegregation of VWD to VWF, and factors including platelet receptor expression variability may play a role in the diagnosis of such patients.37,38 Future investigation may lead to identification of linked gene(s) or other nongenetic factors that are responsible.
The MCMDM-1VWD project has recruited a heterogeneous cohort of index cases and families. Importantly, although one third showed evidence of abnormal circulating plasma VWF, very few cases were clearly classical type 2A or 2B VWD. The presence of certain previously reported mutations (eg, at amino acids 1205, 1315, 1374), the latter often classified as type 2M VWD,39,20 was expected because of their frequency and difficult differential diagnosis with type 1 in the absence of functional tests. Similarly, the presence of the mutation R854Q, which in the homozygous or compound heterozygous situation results in type 2N VWD,40 was excepted because it has previously been shown to occur in up to 1% of the healthy population.41 We believe that the spectrum of VWD phenotype and profile of mutations seen in this cohort indicate those that may be expected when routinely diagnosed type 1 VWD patients are investigated for VWF gene mutations.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
A complete list of the members of the MCMDM-1VWD study group can be found in Document S1, available at the Blood website; click on the “Supplemental Materials” link at the top of the online article.
Contribution: A. Goodeve, F.R., and I.P. initiated and coordinated the study; all authors participated in study design, data collection, and performance of laboratory analyses; A. Goodeve, J.E., G.C., F.R., A.B.F., J.G., R.S., U.B., D.H., S.L., M.H.S., L.B., C.H., A. Guilliatt, W.L., and I.P. analyzed and interpreted results; A. Goodeve., J.E., A.B.F., and I.P. wrote the paper; and all authors checked the final version of the manuscript.
This work was supported by The European Community under the Fifth Framework Programme (QLG1-CT-2000-00387). We thank E. Jennings, M. Makris, H. Powell, M. Walker, L. Marsden, A. Al-Buhairan, S. Joyce, A. Bowyer, J. Anson, D. Hampshire, A. Tosetto, A. Cappelletti, M. Bernardi, K. Bertoncello, R. Bader, M.-T. Canciani, F. Gianniello, E. Fressinaud, A. S. Ribba, A. Stephanian, L. Hilbert, C. Caron, E. Gomez, V. van Marion, J. Lambert, F. Oyen, T. Obser, K. Will, W. Eberl, G. Auerswald, E. Christiansen, K. Drewke, J. Suttnar, J. Dudlova, M. Matyskova, P. Salaj, C. Watson, J. Warren, S. Mughal, S. Enayat, G. Surdhar, and P. Short for their contributions to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal