Abstract
Vascular endothelial growth factor-A is widely used in clinical trials for the treatment of cardiac ischemia. VEGF-A was recently suggested to act in a proinflammatory manner, which could aggravate adjacent atherogenesis in VEGF-A–based therapy. To assess potential bystander effects, VEGF-A was focally overexpressed in advanced atherosclerotic plaques in ApoE−/− mice. Sheer-induced carotid artery plaques were transluminally incubated with Ad.hVEGF-A leading to neointimal overexpression of VEGF-A. Ad.hVEGF-A treatment of pre-existing lesions was seen to promote plaque expansion, with a concomitant increase in macrophage and lipid content, whereas it lowered collagen content. In general, Ad.hVEGF-A–treated plaques displayed a more vulnerable phenotype. VEGF-A overexpression was not accompanied by increased microvessel development in the neointima, suggesting that VEGF-A destabilizes atherosclerotic plaques through an angiogenesis-independent mechanism. Intravital microscopy confirmed that treatment with Ad.hVEGF-A led to an increased monocyte adhesion, which was mediated by a VCAM-1/PECAM-1–dependent pathway. VEGF-A indeed induced a differential expression of VCAM-1 and PECAM-1 in endothelial cells. Our data underline the importance of regular monitoring of stenotic vessels adjacent to the site of VEGF-A application. We propose that VCAM-1/PECAM-1–directed cotherapy may be an efficient strategy to prevent bystander effects of focal VEGF-A therapy in patients suffering from cardiovascular disease.
Introduction
Angiogenesis and inflammation are strongly intertwined processes, and it is suggested that neovascularization may play a pivotal role during the development and progression of chronic inflammatory diseases, such as atherosclerosis.1
Members of the vascular endothelial growth factor family, VEGF-A-E, are the most important and dominant factors during pathophysiological angiogenesis,2,3 and are furthermore indispensable during therapeutic angiogenesis aiming at the formation of collaterals in myocardial or peripheral ischemic tissue.4 VEGF-A, as the most potent angiogenic factor of the VEGF family members, exerts its mitogenic activity via its receptors VEGF-R1 (Flt-1) and VEGF-R2 (Flk-1), which are expressed mainly by endothelial cells, although additionally monocytes and progenitor cells express VEGF-R1. Flk-1, with a strong tyrosine kinase activity, is the major mitogenic mediator of endothelial cells in response to VEGF-A.5 Flt-1 binds VEGF-A at an approximately 10 times higher affinity than Flk-1,6 but its biologic function is not clearly understood. It is believed that Flt-1 may function as a decoy receptor regulating the activity of VEGF-A on the vascular endothelium in a negative fashion by sequestering.7 However, gene targeting studies indicate that Flt-1–mediated signaling may contribute significantly to pathological angiogenesis.8,9 Flt-1 is also present on hematopoietic stem cells,10 and on inflammatory cells, such as monocytes and macrophages,11 regulating chemotaxis of these cells and inducing a differential regulation of proinflammatory cytokines, such as IL-6 and TNF-α in monocytes.12 Moreover, several lines of evidence strongly support the hypothesis that in endothelial cells VEGF-A directly modulates not only angiogenesis-related genes but also proinflammatory molecules such as IL-8 and MCP-1.13,14
VEGF-A is used successfully in clinical and preclinical approaches aimed at promoting therapeutic angiogenesis.15 It is generally administered focally to areas of peripheral or myocardial ischemia by adenoviral gene transfer,16 plasmid transfection,15 or recombinant protein17 to promote the formation of true bypass collaterals, thus improving blood flow to the distal ischemic vascular bed. Indeed promoting proangiogenic therapy appears to be one of the most attractive strategies in cardiac and peripheric ischemia.18 Nevertheless, given the proposed proinflammatory effect of VEGF-A, these approaches might affect the stability of silent atherosclerotic plaques in close proximity of the treated ischemic area, which subsequently could provoke further complications in patients suffering from cardiovascular diseases.
The aim of this study was to investigate the effects of adenovirus-mediated overexpression of VEGF-A in a pre-established atherosclerotic plaque on (1) plaque stability and size, (2) inducibility of neovessel formation, and (3) the inflammatory status of the plaque endothelium influencing the adhesion and transmigration of monocytes in vivo.
We show, for the first time, that focal overexpression of VEGF-A in advanced plaques of ApoE−/− mice induces prolonged adhesion and transmigration of monocytes. Moreover, it is demonstrated that VEGF-A–induced infiltration of monocytes to the plaque is mediated by proinflammatory adhesion molecules VCAM-1 and PECAM-1, but interestingly not by ICAM-1.
This observed induced transmigration is the result of an additive effect of an autocrine stimulation of the endothelium by VEGF-A itself and/or by a paracrine induction of the endothelium through cytokines released by VEGF-A–stimulated monocytes. We assume that these proatherogenic effects induced by VEGF-A administration might influence the success of “therapeutic angiogenesis” in patients suffering from cardiovascular diseases.
Materials and methods
Animals
Female ApoE-deficient mice (n = 21), 10 to 12 weeks old, were obtained from our own breeding stock (Gorleaus Laboratories, Leiden, The Netherlands). Mice were placed on a Western-type diet containing 0.25% cholesterol (Special Diets Services, Witham, United Kingdom). Every second week, blood samples were taken to monitor plasma cholesterol levels using enzymatic procedures (Roche Diagnostics, Mannheim, Germany). High-fat diet and water were provided ad libitum. All animal work was approved by the regulatory authority of Leiden University and performed in compliance with the Dutch government guidelines.
Cell culture and reagents
Human umbilical vein endothelial cells (HUVECs) and human monocytic MonoMac-6 (MM6) cells were cultured as described.19 Cells were cultured in DMEM with 10% FCS (HyClone, Logan, UT) supplemented with 2 mM glutamine, 100 units/mL penicillin, and 0.1 mg/mL streptomycin. Serum-starved cells were obtained by culturing cells in OptiMEM (Invitrogen, Frederick, MD) supplemented with 0.5% FCS overnight. Cells were stimulated with 100 ng/mL recombinant human VEGF-A165 (Abcam, Cambridge, United Kingdom). Conditioned medium from VEGF-A–stimulated MM6 cells was preincubated with 300 ng/mL blocking VEGF-A antibody from R&D Systems (MAB293; Abington, United Kingdom).
Collar placement and transgene expression
Atherosclerotic lesions in carotid arteries were induced by perivascular collar placement in female ApoE−/− mice, as described previously.20 Four and a half weeks after surgery, 20 μL adenoviral suspension at 1 × 1010 pfu/mL for Ad.hVEGF-A (n = 11) and for Ad.empty (n = 10) was instilled transluminally into the common carotid artery via the external carotid as described,20 after which it was drawn off and the external carotid was ligated. After 2½ weeks, mice were killed and the lesions were analyzed histologically with regard to plaque composition and morphology. For ex vivo perfusion and expression experiments, the same experimental setup was applied. Mice were killed 10 days after transluminal incubation.
Tissue harvesting, histology and immunofluorescence
Transverse 5-μm cryosections were prepared from carotid artery specimens, as described previously,20 and stained with hematoxylin (Sigma-Aldrich, St. Louis, MO) and eosin (Merck Diagnostica, West Point, PA). The site of maximal plaque size was selected for morphology. Classification of plaque stability was assessed in a double-blinded manner by the criteria of Virmani et al.21 Fibrous and fibroatheromatous plaques were classified as stable, whereas thin-capped plaques or plaques with cap break or intramural thrombi were perceived as plaques with characteristics of vulnerability.
Collagen was visualized with Picro-sirius red (Direct red 80; Sigma), and lipids with Oil Red O (Sigma Diagnostics). Sections were immunohistochemically stained for the presence of monocytes/macrophages using a rat polyclonal MOMA-2 antibody (diluted 1:10; Research Diagnostics, Flanders, NJ) and for endothelial cells using CD31 (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies. Peroxidase-conjugated goat anti–rat IgG (dilution: 1:100; Sigma, St Louis, MO) was used as secondary antibody, with 3,3′-diamino-benzidine, nitro blue tetrazolium, and 5-bromo-4-chloro-3-indolyl phosphate as enzyme substrates (Sigma). Human VEGF-A was detected by VEGF-A antibody (dilution: 1:100, polyclonal goat antihuman; R&D Systems). Tyramide signal amplification (TSA Biotin system; Perkin Elmer, Warrington, United Kingdom) was used to enhance the signal. Immunoreactivity was detected by the avidin–biotin–horseradish peroxidase system (Vector Laboratories, Burlingame, CA) with DAB as a chromogen (Zymed, South San Francisco, CA) was performed to detect the immunoreactivity. MoMa-2, collagen, and Oil Red O–positive areas were determined by computer-assisted color-gated measurement and related to the total intimal surface area. Images were captured with a Leica DR-ME microscope equipped with a 20× objective lens and a 10× ocular lens and a 10× ocular lens; Leica Qwin software (Leica Imaging Systems) was used.
Adhesion assay under laminar flow conditions
Laminar flow experiments were performed as described.22,23 Confluent, serum-starved HUVECs were treated for 14 hours with VEGF-A (100 ng/mL) or with conditioned medium from VEGF-A–stimulated MM6 cells. MM6 cells (0.5 × 106 cells/mL) were resuspended in Hank balanced salt solution (HHMC) with 0.5% BSA and perfused at 1.5 dyn/cm2 for 5 minutes at 37°C over the endothelial monolayer mounted in a parallel wall flow chamber. Some MM6 cells were preincubated with soluble PECAM-1.Fc, soluble JAM-A.Fc,24 or anti–VLA-4 mAb (clone HP2/1; Immunotech, Miami, FL) or HUVECs incubated with a blocking anti–ICAM-1 Ab prior to perfusion. MM6 cell arrest was analyzed in multiple high-power fields recorded by video microscopy. Cells not moving for more than 30 seconds were defined as adherent.
Ex vivo perfusion of carotid arteries
The carotid bifurcation, including the common carotid artery, internal carotid artery, and external carotid artery branches, was exposed under anesthesia. Branches were ligated with sutures and a polyethylene catheter was inserted through an incision of the common carotid artery distal to a ligation. The artery was perfused with MOPS-buffered saline with 0.5% human serum albumin using a syringe pump. Outflow was enabled by punctures in the external and internal carotid artery. The vessel was cut distal to the sutures and transferred onto a microscope stage (immersion objective 20 ×, Olympus BX51; Olympus, Tokyo, Japan) superfused at 37°C with sodium bicarbonate–buffered saline. MM6 cells (0.5 × 106 cells/mL) labeled with calcein/AM (Molecular Probes, Leiden, The Netherlands) were preincubated with soluble PECAM-1.Fc and soluble JAM-A.Fc, with anti–VLA-4 (clone HP2/1; Immunotech) (in MOPS buffer) at 37°C and perfused at a flow rate of 3 μL/min.24 Adhesive interactions with the atherosclerotic vessel wall were recorded using stroboscopic epifluorescence illumination (Drelloscop 250; Drello, Mönchengladbach, Germany). Cells not moving for more than 30 seconds were considered adherent.
Immunofluorescence
HUVECs were cultured on slides overnight, serum starved for 4 hours, and subsequently induced with VEGF-A for 6 and 14 hours. After fixation in 4% paraformaldehyde, cells were stained with FITC-conjugated anti–hVCAM-1 mAb (clone BBIG-V3; R&D Systems), FITC-conjugated isotype control and anti–PECAM-1 (clone M-20; Santa Cruz Biotechnology), or isotype control (Sigma) detected by biotin-conjugated secondary Ab and fluorescein strepatavidin and mounted with Vectashield containing DAPI (all from Vector Laboratories).
Quantitative real-time polymerase chain reaction (PCR)
For each sample, 0.5 μg total RNA was converted to cDNA with MuLV-Reverse Transcriptase 200 U/μL (MBI-Fermentas, Hanover, MD), with the use of 18mer OligodT (Eurogentec, Seraing, Belgium) and 10 mM dNTP set (MBI-Fermentas).
Gene expression analysis was performed using real-time PCR, 7700 ABIPrism (AppliedBiosystems, Weiterstadt, Germany), and SYBRGreen technology (Eurogentec).
Quantitative gene expression analysis was performed using the following primers (Eurogentec) for hHRPT, 5′-TGACACTGGCAAAACAATGCA-3′ (forward) and 5′-GGTCCTTTTCACCAGCAAGCT-3′ (reverse). Primers for the genes of interest were as follows: hTNFα, 5′-CCCAGGGACCTCTCTCTAATCAG-3′ (forward) and 5′-AGCTGCCCCTCAGCTTGA-3′ (reverse); IL-1β, 5′-ATGATAAGCCCACTCTACAGCTG-3′ (forward) and 5′-GGAACTGGGCAGACTCAAATTC-3′ (reverse); IL6, 5′-TGGCTGAAAAAGATGGATGCTT-3′ (forward) and 5′-AAACTCCAAAAGACCAGTGATGATT-3′ (reverse); MCP-1, 5′-GCTGACCCCAAGCAGAAGT-3′ (forward) and 5′-AGTGAGTGTTCAAGTCTTCGGAGTT-3′ (reverse); PECAM-1, 5′-TCCCCAGAAGCAAAATACTGACA-3′ (forward) and 5′-TCCGATGATAACCACTGCAATAAGT-3′ (reverse); ICAM-1, 5′-GAATCAGTGACTGTCACTCGAGATCT-3′ (forward) and 5′-ACCACAGTGATGATGACAATCTCA-3′ (reverse); and VCAM-1, 5′-GCTCTGTCACTGTAAGC-3′ (forward) and 5′-GTGTTTGCGTACTCTGC-3′ (reverse). Hypoxanthine guanine phosphoribosyl transferase (HPRT) and cyclophilin A were used as the standard housekeeping genes. Relative gene expression (RE) numbers were calculated by subtracting the threshold cycle number (Ct) of the target gene from the average Ct of HPRT and cyclophilin (Cthousekeeping) resulting in a ΔCt (dCt) and raising 2 to the power of minus this difference (2−ΔCt). The average Ct of 2 housekeeping genes was used to exclude that changes in the relative expression were caused by variations in the separate housekeeping gene. Standard deviations were calculated on the basis of the ΔCt.
Statistical analysis
Plaque size, plaque composition parameters, and differences in the change in threshold of cycle number (ΔCt) were compared using the 2-tailed Student t test. Differences in the classification of plaque stability were analyzed in a double-blinded manner with the Yates corrected 2-sided Fisher exact test. Flow cytometry experiments were compared by one-way analysis of variance (ANOVA) followed by Newman-Keuls posttest (InStat software; Graphpad, San Diego, CA). Differences with P values below .05 were considered statistically significant.
Results
Effect of adenoviral VEGF-A gene transfer on atherosclerotic plaques
Previous studies have shown that angiogenesis does aggravate intimal thickening in ApoE−/− mice, but is not essentially required for plaque initiation.1,25 In this study, we have addressed the effects of transient overexpression of VEGF-A in pre-existing plaques in ApoE−/− mice fed a high-cholesterol diet.
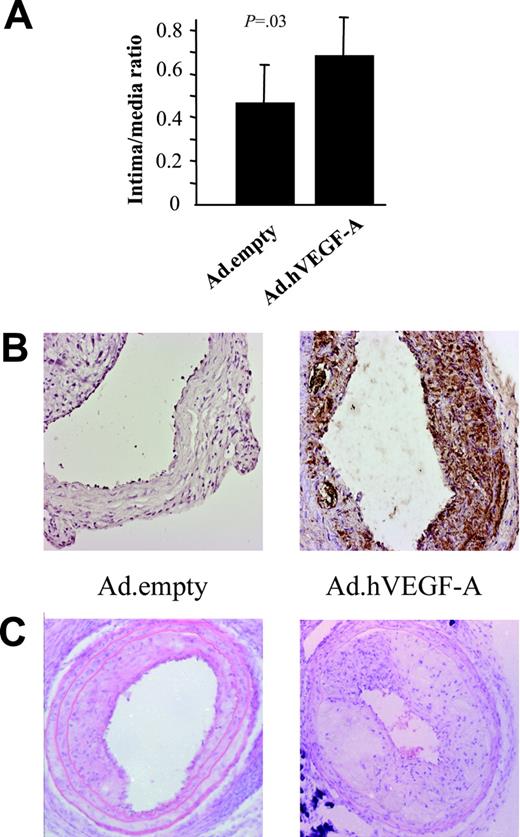
As expected, focal VEGF-A adenoviral gene transfer changed neither cholesterol levels nor body weight (data not shown). Ten days after transluminal transduction, Ad.hVEGF-A–treated, but not Ad.empty-treated, plaques stained positive for human VEGF-A expression (Figure 1B) VEGF-A expression was detected 1½ weeks after transluminal incubation primarily in endothelial cells and to a lesser extent in cap macrophages and smooth muscle cells (SMCs). Additionally, diffuse VEGF-A staining was detected in the media, adverting possible bystander effects of VEGF-A on untransduced surrounding cells. Morphometric analysis of carotid artery 2½ weeks after transduction showed that VEGF-A overexpression indeed acts in a proatherogenic manner and promotes progression (Figure 1). VEGF-A expression in the plaques led to a relative increase in plaque size by 49% (P = .03) (Figure 1A-B). Adenoviral treatment (with Ad.empty or Ad.GFP) or the local incubation regimen per se (sham operation) did not change plaque size compared with untreated collar-induced atherosclerotic plaques (P = .35; data not shown).
Plaque-size analysis of cross-sections of carotid artery specimens obtained from Ad.empty- and Ad.hVEGF-A–treated ApoE−/− mice. Error bars represent standard deviation (n = 10-12). (A) Plaque sizes of Ad.hVEGF-A–treated compared with that of Ad.empty control mice are significantly increased by 49% (P = .03). (B) Ad.hVEGF-A–treated plaques (right panel) showed higher expression of the transgene at 10 days after transduction than Ad.empty-treated plaques. Please note that expression is largely confined to the intima and diffuse with a higher density in the cap region. (C) Hematoxylin/eosin staining of a typical Ad.empty– and Ad.hVEGF-A–treated atherosclerotic plaque. Error bars indicate standard deviation (SD).
Plaque-size analysis of cross-sections of carotid artery specimens obtained from Ad.empty- and Ad.hVEGF-A–treated ApoE−/− mice. Error bars represent standard deviation (n = 10-12). (A) Plaque sizes of Ad.hVEGF-A–treated compared with that of Ad.empty control mice are significantly increased by 49% (P = .03). (B) Ad.hVEGF-A–treated plaques (right panel) showed higher expression of the transgene at 10 days after transduction than Ad.empty-treated plaques. Please note that expression is largely confined to the intima and diffuse with a higher density in the cap region. (C) Hematoxylin/eosin staining of a typical Ad.empty– and Ad.hVEGF-A–treated atherosclerotic plaque. Error bars indicate standard deviation (SD).
VEGF-A and its effect on angiogenesis in the atherosclerotic plaque
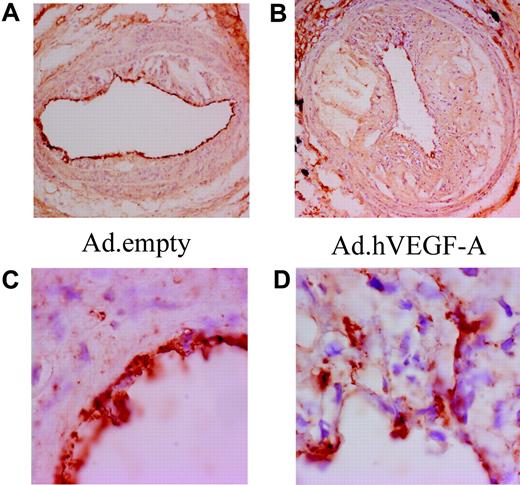
To establish the angiogenic activity of VEGF-A overexpression in atherosclerotic plaques, a CD31 staining was performed (Figure 2) While we incidentally noted the presence of microcapillaries in advanced carotid artery plaques, much to our surprise VEGF-A overexpression did not lead to a significant increase in neovessel formation (Figure 2 A,C). Morphologic analysis of the luminal endothelial cells revealed that VEGF-A overexpression caused characteristic irregularities in the endothelial cell layer and seemed to induce angiogenic sprouting of the endothelial cells toward the inner atheroma (Figure 2B,D). This may significantly impact the functionality of the endothelium in regard to the penetrability for adhering monocytes and plasma contents (eg, lipoproteins). These data point to a potentially destabilizing effect of VEGF-A, which is effected not only via an angiogenesis-dependent, but also by an angiogenesis-independent, mechanism.
Ad.hVEGF-A treatment results in clear irregularities in endothelial morphology and arrangement. CD31 staining in Ad.empty–treated (A,C) and Ad.hVEGF-A–treated (B,D) plaques shows weakly stained microcapillaries in advanced carotid artery lesions. VEGF-A does induce endothelial cell sprouting from the lumen to the central atheroma in Ad.hVEGF-A–treated plaques (D); these endothelial irregularities are not observed in Ad.empty–treated plaques. Panels C and D are high-power views of the vessel segments shown in panels A and B, respectively. Original magnification: panels A and B, ×40; panels C and D, ×100.
Ad.hVEGF-A treatment results in clear irregularities in endothelial morphology and arrangement. CD31 staining in Ad.empty–treated (A,C) and Ad.hVEGF-A–treated (B,D) plaques shows weakly stained microcapillaries in advanced carotid artery lesions. VEGF-A does induce endothelial cell sprouting from the lumen to the central atheroma in Ad.hVEGF-A–treated plaques (D); these endothelial irregularities are not observed in Ad.empty–treated plaques. Panels C and D are high-power views of the vessel segments shown in panels A and B, respectively. Original magnification: panels A and B, ×40; panels C and D, ×100.
Effect of transluminal VEGF-A gene delivery on the composition of the atherosclerotic plaque
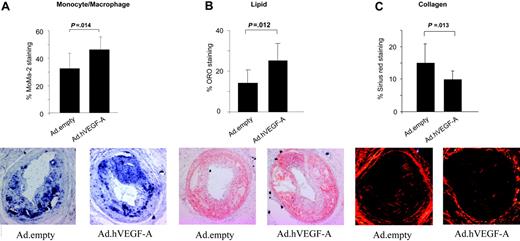
To further evaluate the proatherogenic effects of VEGF-A gene delivery, carotid artery plaques were analyzed for monocyte/macrophage staining. Indeed, VEGF-A was seen to increase monocyte/macrophage content by 40% (P = .014) (Figure 3A) Similarly, VEGF-A overexpression led to a 66% elevation in lipid content (P = .012) (Figure 3B). A more detailed look at the sections did not reveal any significant differences in core size and in absolute macrophage cell size, underlining that the increased lipid-positive staining is attributable mainly to the lipid-rich necrotic core. Furthermore, VEGF-A overexpression significantly reduced plaque collagen content by 33% (P = .013) (Figure 3C), which seemed attributable mainly to a decrease in cap collagen in Ad.hVEGF-A– versus Ad.empty-treated plaques. Thus, the Ad.hVEGF-A–treated plaques generally displayed more features of enhanced vulnerability than control plaques.
Immunohistochemical analysis of Ad.hVEGF-A– and Ad.empty-transduced plaques on plaque composition and stability. The content was calculated as percentage of stained area relative to the total plaque size. (A) In Ad.hVEGF-A–treated plaques, MoMa2 staining, and thus macrophage content, was markedly increased by 40% (P = .014). (B) Analysis of Oil Red O–stained sections reveals a 66% increase in lipid content in Ad.hVEGF-A–treated plaques (P = .012). (C) Picro-sirius red–stained sections from Ad.empty controls and Ad.hVEGF-A–treated plaques. VEGF-A overexpression led to a significant decrease of 33% in plaque collagen content (P = .013). Error bars indicate SD.
Immunohistochemical analysis of Ad.hVEGF-A– and Ad.empty-transduced plaques on plaque composition and stability. The content was calculated as percentage of stained area relative to the total plaque size. (A) In Ad.hVEGF-A–treated plaques, MoMa2 staining, and thus macrophage content, was markedly increased by 40% (P = .014). (B) Analysis of Oil Red O–stained sections reveals a 66% increase in lipid content in Ad.hVEGF-A–treated plaques (P = .012). (C) Picro-sirius red–stained sections from Ad.empty controls and Ad.hVEGF-A–treated plaques. VEGF-A overexpression led to a significant decrease of 33% in plaque collagen content (P = .013). Error bars indicate SD.
To substantiate this notion, we have categorized plaques for stability according to the criteria of Virmani et al.21 Fibrous and fibroatheromatous plaques were classified as stable, and thin-capped plaques or plaques with cap break or intramural thrombi were considered unstable (Table 1). In keeping with the observed shift in plaque composition, VEGF-A overexpression led to a significant switch from a stable to a vulnerable plaque phenotype (P = .03).
Lesions
| . | No. of lesions . | ||
|---|---|---|---|
| . | Stable . | Unstable . | Total . |
| Ad.empty | 8 | 2 | 10 |
| Ad.hVEGF-A | 3 | 8 | 11 |
| Total | 11 | 10 | 21 |
| . | No. of lesions . | ||
|---|---|---|---|
| . | Stable . | Unstable . | Total . |
| Ad.empty | 8 | 2 | 10 |
| Ad.hVEGF-A | 3 | 8 | 11 |
| Total | 11 | 10 | 21 |
Lesions were categorized according to their morphologic features for stability (by 2 blinded observers). Fibrous lesions and atheromatous plaques were considered stable, whereas thin-capped plaques and plaques with adverse events (eg, intraplaque hemorrhage, cap break, or intramural thrombi) were considered unstable. VEGF-A overexpression led to a higher prevalence of unstable plaques (P = .03).
VEGF-A acts in an autocrine and paracrine manner on the endothelium
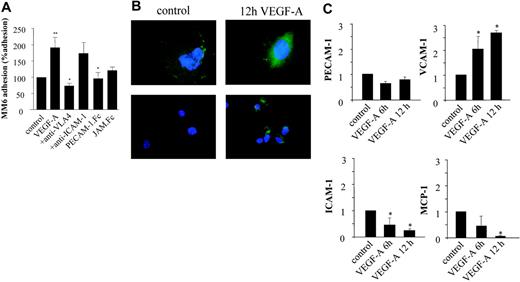
Treatment of monocytes with VEGF-A was reported to stimulate not only chemotactic migration but also the expression of proinflammatory cytokines.13,14 In turn, these cytokines can activate the endothelium and lead to an up-regulation of specific adhesion molecules and thus to increased recruitment of monocytes. We have mapped the relative contribution and cooperativity of autocrine versus paracrine activation of endothelial cells by VEGF-A. Hereto, we analyzed the effect of VEGF-A (100 ng/mL; autocrine) (Figure 4) and VEGF-A–conditioned MM6 medium (paracrine) (Figure 5) on the capacity of human monocytic MM6 cells to adhere to serum-starved confluent human umbilical vein endothelial cells (HUVECs) in an in vitro lamina flow experiment. The adherence of quiescent MM6 cells to VEGF-A–activated endothelial cells was significantly increased and, in fact, was 2-fold enhanced compared with the untreated control group (P = .027). Preincubation with blocking antibodies for VCAM-1 and PECAM-1 completely abrogated the VEGF-A–mediated induction of MM6 cell adherence, whereas inhibition of ICAM-1 and JAM-1 did not have a significant inhibiting effect (Figure 4A).
Autocrine effects of VEGF-A on the adherence of Monomac6 (MM6) cells to HUVECs. (A) Serum-starved HUVECs were treated for 24 hours with VEGF-A (100 ng/mL) and perfused with Monomac 6 cells treated with a blocking anti–VLA-4 antibody, sPECAM-1.Fc, or sJAM.Fc (10 μg/mL, 30 min). Some HUVECs were treated with anti–ICAM-1 Ab prior to perfusion. Cell arrest and spreading were analyzed in multiple high-power fields recorded by video microscopy. Cells not moving for more than 30 seconds were defined as adherent. VEGF-A significantly induced the adherence of MM6 cells at a shear rate of 1.5 dyne/cm2. Values represent mean ± SEM of 3 to 5 experiments performed in triplicate; *P < .05 versus control and **P < .05 versus VEGF-A. (B) Immunofluorescence microscopy of HUVECs treated with VEGF-A (10 ng/mL) for 12 hours after staining for endothelial cell marker PECAM-1 and VCAM-1. Stimulation with VEGF-A induces redistribution of constitutively expressed PECAM-1 to the apical side of the endothelial cells. No constitutive expression of VCAM-1 is observed; VCAM-1 expression is induced by VEGF-A exposure. (C) Quantitative real-time reverse-transcription–polymerase chain reaction analysis of total RNA isolated from HUVECs confirmed that PECAM-1 expression is not changed after 6- and 12-hour VEGF treatment. The expression of VCAM-1 mRNA is significantly up-regulated, and that of ICAM-1 significantly down-regulated. MCP-1 mRNA expression level is almost completely inhibited by VEGF-A. Levels are normalized to that of hHRPT as well as cyclophilin A mRNA. *P < .05 versus control. Error bars indicate SD.
Autocrine effects of VEGF-A on the adherence of Monomac6 (MM6) cells to HUVECs. (A) Serum-starved HUVECs were treated for 24 hours with VEGF-A (100 ng/mL) and perfused with Monomac 6 cells treated with a blocking anti–VLA-4 antibody, sPECAM-1.Fc, or sJAM.Fc (10 μg/mL, 30 min). Some HUVECs were treated with anti–ICAM-1 Ab prior to perfusion. Cell arrest and spreading were analyzed in multiple high-power fields recorded by video microscopy. Cells not moving for more than 30 seconds were defined as adherent. VEGF-A significantly induced the adherence of MM6 cells at a shear rate of 1.5 dyne/cm2. Values represent mean ± SEM of 3 to 5 experiments performed in triplicate; *P < .05 versus control and **P < .05 versus VEGF-A. (B) Immunofluorescence microscopy of HUVECs treated with VEGF-A (10 ng/mL) for 12 hours after staining for endothelial cell marker PECAM-1 and VCAM-1. Stimulation with VEGF-A induces redistribution of constitutively expressed PECAM-1 to the apical side of the endothelial cells. No constitutive expression of VCAM-1 is observed; VCAM-1 expression is induced by VEGF-A exposure. (C) Quantitative real-time reverse-transcription–polymerase chain reaction analysis of total RNA isolated from HUVECs confirmed that PECAM-1 expression is not changed after 6- and 12-hour VEGF treatment. The expression of VCAM-1 mRNA is significantly up-regulated, and that of ICAM-1 significantly down-regulated. MCP-1 mRNA expression level is almost completely inhibited by VEGF-A. Levels are normalized to that of hHRPT as well as cyclophilin A mRNA. *P < .05 versus control. Error bars indicate SD.
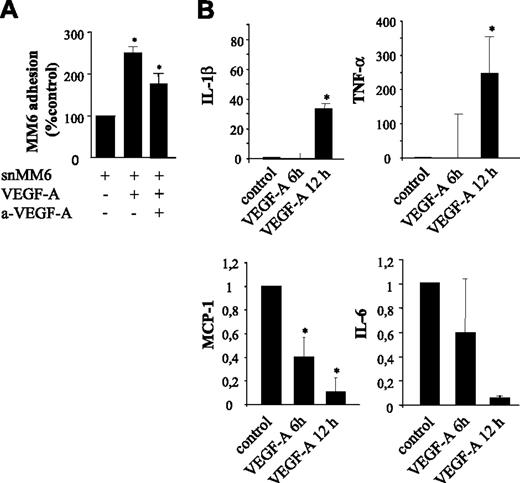
Paracrine effects of VEGF-A on the adherence of Monomac6 (MM6) cells to HUVECs. (A) Endothelial HUVEC monolayer was stimulated with the supernatant of MM6 cells (snMM6) treated with VEGF-A (same concentration). Note that with snMM6 the adherence of quiescent MM6 cells is significantly increased. Neutralization of VEGF-A by neutralizing antibodies in the supernatant of VEGF-A–treated MM6 cells significantly decreases the adherence of MM6 cells, which is still significantly increased in comparison with the control. *P < .05. (B) Quantitative RT-PCR to map paracrine factors. Expression levels of IL-1β and TNF-α are increased, whereas IL-6 and MCP1 expression is almost completely inhibited by VEGF-A. *P < .05 versus control. Error bars indicate SD.
Paracrine effects of VEGF-A on the adherence of Monomac6 (MM6) cells to HUVECs. (A) Endothelial HUVEC monolayer was stimulated with the supernatant of MM6 cells (snMM6) treated with VEGF-A (same concentration). Note that with snMM6 the adherence of quiescent MM6 cells is significantly increased. Neutralization of VEGF-A by neutralizing antibodies in the supernatant of VEGF-A–treated MM6 cells significantly decreases the adherence of MM6 cells, which is still significantly increased in comparison with the control. *P < .05. (B) Quantitative RT-PCR to map paracrine factors. Expression levels of IL-1β and TNF-α are increased, whereas IL-6 and MCP1 expression is almost completely inhibited by VEGF-A. *P < .05 versus control. Error bars indicate SD.
Next we investigated if VEGF-A was able to induce a differential regulation of adhesion molecule expression in endothelial cells by immunofluorescence studies as well as expression analysis of VEGF-A–treated HUVECs. PECAM-1 was shown to be constitutively expressed on nonstimulated cells (Figure 4B). It was diffusely distributed over the membrane with focal points of enhanced expression at intercellular junctions, reflecting its important function as a junctional protein in endothelial cell-cell contact.26,27 In VEGF-A–stimulated endothelial cells, however, the focal PECAM-1 staining at intercellular junctions was lost and its expression seen to redistribute to the apical surface, where it may engage in leukocyte arrest and diapedesis. Accordingly, RNA expression levels of PECAM-1 did not change in VEGF-A–treated HUVECs (Figure 4C).
Conversely, VCAM-1 expression was strongly regulated by VEGF-A. While VCAM-1 was virtually undetectable in nonactivated endothelial cells by immunostaining (Figure 4B), its expression was highly up-regulated upon stimulation with VEGF-A. In keeping with these results, Taqman analysis showed a strong up-regulation of VCAM-1 expression at just 6 hours, which was even more pronounced at 12 hours of stimulation. Furthermore, immunostaining indicated that ICAM-1 expression was not altered in VEGF-A–treated versus untreated endothelial cells (data not shown). In fact, Taqman analysis showed that ICAM-1 expression was even down-regulated in VEGF-A–treated endothelial cells. Since monocyte chemotactic protein-1 (MCP-1) was reported to play a pivotal role in atherogenesis, we determined if expression levels of this chemoattractant were also affected by VEGF-A treatment. Surprisingly, VEGF-A treatment led to a dramatic 95% down-regulation of MCP-1 expression in endothelial cells (P < .001).
Paracrine stimulation of serum-starved HUVECs by VEGF-A was even more pronounced (Figure 5A). The adhesion of quiescent MM6 cells to HUVECs that had been incubated with conditioned medium from VEGF-A–stimulated MM6 cells was increased by 260% versus 80% for VEGF-A–stimulated HUVECs (P = .002) (Figure 4A). These data suggest that VEGF-A–treated monocytes indeed produce mitogens, which, in concert with VEGF-A, further augment monocyte adhesion. Blocking recombinant VEGF-A in the conditioned MM6 medium with neutralizing antibodies gave a partial (50%) reduction of MM6 cell adherence compared with conditioned medium treatment per se, but MM6 cell adherence was still significantly (80%) higher compared with that of untreated HUVECs. In conclusion, VEGF-A strongly promotes MM6 cell adhesion to HUVECs not only in an autocrine manner but also, and to a similar extent, by active paracrine factors.
To map the paracrine factors, we performed expression analysis of VEGF-A–treated MM6 cells. VEGF-A–stimulated MM6 cells displayed a 32-fold up-regulation of IL-1β (Figure 5B) and a 250-fold up-regulation of TNF-α (Figure 5C), both strong inducers of inflammatory responses in endothelial cells. Similar to the autocrine down-regulation of MCP-1 by VEGF-A, this chemoattractant was strongly down-regulated in VEGF-A–treated MM6 cells as well (Figure 5D). Furthermore, monocyte expression of IL-6, a strong proinflammatory mediator, was further significantly reduced in response to VEGF-A (Figure 5E).
VEGF-A–induced mononuclear cell recruitment in vivo is mediated via VCAM-1 and PECAM-1
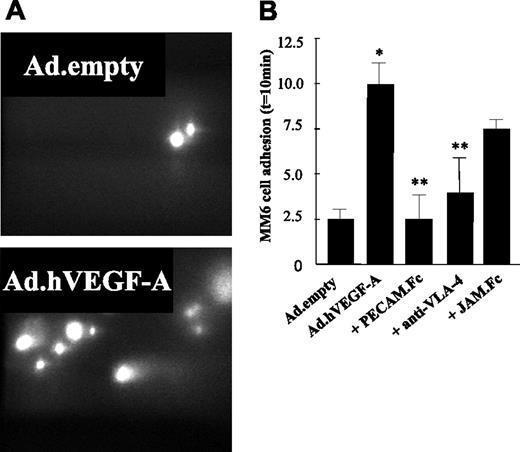
To further elucidate the pathways underlying the enhanced plaque monocyte content, carotid arteries containing Ad.hVEGF-A (or Ad.empty)–treated plaques were perfused ex vivo. In line with the in vitro data, MM6 adhesion to the atherosclerotic vessel wall in Ad.hVEGF-A–treated animals was induced by more than 4-fold compared with those of Ad.empty controls (Figure 6A-B) Preincubation of MM6 cells with soluble JAM-1.Fc did not significantly reduce its adhesion to the VEGF-A–overexpressing atherosclerotic vessel wall. By contrast, we demonstrate here that VEGF-A–induced adhesion of MM6 cells could be profoundly inhibited by antibody blockade of VLA-4 and completely blocked by soluble PECAM-1.Fc, confirming the in vitro flow cytometry results. Thus, these data are the first to show that overexpression of VEGF-A in an atherosclerotic plaque strongly increases the adherence of circulating monocytes in vivo and that this effect is orchestrated predominantly by an inflammatory response of the endothelium, which is mediated by VCAM-1 and PECAM-1.
Ex vivo perfusion of murine carotid arteries with monocytic Monomac 6 cells (MM6). Ad.empty– and Ad.hVEGF-A–treated arteries were perfused with MM6 cells labeled with calcein/AM at a flow rate of 3 μL/min. Some monocytic cells were preincubated with sJAM.Fc, sPECAM-1.Fc, and anti–VLA-4 at 37°C for 30 minutes. Adhesive interactions with the atherosclerotic vessel wall were recorded using epifluorescence illumination and cells not moving for more than 30 seconds were defined as adherent. (A) MM6 cells adhere to Ad.hVEGF-A–treated (bottom panel) and Ad.empty–treated (top panel) carotid arteries after 10 minutes of perfusion. Flow direction from was right to left. (B) Quantification of adherent cells reveals that VEGF-A overexpression led to a significant increase in the adherence of MM6 cells, which could be blocked by pretreatment of MM6 cells with anti–VLA-4 and sPECAM-1.Fc, but not by sJAM.Fc. *P < .05 versus Ad.empty; **P < .05 versus Ad.hVEGF-A. Error bars indicate standard deviation.
Ex vivo perfusion of murine carotid arteries with monocytic Monomac 6 cells (MM6). Ad.empty– and Ad.hVEGF-A–treated arteries were perfused with MM6 cells labeled with calcein/AM at a flow rate of 3 μL/min. Some monocytic cells were preincubated with sJAM.Fc, sPECAM-1.Fc, and anti–VLA-4 at 37°C for 30 minutes. Adhesive interactions with the atherosclerotic vessel wall were recorded using epifluorescence illumination and cells not moving for more than 30 seconds were defined as adherent. (A) MM6 cells adhere to Ad.hVEGF-A–treated (bottom panel) and Ad.empty–treated (top panel) carotid arteries after 10 minutes of perfusion. Flow direction from was right to left. (B) Quantification of adherent cells reveals that VEGF-A overexpression led to a significant increase in the adherence of MM6 cells, which could be blocked by pretreatment of MM6 cells with anti–VLA-4 and sPECAM-1.Fc, but not by sJAM.Fc. *P < .05 versus Ad.empty; **P < .05 versus Ad.hVEGF-A. Error bars indicate standard deviation.
Discussion
VEGF-A–based angiogenic therapy has shown to hold promise as a therapeutic modality to restore blood flow in cardiac or peripheral ischemic tissue and is now being evaluated in clinical trials. Generally, focal delivery protocols are applied to effect elevated intramyocardial or intracoronary levels of VEGF-A at the site of ischemia, which may theoretically be accompanied by adverse side effects on adjacent atherosclerotic vessel segments. To address this notion, we have mimicked clinical practice by overexpressing VEGF-A in atherosclerotic carotid artery plaques in ApoE−/− mice that were induced by promoting low sheer at the common carotid proximal to a slightly constrictive collar.20 Our study is the first to demonstrate that indeed focal exposure of advanced plaques to VEGF-A will promote plaque expansion, a phenomenon that we did not observe after overexpression of inert (eg, GFP) nor established proatherogenic factors, such as IL-18 or MMP-9.28,29 Of more importance, VEGF-A overexpression triggered sustained influx of monocytes and (cap) collagen erosion, even without altering the angiogenic status of the plaque. Furthermore, we show here that the renewed influx of monocytes to the plaque upon sustained exposure of VEGF-A is orchestrated by paracrine as well as autocrine stimulation of endothelial adhesion molecule VCAM-1 and PECAM-1 activity.
Previous studies have fueled the hypothesis that recruitment of circulating monocytes occurs during initiation rather than expansion of plaques, where proliferation of intimal macrophages prevails.30 The increased macrophage content of VEGF-A–overexpressing plaques is suggested to be primarily attributable to an enhanced influx of monocytes, implying that exposure of the plaque to VEGF-A may alter its chemotactic repertoire. The increased attraction of circulating monocytes may be mediated by the chemotactic activity of VEGF-A on Flt-1+ monocytes,11 and by a shifted pattern of adhesion molecule activation on the plaque endothelium. In agreement, our flow adhesion experiments and ex vivo perfusion microscopy revealed that VEGF-A promoted monocyte arrest and that this effect could be blunted by blocking VCAM-1 and PECAM-1. By contrast, inhibition of ICAM-1 and JAM-A, both key players in leukocyte adherence and transmigration to diet-induced early lesions,24 had no effect. While the genetic deletion of JAM-A has been shown to reduce neointimal hyperplasia after injury,31 its relevance for the development of advanced plaques has not yet been elucidated. Similarly, ICAM-1 has been reported to be pivotal in plaque initiation, while its effect on plaque progression is controversial.32,33 Thus, VEGF-A appears to selectively activate the VCAM-1/PECAM-1 pathway, leaving that of ICAM-1/JAM-A unaffected. First, ICAM-1 and/or JAM-A may already be fully operational in advanced plaques. Indeed, TNF-α and IFN-γ were reported to induce redistribution of JAM-A on endothelial cells from a junctional to the apical surface to support monocyte adherence and transmigration.34,35 Regardless of effects on expression, VEGF-A could interfere with the redistribution of JAM-A induced by inflammatory stimuli. Second, as has been shown previously, the recruitment of mononuclear cells to early atherosclerotic endothelium is primarily mediated by VCAM-1 and a contribution of JAM-A can be observed only after blocking of VCAM-1/VLA-4. Similarly, effects of JAM-A in mononuclear cell recruitment to VEGF-A–transduced carotid arteries might be unmasked only after VCAM-1 blockade.
We show that binding of VEGF-A to Flt-1 on monocytes markedly alters the expression of inflammatory cytokines relevant to atherosclerosis. VEGF-A strongly increased the expression of TNF-α and IL-1β in monocytes, whereas that of MCP-1 and IL-6 was decreased. The VEGF-A–induced shift in expression pattern of macrophage-derived cytokines probably underlies the observed paracrine hyperactivation of endothelial cells.36-38 Both paracrine and autocrine activation of endothelial cells by VEGF-A implicated the VCAM-1/PECAM-1 pathway. The involvement of parallel signaling pathways in VEGF-A activity implies that the regulation of adhesion molecule expression pattern on the endothelium is finely tuned. Although the relative impact of the separate VEGF-A pathways in vivo still needs to be firmly established, our in vitro and in vivo data suggest that the paracrine signaling cascade could contribute considerably to the proinflammatory effect of VEGF-A. Further studies on VEGF-A signaling in vivo are of high importance, since some of the VEGF-A responses are beneficial, whereas others, such as the reinforcement of ongoing inflammatory processes by TNF-α and IL-1β, will be deleterious during “therapeutic angiogenesis.” Furthermore, our data suggest that MCP-1 and IL-6 do not partake in the paracrine or autocrine effects precipitated by VEGF-A treatment.
Plaque composition
Our data show a significant reduction in plaque collagen content after Ad.hVEGF-A treatment. This may be ascribed to an impaired production of collagen by vascular SMCs (vSMCs) due to vSMC erosion or a phenotypic switch39,40 or, more likely, to an elevated production of collagenases by VEGF-A–activated endothelial and mononuclear cells.41 We did not observe any effects on plaque vSMC content, and a further phenotypic switch of the dedifferentiated cap vSMCs in advanced plaques seems not very plausible. Moreover, VEGF-A is unlikely to exert a direct autocrine effect on vSMCs, which lack VEGF-A receptors. Still we cannot exclude that vSMCs will be affected in a paracrine manner by inflammatory mediators secreted by mononuclear cells in response to VEGF-A. Nonetheless, the net resultant of these effects is that VEGF-A–exposed plaques were clearly displaying more features of enhanced vulnerability.
Neoangiogenesis
Surprisingly, VEGF-A overexpression in the plaque did not markedly promote neovascularization. However, a closer look at the plaque endothelium revealed striking irregularities in endothelial cell morphology and arrangement of Ad.hVEGF-A–treated plaques, pointing to a mitogenic response and endothelial dysfunction. Conceivably, the close proximity of the VEGF-A source to the endothelium and the lack of a VEGF-A gradient could limit the extent of sprouting. In part, it could also reflect a technologic shortcoming of the immunohistochemical analysis, as several groups have reported the sharp down-regulation of CD31 in activated endothelial cells42,43 as present in atheroma-permeating microcapillaries. Nevertheless, our results leave unimpeded that transient VEGF-A overexpression can destabilize advanced atherosclerotic lesions even without markedly affecting neoangiogenesis. It is expected that a potential effect of VEGF-A on plaque neovascularization, as has been reported for human plaques,44 would render the plaque even more unstable and rupture-prone causing further complications.
The therapeutic potential of recombinant VEGF-A protein or VEGF-A gene transfer to promote the formation of collateral vessels assuring re-establishment of the blood flow in ischemic tissue of patients suffering from coronary artery disease (CAD) has been assessed at a preclinical level and in various clinical trials (KAT, VIVA, Euroinject One),15-17 showing that short-term treatment was tolerated well. The issue of a potential proinflammatory effect of the therapeutic VEGF-A on undiagnosed latent atherosclerosis has been addressed in a few recent studies.1,13,45 Whereas 2 studies pointed to a proatherogenic effect of VEGF-A in ApoE−/− mice and rabbits fed a high-fat diet, Leppanen et al45 showed that VEGF-A did not affect atherogenesis in LDLr−/− ApoB48 mice, and this result was ascribed to the model used. Furthermore, we assume that VEGF-A concentrations in the atherosclerotic plaque differ strongly between these 2 models of systemic administration and focal overexpression of Ad.hVEGF-A, causing the observed proatherogenic effects. Our study is the first to investigate effects of focal exposure of advanced plaques to VEGF-A, which may be more reflective of topical Ad.VEGF-A or plasmid-based therapy in the ischemic tissue. We thus believe that the administration route as well as the progression stage of the studied plaques favors synergistic paracrine/autocrine activation of the plaque, which may translate to the grave effects.
Thus, our data underscore the importance of a regular check-up of stenotic vessels adjacent to the ischemic tissue in angiogenic therapy as focal VEGF-A can aggravate inflammatory processes in stenotic lesions leading to progressive plaque destabilization, and possibly rupture and additional complications. Furthermore, our data raise the intriguing option that VCAM-1– or PECAM-1–directed cotherapy could be an effective strategy to prevent adverse side effects of VEGF-A–based angiogenic therapy.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Contribution: M.L. designed and performed research, and wrote the paper; A.Z. contributed vital new reagents and performed research; R.d.N. contributed practically and analyzed data, S.C.d.J., I.B., and C.v.d.L. contributed practically; I.K. and E.A.L. contributed analytical tools; T.J.C.v.B. reviewed the paper; S.Y.-H. contributed new reagents; C.W. designed research; E.A.L.B. designed research, wrote the paper, and reviewed the paper.
Acknowledgments
We wish to thank Vivian de Waard and Renate Hofer-Warbinek for valuable corrections and suggestions to the paper and Kiril Bidzhekov for technical support.
This work was supported by grants of the European community (MEIF-CT-2003-500034), the Netherlands Heart Foundation (2003T201), and the Molecular Cardiology Program (M93.001). The authors belong to the European Vascular Genomics Network (http://www.evgn.org), a Network of Excellence supported by the European Community's Sixth Framework Program for Research Priority 1 (Life Sciences, Genomics, and Biotechnology for Health; contract LSHM-CT-2003-503254).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal