Abstract
The T-cell protein tyrosine phosphatase (TC-PTP) is a negative regulator of the Jak/Stat cytokine signaling pathway. Our study shows that the absence of TC-PTP leads to an early bone marrow B-cell deficiency characterized by hindered transition from the pre-B cell to immature B-cell stage. This phenotype is intrinsic to the B cells but most importantly due to bone marrow stroma abnormalities. We found that bone marrow stromal cells from TC-PTP−/− mice have the unique property of secreting 232-890 pg/mL IFN-γ. These high levels of IFN-γ result in 2-fold reduction in mitotic index on IL-7 stimulation of TC-PTP−/− pre-B cells and lower responsiveness of IL-7 receptor downstream Jak/Stat signaling molecules. Moreover, we noted constitutive phosphorylation of Stat1 in those pre-B cells and demonstrated that this was due to soluble IFN-γ secreted by TC-PTP−/− bone marrow stromal cells. Interestingly, culturing murine early pre-B leukemic cells within a TC-PTP–deficient bone marrow stroma environment leads to a 40% increase in apoptosis in these malignant cells. Our results unraveled a new role for TC-PTP in normal B lymphopoiesis and suggest that modulation of bone marrow microenvironment is a potential therapeutic approach for selected B-cell leukemia.
Introduction
The T-cell protein tyrosine phosphatase (TC-PTP) is an intracellular enzyme encoded by Ptpn2. It is ubiquitously expressed, with highest levels in hematopoietic tissues (for a review, see Bourdeau et al1 ). TC-PTP−/− mice appear physically normal until 10 to 14 days of age, at which time they progressively develop tissue mononuclear cell infiltrates.2 Elevated levels of IFN-γ can be measured at 19 days of age,3 and the animals die between 21 and 35 days of age. TC-PTP−/− mice display defective hematopoiesis and immune function, characterized by anemia and splenomegaly secondary to sequestration of erythrocytes and accumulation of myeloid cells.2 TC-PTP was also shown to interact with TRAF2 downstream of the TNF proinflammatory cytokine. This interaction inactivated Src and suppressed MAPK signaling.4 TC-PTP has been identified as a critical regulator of colony-stimulating factor 1 (CSF-1) signaling and mononuclear phagocyte development. On CSF-1 stimulation, a deficiency in TC-PTP leads to enhanced tyrosine phosphorylation of the Grb2/Gab2/Shp2 complex by the CSF-1 receptor and increased activation of Erk.5 These results identified TC-PTP as a key modulator of inflammatory signals as well as macrophage and lymphocyte functions
TC-PTP has been shown to control cytokine signaling events by its negative action on the Janus kinase (Jak) and signal transducer and activator of transcription (Stat) pathways (reviewed by Bourdeau et al1 ). This cascade is crucial for hematopoietic development and cellular response to growth factors.6 Using an in vitro approach, TC-PTP substrate-trapping mutant D/A was shown to interact with Jak1 and Jak3.7 Stat1, Stat3, and Stat5a/5b were also identified as substrates for TC-PTP.8–10 It is clear that TC-PTP is a key director of several cytokine-signaling pathways, and thus may be involved in the development of multiple hematopoietic lineages.
Here, we report that bone marrow stromal cells from TC-PTP−/− mice secrete abnormally high levels of IFN-γ, resulting in constitutive phosphorylation of Stat1 in pre-B cells and altered B-cell development in the bone marrow. Our findings are novel and reflect the therapeutic potential of TC-PTP as a modulator of bone marrow stroma microenvironment that could be exploited in leukemia and other immune disorders.
Materials and methods
Mice
Generation of TC-PTP mutant mice was described previously.2 Experiments were performed with mice on a mixed Balb/c-129SJ background and with mice backcrossed for 8 generation on Balb/c. All procedures were approved by the McGill University Research and Ethics Animal Committee, and experiments were carried out according to the Canadian Council on Animal Care ethical regulations.
Cell lines
The murine pre-B lymphoblastic leukemia cell lines ABE 8.1/2 and 70Z/3 were purchased from American Type Culture Collection (Manassas, VA) and were cultured according to the manufacturer's recommendations.
Methylcellulose hematopoietic colony-forming assay
Bone marrow cells from postnatal day 1 (P1), 4 (P4), and 7 (P7) were isolated from TC-PTP+/+ and TC-PTP−/− animals. Cells (5 × 104) were cultured in duplicate in 1% methylcellulose medium M3630 (StemCell Technologies, Vancouver, BC, Canada) to generate pre-B–cell colonies. Granulocyte, monocyte, erythroid cell, and megakaryocyte colonies were obtained by incubation of 1 × 104 bone marrow cells in 1% methylcellulose medium M3434 (StemCell Technologies). Colonies were scored after 7 days for pre-B cells, and other at 14 days under and inverted microscope at × 40 magnification.
Primary bone marrow stromal cell cultures
Bone marrow stroma was isolated by lineage depletion (CD3+, CD4+, CD5+, CD8+, CD11b+, CD19+, DX5+, Sca-1+, Ter119+ cells) using magnetic beads (EasySep, StemCell Technologies) and cultured in Iscove modified Dulbecco media supplemented with 10% FBS and antibiotics. Confluent stromal cells devoid of hematopoietic cells were obtained by serial passage. Experiments were performed using only fresh cells from passage 5 or 6.
Generation of bone marrow pre-B–cell cultures
Murine bone marrow cells obtained at P7 were cultured in the presence of 20 ng/mL mouse recombinant IL-7 (StemCell Technologies). After 5 to 10 days, nonadherent cells were on average more than 90% pre-B cells.
Flow cytometry
Fresh bone marrow cell suspensions were obtained from TC-PTP+/+ and TC-PTP−/− mice from P1 to P7. Antibodies directly conjugated with FITC, PE, PECy5, PECy7, or APC were purchased from BD Biosciences (Mississauga, ON, Canada) or Biolegend (Vineland, ON, Canada). Cell surface staining and flow cytometry analysis were performed as previously described.11 Intracellular staining was achieved by fixing cells in 1.5% formaldehyde for 10 minutes and permeabilizing the cell membrane with 0.05% Triton for 10 minutes. Phospho-Stat1-PE antibody and rat anti–mouse IFN-γ was purchased from BD Biosciences. Rabbit anti–mouse Stat1 antibody was obtained from Cell Signaling Technology (Danvers, MA), and anti–rabbit or anti–rat FITC conjugate from Invitrogen-Molecular Probes (Burlington, ON, Canada). Apoptosis was detected using annexin V–FITC and propidium iodide staining according to the manufacturer's instructions (Biosource, Camarillo, CA).
Proliferation assay and mitotic index
Bone marrow cells obtained at P7 were labeled with 5 μM CFDA-SE (Invitrogen-Molecular Probes) and cultured for 5 days in the presence of 20 ng/mL mouse recombinant IL-7 (StemCell Technologies). Nonadherent cells were analyzed by flow cytometry. The mitotic activity was estimated from the flow cytometry data assuming that 2 cells of a given CFDA-SE intensity arose from the single mitosis of a cell possessing a CFDA-SE intensity immediately greater. The division index = (100−Y)/Y, where Y (%) = x0 + x1/2 + x2/4 + x3/8 + x4/16 + x5/32 + x6/64 + x7/128 and x0 represents the percentage of B cells that have not divided.12
Western blot and quantitation
Bone marrow pre-B cells were collected and starved for 2 hours. Cells were stimulated for 5 minutes at 37°C with 20 ng/mL mouse recombinant IL-7 or 500 U/mL IFN-γ (R&D Systems, Minneapolis, MN). Unstimulated and stimulated cells were lysed in 150 mM NaCl/50 mM Tris HCl, pH 7.5/0.5% NP-40/0,13% sodium deoxycholate/1 mM Na3VO4/10 mM NaF supplemented with Complete EDTA-free protease inhibitor mixture (Roche, Mississauga, ON, Canada). Protein samples were resolved on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to immunoblotting. Phosphorylated Jak1, Stat1, and Stat5 proteins were detected using polyclonal antibodies from Cell Signaling Technology. Total Jak1, Stat1, and Stat5 proteins were detected using polyclonal antibodies from (Santa Cruz Biotechnology, Santa Cruz, CA). β-Actin monoclonal antibody was purchased from Sigma (Oakville, ON, Canada) and TC-PTP was detected with monoclonal antibody 3E2. Primary antibodies were followed by horseradish peroxidase-conjugated goat anti–rabbit or mouse IgG (Jackson ImmunoResearch Laboratories, Bar Harbor, ME). Blots were revealed using Western lightning chemiluminescent substrate (Perkin Elmer, Boston, MA). Bands obtained on film were scanned, digitized, and quantitated using National Institutes of Health image software. Mean intensity values were calculated and compared between TC-PTP+/+ and TC-PTP−/− animals.
RT-PCR
Total RNA was extracted from purified pre-B cells, stromal cells, and spleen tissue from TC-PTP+/+ and TC-PTP−/− mice by using TRIzol (Invitrogen-Molecular Probes), and first-strand cDNA synthesis was obtained from 1 μg RNA with random hexamers using SuperScript II reverse transcriptase (Invitrogen-Molecular Probes) according to the manufacturer's instructions. Aliquots of 2 μL of the reverse transcription (RT) reaction were amplified using 1 U AmpliTaq Gold (Applied Biosystems, Streetsville, ON, Canada) and one or 2 sets of synthesized primers under the following polymerase chain reaction (PCR) conditions: 5 minutes at 95°C, 30 seconds at 94°C, 30 seconds at 55°C, and 30 seconds at 72°C for 30 cycles, 2 minutes at 72°C) in a 25-μL reaction mixture. Primers (20 pmol each): 5′IFN-γ 5′-ATCCTTTGGACCCTCTGACTT-3′; 3′IFN-γ, 5′-TAATCTGGCTCTGCAGGATTT-3′; 5′β-actin, 5′-CCCAGATCATGTTTGAGACCT-3′; 3′β-actin, 5′-CAGGCAGCTCATAGCTCTTCT-3′. Reaction products were separated by 1% agarose gel electrophoresis and detected with ethidium bromide staining.
ELISA
IFN-γ secreted by bone marrow stromal cells was measured using 24-hour culture supernatant with OptEIA mouse IFN-γ enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (BD Biosciences). Absorbance was measured at 450 nm on an ELISA plate reader and standardized with a recombinant mouse IFN-γ standard curve.
Coculture experiments
Confluent bone marrow stromal cell cultures were obtained from TC-PTP+/+ and TC-PTP−/− mice. Culture media was replaced 24 hours before the experiment and anti–mouse IFN-γ monoclonal antibody (BD Biosciences) was added to the appropriate cultures. Pre-B cells were collected and starved for 2 hours. Pre-B cells (400 000 cells) were then stimulated for 5 minutes or 15 minutes at 37°C with bone marrow stromal cell culture supernatant. Similarly, 1 × 106 leukemic pre-B cells were cocultured for 24 hours with supernatant harvested from TC-PTP+/+ and TC-PTP−/− stromal cells.
Statistical analysis
All statistical differences were determined by 2-tailed, unpaired Student t test or ANOVA analysis.
Results
Cell autonomous and bone marrow stroma–dependent early developmental B-cell defect
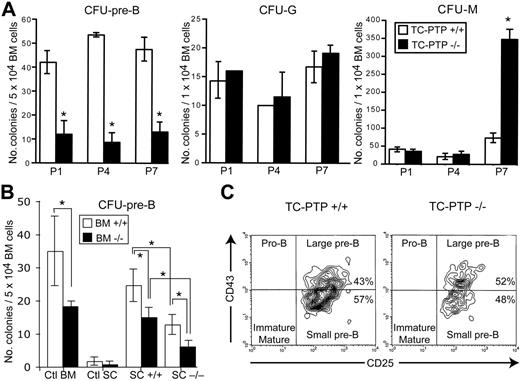
To identify the earliest hematopoietic defect in TC-PTP−/− mice, bone marrow cells were isolated from TC-PTP+/+ and TC-PTP−/− animals at P1, P4, and P7, and analyzed by colony assays (Figure 1A). A 4-fold decrease in the number of pre-B–cell colonies (CFU–pre-B) was observed as early as P1 in TC-PTP−/− cultures, whereas the granulocyte population (CFU-G) appeared normal throughout the first 7 days of life. A defect in the monocyte population (CFU-M) became detectable at P7 in TC-PTP−/− mice, when a 6-fold increase in this population was observed compared to control (Figure 1A). These results suggest that the first defect seen in TC-PTP−/− mice resides in the B-cell population, thus implicating TC-PTP in the regulation of B-cell development.
Bone marrow colony-forming assays. (A) Whole bone marrow was harvested from wild-type (WT; □) and TC-PTP−/− (KO; ▪) mice at P1 (3 WT, 3 KO), P4 (2 WT, 4 KO), and P7 (4 WT, 3 KO) and incubated in methylcellulose medium. The total number of colonies per culture dish is reported as mean ± SD. *P < .002. (B) Whole bone marrow was harvested from wild-type (WT; □) and TC-PTP−/− (KO; ▪) mice at P7 (8 WT, 6 KO). Cells were cultured in methylcellulose medium supplemented with IL-7 in uncoated culture dishes (Ctl BM) or on monolayers of bone marrow stromal cells from WT mice (SC +/+) or KO mice (SC −/−). As control, marrow stromal cells were cultured with methylcellulose medium containing IL-7 without bone marrow cells (Ctl SC). The total number of colonies per culture dish is reported as mean ± SD. *P < .002. (C) Flow cytometry analysis of CFU–pre-B cells recovered from methylcellulose cultures. Analysis was gated on B220+ cells (95% B220+ for TC-PTP+/+ and 94% B220+ for TC-PTP−/−) to include only B cells. Representative data are shown for flow cytometry of TC-PTP+/+ and TC-PTP−/− cultures. All experiments were repeated at least 3 times.
Bone marrow colony-forming assays. (A) Whole bone marrow was harvested from wild-type (WT; □) and TC-PTP−/− (KO; ▪) mice at P1 (3 WT, 3 KO), P4 (2 WT, 4 KO), and P7 (4 WT, 3 KO) and incubated in methylcellulose medium. The total number of colonies per culture dish is reported as mean ± SD. *P < .002. (B) Whole bone marrow was harvested from wild-type (WT; □) and TC-PTP−/− (KO; ▪) mice at P7 (8 WT, 6 KO). Cells were cultured in methylcellulose medium supplemented with IL-7 in uncoated culture dishes (Ctl BM) or on monolayers of bone marrow stromal cells from WT mice (SC +/+) or KO mice (SC −/−). As control, marrow stromal cells were cultured with methylcellulose medium containing IL-7 without bone marrow cells (Ctl SC). The total number of colonies per culture dish is reported as mean ± SD. *P < .002. (C) Flow cytometry analysis of CFU–pre-B cells recovered from methylcellulose cultures. Analysis was gated on B220+ cells (95% B220+ for TC-PTP+/+ and 94% B220+ for TC-PTP−/−) to include only B cells. Representative data are shown for flow cytometry of TC-PTP+/+ and TC-PTP−/− cultures. All experiments were repeated at least 3 times.
To assess the individual contribution of cell autonomous and bone marrow microenvironment defects in causing perturbed B lymphopoiesis, primary bone marrow stromal cells were evaluated for their ability to support hematopoiesis. Wild-type or TC-PTP–deficient bone marrow stromal cells were isolated and cocultured with either TC-PTP+/+ or TC-PTP−/− hematopoietic cells. These stromal cells were CD34+, Sca-1−, CD31−, CD11b−, CD11c−, CD49b−, CD3−, B220− as demonstrated in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). The differentiation potential of conditioned progenitor cells into pre-B cells was then assayed (Figure 1B). Compared to wild-type cocultures, a 2-fold decrease in the number of pre-B–cell colonies was observed when TC-PTP−/− bone marrow was incubated on wild-type stroma. Moreover, when normal bone marrow was incubated on TC-PTP−/− stroma, a similar 2-fold decrease in the number of colonies was observed compared to wild-type cocultures. Thus, bone marrow stroma and B-cell autonomous defects appear to contribute equally to impaired B lymphopoiesis, at least under in vitro culture conditions. When TC-PTP−/− hematopoietic cells were cocultured with TC-PTP−/− stroma, a further decrease in the number of pre-B colonies was observed (Figure 1B) indicating the defects are additive. Phenotypic characterization of the colonies by flow cytometry confirmed their pre-B identity (B220+, CD43+, CD25+, large pre-B; B220+, CD43−, CD25+, small pre-B) as well as similar proportion of pure B220+ colonies and pre-B subsets (Figure 1C). Therefore, the B-cell defect observed in TC-PTP−/− mice results from both cell autonomous and bone marrow microenvironment abnormalities.
Altered distribution of B-cell subsets in TC-PTP–deficient mice
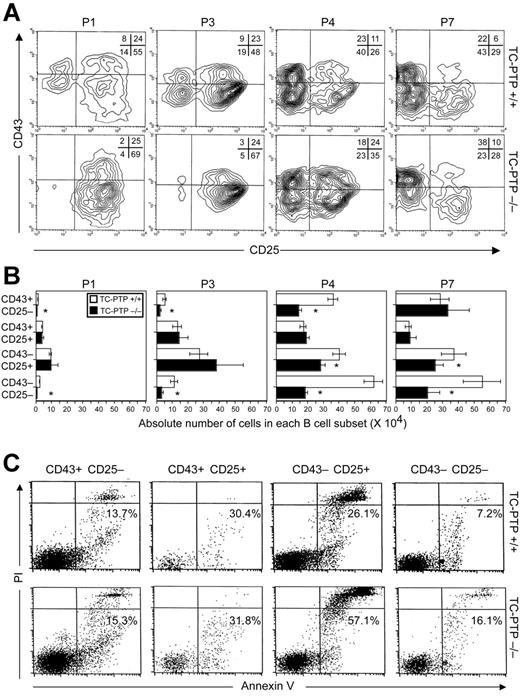
Having determined the developmental age at which defective B lymphopoiesis occurs, we identified which developmental stage is affected by the absence of TC-PTP. Differential expression of cell surface markers was used to subdivide bone marrow B cells into pro-B–cell (B220+, CD43+, CD25−), large pre-B–cell (B220+, CD43+, CD25+), small pre-B–cell (B220+, CD43−, CD25+), and immature/mature B-cell (B220+, CD43−, CD25−) subpopulations (Figure 2A). In TC-PTP+/+ marrow, approximately 75% of cells were in pre-B stages of development until P3, reflecting persistence of fetal hematopoiesis. After P4, B cells progressed to the immature stage of development, indicating transition to adult-type B lymphopoiesis (Figure 2A). In TC-PTP−/− bone marrow, B-cell precursors readily progressed to the pre-B stage but were hindered in their transition to the immature B-cell stage, as demonstrated by a profound reduction in the CD25−CD43− cell population in TC-PTP−/− marrow compared to control. Concomitantly, a relative increase in the pro-B–cell population was observed in adult TC-PTP−/− bone marrow at P7 (Figure 2A). Because the CD25−CD43− B-cell fraction contained both immature and mature recirculating B cells, further analysis of this population with IgM and IgD showed a 50% decrease in immature (IgM+, IgD−), and, as reported by You-Ten et al,2 fewer B cells are recirculating back to the bone marrow (IgM+, IgD+; Figure S2A). Therefore, the transition from the pre-B to immature B stage of development is impaired in the absence of TC-PTP.
B-cell development in TC-PTP+/+ and TC-PTP−/− bone marrow. Whole bone marrow was harvested from TC-PTP+/+ (WT) and TC-PTP−/− (KO) mice at P1 (3 WT, 3 KO), P3 (3 WT, 3 KO), P4 (4 WT, 4 KO), and P7 (11 WT, 6 KO). (A) Cells were stained for flow cytometry and analysis was gated on B220+ cells to include only B cells (∼20% B220+ at P1, P3 and ∼25% B220+ at P4, P7). Representative data are shown for each time point. The percentage of each subpopulation is shown in the upper right quadrant. (B) Absolute cell counts for each B-cell subset from TC-PTP+/+ (□) and TC-PTP−/− (▪) are illustrated. Cell counts were derived by multiplying the percentage of total cells, obtained by flow cytometry for each subpopulation, by the whole marrow cell count, obtained by manual counting with a hemocytometer. Cell counts are reported as mean ± SD. *P < .01. (C) Whole bone marrow was harvested from TC-PTP+/+ (WT) and TC-PTP−/− (KO) mice at P7 (5 WT, 5 KO). Cells (1 × 106) were stained for surface expression of CD25, CD43, and B220, and with annexin V and propidium iodide (PI), and analyzed by flow cytometry. Analysis was gated on B220+ cells to include only B cells. The combined percentage of preapoptotic (annexin V+, PI−) and apoptotic (annexin V+, PI+) cells is provided. Representative data are shown for each subset. All experiments were repeated at least 3 times.
B-cell development in TC-PTP+/+ and TC-PTP−/− bone marrow. Whole bone marrow was harvested from TC-PTP+/+ (WT) and TC-PTP−/− (KO) mice at P1 (3 WT, 3 KO), P3 (3 WT, 3 KO), P4 (4 WT, 4 KO), and P7 (11 WT, 6 KO). (A) Cells were stained for flow cytometry and analysis was gated on B220+ cells to include only B cells (∼20% B220+ at P1, P3 and ∼25% B220+ at P4, P7). Representative data are shown for each time point. The percentage of each subpopulation is shown in the upper right quadrant. (B) Absolute cell counts for each B-cell subset from TC-PTP+/+ (□) and TC-PTP−/− (▪) are illustrated. Cell counts were derived by multiplying the percentage of total cells, obtained by flow cytometry for each subpopulation, by the whole marrow cell count, obtained by manual counting with a hemocytometer. Cell counts are reported as mean ± SD. *P < .01. (C) Whole bone marrow was harvested from TC-PTP+/+ (WT) and TC-PTP−/− (KO) mice at P7 (5 WT, 5 KO). Cells (1 × 106) were stained for surface expression of CD25, CD43, and B220, and with annexin V and propidium iodide (PI), and analyzed by flow cytometry. Analysis was gated on B220+ cells to include only B cells. The combined percentage of preapoptotic (annexin V+, PI−) and apoptotic (annexin V+, PI+) cells is provided. Representative data are shown for each subset. All experiments were repeated at least 3 times.
Absolute cell counts derived from the percentage of B220+ cells obtained by flow cytometry (∼20% at P1, P3 and ∼25% at P4, P7) further substantiate these findings (Figure 2B). At P1 and P3, when fetal hematopoiesis is still occurring, the number of pro-B cells in TC-PTP−/− marrow was reduced significantly compared to control. In addition, whereas the number of immature/mature B cells increased in TC-PTP+/+ marrow between P1 and P3, there was only a modest increase in this population in TC-PTP−/− marrow, whereas small pre-B cells accumulated abnormally. Between P4 and P7, following transition to adult-type hematopoiesis, the number of pro-B and large pre-B cells progressively normalized in TC-PTP−/− marrow. However, transition from the pre-B– to immature/mature B-cell stage remained impaired. Indeed, whereas the number of immature/mature B cells increased 1.5-fold compared to small pre-B cells in wild-type bone marrow, a 1.5-fold reduction following this transition was observed in TC-PTP−/− marrow (Figure 2B). This reduction is confirmed by the isolation of pure immature B cells from mature recirculating B cells (Figure S2B). Moreover, small pre-B cells were fewer in number in TC-PTP−/− marrow compared to control, suggesting cell loss at this stage of development (Figure 2B). These results demonstrate that the B-cell defect resides in the small pre-B–cell and immature B-cell subpopulations. Increased apoptosis explains, at least in part, the cellular loss at these specific stages of B-cell development as demonstrated by flow cytometry after staining each B-cell subset with annexin V and propidium iodide (Figure 2C).
Reduced B-cell proliferation in response to IL-7 stimulation
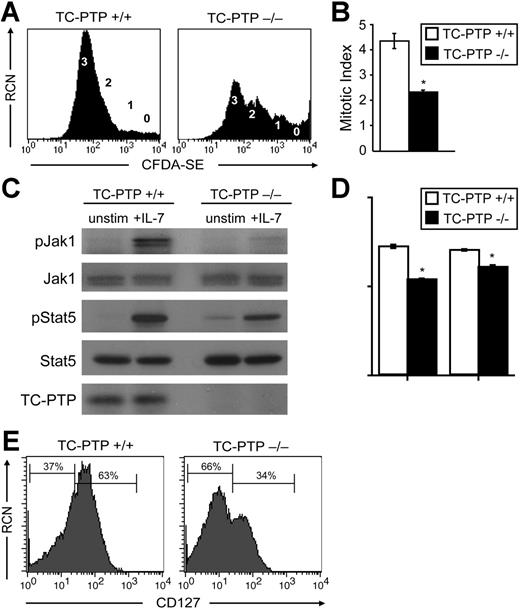
To better characterize the molecular mechanism underlying the B-cell defect, we isolated bone marrow from P7 animals and generated nearly pure pre-B cells in the presence of IL-7. After 5 to 10 days in culture, although fewer cells were produced in TC-PTP−/− cultures (Figure S3A), the proportion of B220+ cells were identical to control (> 95%) and the majority of cells were pre-B (Figure S3B). We monitored 5-day proliferation with the cell tracer CFDA-SE in the presence of IL-7. Analysis by flow cytometry showed that more than 75% of B cells from TC-PTP+/+ mice had divided 3 times, whereas less than 45% from TC-PTP−/− animals had done so. A significant number of B cells had not divided (“0”) or had undergone 2 or fewer cell divisions (Figure 3A). Similar results were obtained when cells were labeled from day 5 to day 10 (Figure S3C). The mitotic index can be calculated from the flow cytometry data12 (Figure 3B). By this method, a near 2-fold decrease in mitotic index was calculated between wild-type and TC-PTP–deficient mice (4.34 ± 0.31 versus 2.32 ± 0.06), further demonstrating the reduced proliferation in response to IL-7 in TC-PTP−/− B cells (Figure 3B). Cellular loss in this in vitro system by apoptosis of pre-B cells was also additive to the decreased proliferation (Figure S3B).
Effects of IL-7 stimulation and IL-7 receptor expression in B cells. (A-B) Whole bone marrow was harvested from TC-PTP+/+ (WT) and TC-PTP−/− (KO) mice at P7 (7 WT, 3 KO). Pre-B cells, generated in vitro as described in “Materials and methods,” were labeled with CFDA-SE for 5 days and stained for surface expression of B220 for flow cytometry. Analysis was gated on B220+ cells to include only B cells. Purity and yield are described in Figure S3. (A) Relative cell number (RCN) is plotted against CFDA-SE fluorescence. The number of cell divisions is indicated next to the corresponding CFDA-SE peak. (B) The mitotic index of TC-PTP+/+ (□) and TC-PTP−/− (▪) B cells is reported as mean ± SD. *P < .001. (C-D) Whole bone marrow was harvested from TC-PTP+/+ (WT) and TC-PTP−/− (KO) mice at P7 (4 WT, 4 KO). Pre-B cells were cultured in vitro, as described in “Materials and methods,” and purity and yield are described in Figure S3. Pre-B cells were harvested and starved prior to stimulation with IL-7 (+IL-7) for 5 minutes or left untreated (unstim). Cell lysates were prepared and fractionated on 8% SDS-PAGE. (C) Western blot analysis of phosphorylated Jak1 (pJak1), total Jak1 (Jak1), phosphorylated Stat5 (pStat5), total Stat5 (Stat5), and TC-PTP was performed. Each sample contained 17 μg protein. (D) Quantitation of Western blot analysis. □, TC-PTP+/+; ▪, TC-PTP−/−. Fold increase in the density of pJak1 and pStat5 are provided relative to unstimulated controls and after normalization to total density of Jak1 and Stat5 protein, respectively. Data are provided as mean ± SD. *P < .01. (E) Whole bone marrow was harvested from TC-PTP+/+ and TC-PTP−/− mice at P7. Ex vivo cells (1 × 106) were stained for surface expression of B220, CD25 and CD43, and CD127 (IL-7Rα) and analyzed by flow cytometry. Analysis was gated on B220+, CD43+ or CD43−, and CD25+ cells to include only pre-B cells. RCN is plotted against CD127 fluorescence. The percentage of CD127− and CD127+ cells is indicated. All experiments were repeated at least 3 times.
Effects of IL-7 stimulation and IL-7 receptor expression in B cells. (A-B) Whole bone marrow was harvested from TC-PTP+/+ (WT) and TC-PTP−/− (KO) mice at P7 (7 WT, 3 KO). Pre-B cells, generated in vitro as described in “Materials and methods,” were labeled with CFDA-SE for 5 days and stained for surface expression of B220 for flow cytometry. Analysis was gated on B220+ cells to include only B cells. Purity and yield are described in Figure S3. (A) Relative cell number (RCN) is plotted against CFDA-SE fluorescence. The number of cell divisions is indicated next to the corresponding CFDA-SE peak. (B) The mitotic index of TC-PTP+/+ (□) and TC-PTP−/− (▪) B cells is reported as mean ± SD. *P < .001. (C-D) Whole bone marrow was harvested from TC-PTP+/+ (WT) and TC-PTP−/− (KO) mice at P7 (4 WT, 4 KO). Pre-B cells were cultured in vitro, as described in “Materials and methods,” and purity and yield are described in Figure S3. Pre-B cells were harvested and starved prior to stimulation with IL-7 (+IL-7) for 5 minutes or left untreated (unstim). Cell lysates were prepared and fractionated on 8% SDS-PAGE. (C) Western blot analysis of phosphorylated Jak1 (pJak1), total Jak1 (Jak1), phosphorylated Stat5 (pStat5), total Stat5 (Stat5), and TC-PTP was performed. Each sample contained 17 μg protein. (D) Quantitation of Western blot analysis. □, TC-PTP+/+; ▪, TC-PTP−/−. Fold increase in the density of pJak1 and pStat5 are provided relative to unstimulated controls and after normalization to total density of Jak1 and Stat5 protein, respectively. Data are provided as mean ± SD. *P < .01. (E) Whole bone marrow was harvested from TC-PTP+/+ and TC-PTP−/− mice at P7. Ex vivo cells (1 × 106) were stained for surface expression of B220, CD25 and CD43, and CD127 (IL-7Rα) and analyzed by flow cytometry. Analysis was gated on B220+, CD43+ or CD43−, and CD25+ cells to include only pre-B cells. RCN is plotted against CD127 fluorescence. The percentage of CD127− and CD127+ cells is indicated. All experiments were repeated at least 3 times.
The phosphorylation status of Jak1 and Stat5, which are implicated in signal transduction through the IL-7R, was assessed in cultured B cells by Western blot analysis (Figure 3C). As expected, on IL-7 stimulation of its receptor, phosphorylation of Jak1 and Stat5 was observed in TC-PTP+/+ B cells. Surprisingly, a marked reduction in the phosphorylation of Jak1 and Stat5 was seen in B cells from TC-PTP−/− mice. No difference was observed in the total amount of Jak1 and Stat5 protein in each sample (Figure 3C). Quantitation of the relative density of phosphorylated Jak1 normalized on total Jak1 protein revealed a 25% reduction in phosphorylated Jak1 in TC-PTP−/− animals. Similarly, the density of phosphorylated Stat5 in TC-PTP−/− mice was diminished by 14% when normalized on total Stat5 protein (Figure 3D). Thus, despite the absence of a known negative regulator of Jak1 and Stat5,7 hypophosphorylation of both proteins was observed in B cells from TC-PTP−/− mice, pointing to an additional indirect effect of this phosphatase. Because Jak1 binds directly to the activated IL-7R, hypophosphorylation of this protein suggests that abnormal trafficking or function of the IL-7R might be the cause of the observations described. Therefore, surface expression of CD127 (IL-7Rα) on B cells was measured by flow cytometry (Figure 3E). A 54% reduction in the number of B cells expressing CD127 was observed in TC-PTP−/− mice compared to control B cells. Thus, reduction in surface expression of IL-7R is associated with hypophosphorylation of Jak1 and Stat5 and reduced proliferation in response to IL-7 stimulation in B cells from TC-PTP–deficient mice, pointing to an indirect effect of TC-PTP on IL-7R.
Hyperphosphorylation of Stat1 in TC-PTP–deficient B cells
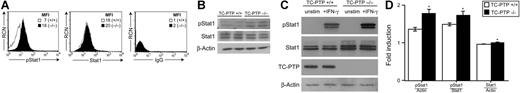
It has been reported that IFN-γ decreases responsiveness of B cells to IL-7.13 Moreover, our group has recently shown that TC-PTP−/− mice progressively develop a systemic inflammatory disease associated with elevated serum IFN-γ levels.3 Accordingly, abnormal IFN-γ stimulation may be responsible for the impaired responsiveness of TC-PTP−/− B cells to IL-7. To study this possibility, we first examined Stat1 phosphorylation in freshly isolated P7 bone marrow B cells from TC-PTP+/+ and TC-PTP−/− mice, since this protein becomes phosphorylated on activation of the IFN-γR. Using flow cytometry, we determined that pre-B cells from TC-PTP−/− mice showed a 2.5-fold increase in Stat1 phosphorylation compared to TC-PTP+/+ animals (mean fluorescent intensity [MFI] 18 versus 7; Figure 4A). Equivalent amounts of Stat1 protein were detected (MFI, 20 versus 18). We confirmed these results by Western blot analysis (Figure 4B). Our data suggest that Stat1 is constitutively activated in B cells from TC-PTP−/− mice, supporting the notion that abnormal IFN-γ signaling may be occurring in these animals.
Stat1 phosphorylation in bone marrow B cells. (A) Whole bone marrow was harvested from TC-PTP+/+ (□) and TC-PTP−/− (▪) mice at P7. Ex vivo cells were stained for surface expression of B220 and intracellular expression of Stat1 and phosphorylated Stat1 (pStat1), for flow cytometry analysis. Nonspecific IgG was used as negative control for intracellular staining. Analysis was gated on B220+ cells to include only B cells. RCN is plotted against pStat1, Stat1, or IgG fluorescence. MFI is indicated. (B) Whole bone marrow was harvested from TC-PTP+/+ (WT) and TC-PTP−/− (KO) mice at P7 (3 WT, 3 KO). Ex vivo pre-B–cell lysates were prepared and fractionated on 8% SDS-PAGE. Western blot analysis of phosphorylated Stat1 (pStat1), total Stat1 (Stat1), and β-actin was performed in duplicate. Each sample contained 15 μg protein. (C) Whole bone marrow was harvested from TC-PTP+/+ and TC-PTP−/− mice at P7 (4 WT, 3 KO). Pre-B cells were cultured in vitro as described in “Materials and methods”; purity and yield are described in Figure S3. Pre-B cells were harvested and starved prior to stimulation with IFN-γ (+IFN-γ) for 5 minutes or left untreated (unstim). Cell lysates were prepared and fractionated on 8% SDS-PAGE. Western blot analysis of phosphorylated Stat1 (pStat1), total Stat1 (Stat1), TC-PTP, and β-actin was performed. Each sample contained 3.25 μg protein. (D) Quantitation of Western blot analysis. □, TC-PTP+/+; ▪, TC-PTP−/−. (D left panel) Fold increase in the density of pStat1 is provided relative to unstimulated controls and after normalization to density of β-actin (Actin) and total density of Stat1. (D right panel) Relative density of total Stat1 protein before and after normalization to total density of β-actin. Data are provided as mean ± SD. *P < .01. All experiments were repeated at least 3 times. All experiments were repeated at least 3 times.
Stat1 phosphorylation in bone marrow B cells. (A) Whole bone marrow was harvested from TC-PTP+/+ (□) and TC-PTP−/− (▪) mice at P7. Ex vivo cells were stained for surface expression of B220 and intracellular expression of Stat1 and phosphorylated Stat1 (pStat1), for flow cytometry analysis. Nonspecific IgG was used as negative control for intracellular staining. Analysis was gated on B220+ cells to include only B cells. RCN is plotted against pStat1, Stat1, or IgG fluorescence. MFI is indicated. (B) Whole bone marrow was harvested from TC-PTP+/+ (WT) and TC-PTP−/− (KO) mice at P7 (3 WT, 3 KO). Ex vivo pre-B–cell lysates were prepared and fractionated on 8% SDS-PAGE. Western blot analysis of phosphorylated Stat1 (pStat1), total Stat1 (Stat1), and β-actin was performed in duplicate. Each sample contained 15 μg protein. (C) Whole bone marrow was harvested from TC-PTP+/+ and TC-PTP−/− mice at P7 (4 WT, 3 KO). Pre-B cells were cultured in vitro as described in “Materials and methods”; purity and yield are described in Figure S3. Pre-B cells were harvested and starved prior to stimulation with IFN-γ (+IFN-γ) for 5 minutes or left untreated (unstim). Cell lysates were prepared and fractionated on 8% SDS-PAGE. Western blot analysis of phosphorylated Stat1 (pStat1), total Stat1 (Stat1), TC-PTP, and β-actin was performed. Each sample contained 3.25 μg protein. (D) Quantitation of Western blot analysis. □, TC-PTP+/+; ▪, TC-PTP−/−. (D left panel) Fold increase in the density of pStat1 is provided relative to unstimulated controls and after normalization to density of β-actin (Actin) and total density of Stat1. (D right panel) Relative density of total Stat1 protein before and after normalization to total density of β-actin. Data are provided as mean ± SD. *P < .01. All experiments were repeated at least 3 times. All experiments were repeated at least 3 times.
To directly test the effect of IFN-γ on TC-PTP−/− B cells, we stimulated starved pre-B cells with 500 U/mL IFN-γ, following which lysates were prepared for Western blot analysis (Figure 4C). On IFN-γ stimulation, phosphorylation of Stat1 was observed in B+/+ cells. However, an even greater phosphorylation of Stat1 was seen in B−/− cells. Total Stat1 protein in TC-PTP−/− B cells was mildly increased compared to control, whereas β-actin levels were equivalent in all samples. Quantitation of the relative density of phosphorylated Stat1 protein revealed a 24%-fold induction in phosphorylated Stat1 in TC-PTP−/− animals when normalized on β-actin, and 14% when normalized on total Stat1 protein (Figure 4D). The density of Stat1 in TC-PTP−/− mice was increased by 5% after normalizing for β-actin content (Figure 4D). Similar experiments were conducted using IFN-α, another known activator of Stat1, with no measurable increase in phosphorylated Stat1 expression (data not shown). Together, these results indicate that the absence of TC-PTP enhances IFN-γ–induced Stat1 phosphorylation in B cells.
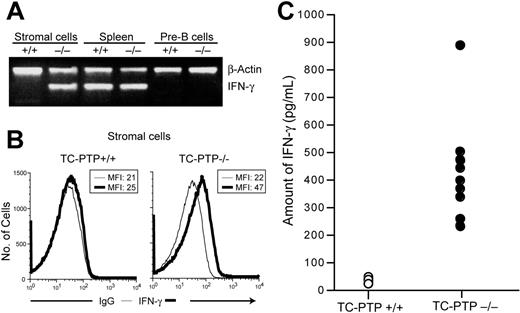
Abnormal secretion of IFN-γ by the bone marrow stroma in TC-PTP–deficient mice
The observation that B cells from TC-PTP−/− mice do not contain phosphorylated Stat1 when deprived of serum and stroma-derived stimuli, whereas hyperphosphorylation of Stat1 results when these cells are cocultured with purified TC-PTP−/− bone marrow stroma or on stimulation with IFN-γ, but not other cytokines, suggests that marrow stromal cells might secrete IFN-γ. To determine if this is the case, we assessed the expression of IFN-γ mRNA in purified bone marrow stromal cells (Figure S1) and pre-B cells by semiquantitative RT-PCR (Figure 5A). IFN-γ transcripts could not be detected in pre-B cells of either TC-PTP+/+ or TC-PTP−/− mice but were readily detectable in the spleen of both animals. TC-PTP+/+ stroma did not express detectable levels of IFN-γ mRNA. However, abundant expression was observed in TC-PTP−/− stroma. Intracellular flow cytometry also detected IFN-γ in stromal cell cultures; a nearly 2-fold shift in MFI can be detected in TC-PTP−/− bone marrow stromal cells (Figure 5B). The amount of IFN-γ secreted by primary bone marrow stromal cell cultures was quantitated by ELISA (Figure 5C). TC-PTP−/− bone marrow stromal cells secreted between 232 and 890 pg/mL IFN-γ after 24 hours in culture, whereas the concentration of IFN-γ in wild-type cultures barely exceeded the detection limit of the assay (40 ± 6 pg/mL). Thus, we observed a 12-fold increase in IFN-γ secretion by TC-PTP−/− bone marrow stromal cells compared to control. Taken together, these results suggest that abnormal secretion of IFN-γ by the bone marrow stromal cells could result in Stat1 hyperphosphorylation and decreased IL-7 responsiveness.
Secretion of IFN-γ by TC-PTP−/− bone marrow stromal cells. (A) Semiquantitative RT-PCR analysis of IFN-γ expression. cDNA was prepared from purified bone marrow stromal cells and pre-B cells and from total spleen. The presence of IFN-γ and β-actin transcripts was assayed by PCR in the same reaction. (B) Bone marrow stromal cell cultures (passage 5) from TC-PTP+/+ and TC-PTP−/− mice at P7 were stained for surface expression of markers shown in Figure S1 and intracellular expression of IFN-γ for flow cytometry analysis. Nonspecific IgG was used as negative control for intracellular staining. RCN is plotted against IgG (thin line) or IFN-γ (thick line) fluorescence. MFI is indicated. (C) Quantitation of IFN-γ secretion by ELISA. Primary bone marrow stromal cell cultures were established. After 5 or 6 passages, the culture medium was replaced and the concentration of IFN-γ was measured by ELISA 24 hours later. Data from 10 TC-PTP+/+ (○) and 9 TC-PTP−/− (•) stromal cell cultures are represented. The difference between the 2 groups was significant (P < .02). All experiments were repeated at least 3 times.
Secretion of IFN-γ by TC-PTP−/− bone marrow stromal cells. (A) Semiquantitative RT-PCR analysis of IFN-γ expression. cDNA was prepared from purified bone marrow stromal cells and pre-B cells and from total spleen. The presence of IFN-γ and β-actin transcripts was assayed by PCR in the same reaction. (B) Bone marrow stromal cell cultures (passage 5) from TC-PTP+/+ and TC-PTP−/− mice at P7 were stained for surface expression of markers shown in Figure S1 and intracellular expression of IFN-γ for flow cytometry analysis. Nonspecific IgG was used as negative control for intracellular staining. RCN is plotted against IgG (thin line) or IFN-γ (thick line) fluorescence. MFI is indicated. (C) Quantitation of IFN-γ secretion by ELISA. Primary bone marrow stromal cell cultures were established. After 5 or 6 passages, the culture medium was replaced and the concentration of IFN-γ was measured by ELISA 24 hours later. Data from 10 TC-PTP+/+ (○) and 9 TC-PTP−/− (•) stromal cell cultures are represented. The difference between the 2 groups was significant (P < .02). All experiments were repeated at least 3 times.
The IFN-γ secreted by TC-PTP−/− bone marrow stromal cells directly affects homeostasis of B cells
We have demonstrated that bone marrow stromal cells from TC-PTP−/− mice secrete significant amounts of IFN-γ. To directly assess if this cytokine secretion is responsible for the hyperphosphorylation of Stat1 in pre-B cells, we harvested pre-B cells from TC-PTP+/+ and TC-PTP−/− mice at P7 and stimulated them in vitro with the culture supernatant of either wild-type or TC-PTP–deficient bone marrow stromal cells with or without the presence of IFN-γ–capturing antibodies. We analyzed our results by intracellular fluorescent staining and flow cytometry comparing the unstimulated to the stimulated pStat1 as well as total Stat1 proteins for each culture condition (Figure 6A). When the culture supernatant from TC-PTP+/+ stromal cells (with or without IFN-γ antibodies) was harvested to stimulate TC-PTP+/+ or TC-PTP−/− pre-B cells, no shift in the pStat1 MFI could be observed between unstimulated and stimulated samples (Figure 6A top). However, when TC-PTP+/+ pre-B cells were incubated with the culture supernatant of TC-PTP−/− bone marrow stromal cells, a marked increase in MFI was observed indicating phosphorylation of Stat1 under these conditions. This increase in MFI is restored to basal levels when excess of IFN-γ antibodies have efficiently sequestered all molecules (Figure 6A bottom far left and left panels). Moreover, when TC-PTP−/− pre-B cells were incubated with the culture supernatant of TC-PTP−/− bone marrow stromal cells, a further shift in MFI was noted compared to TC-PTP+/+ pre-B cells. These results reflect the hyperphosphorylation status of Stat1 in TC-PTP−/− B cells. This augmentation in MFI is back to unstimulated values when IFN-γ capturing antibodies were used (Figure 6A bottom right and far right panels). The phosphorylation of Stat1 in both TC-PTP+/+ and TC-PTP−/− B cells was comparable to controls where exogenous IFN-γ (500 pg/mL) was added to stimulate the B cells (control panels). Total Stat1 protein levels were unchanged by IFN-γ stimulation in all coculture conditions (Figure 6A inset panels).
Stat1 response analysis of pre-B cells stimulated with bone marrow stromal cell supernatants. (A) Cultured pre-B cells (pool of either WT [B+/+] or KO [B−/−]) were stimulated 5 minutes with the supernatant of P7 TC-PTP+/+ or TC-PTP−/− bone marrow stromal cells cultures (with or without the presence of anti–IFN-γ antibody [α-IFN-γ]; 5 WT, 5 KO stroma). Intracellular expression of Stat1 and phosphorylated Stat1 (pStat1) was then analyzed by flow cytometry. The relative cell number (RCN) is plotted against pStat1 and Stat1 (inset) for unstimulated (time 0, dotted line) and stimulated (5 minutes, bold line) pre-B cells. (B) Normalization of the MFI of pStat1 versus Stat1 obtained by flow cytometry was obtained for each B cell/stromal cell supernatant stimulation condition. Data are provided as pStat1/Stat1 ratio ± SEM. *P < .02. (C) Leukemic pre-B–cell lines ABE 8.1/2 and 70Z/3 were cultured 24 hours in the presence of either TC-PTP+/+ or TC-PTP−/− bone marrow stromal cell culture supernatant (stroma +/+ or stroma −/−). Cells were stained with annexin V and propidium iodide (PI) for flow cytometry analysis. The combined percentage of preapoptotic (annexin V+, PI−) and apoptotic (annexin V+, PI+) cells is provided. Representative data are shown for each subset. (D) Quantitation of annexin V+ in leukemic cells; □, leukemic cells incubated with TC-PTP+/+ stroma supernatant (n = 4); ▪, leukemic cells incubated with TC-PTP−/− stroma supernatant (n = 4). Fold increase in the number of apoptotic annexin V+ cells ± SEM is provided relative to time 0 leukemic culture. **P < .005. All experiments were repeated at least 3 times.
Stat1 response analysis of pre-B cells stimulated with bone marrow stromal cell supernatants. (A) Cultured pre-B cells (pool of either WT [B+/+] or KO [B−/−]) were stimulated 5 minutes with the supernatant of P7 TC-PTP+/+ or TC-PTP−/− bone marrow stromal cells cultures (with or without the presence of anti–IFN-γ antibody [α-IFN-γ]; 5 WT, 5 KO stroma). Intracellular expression of Stat1 and phosphorylated Stat1 (pStat1) was then analyzed by flow cytometry. The relative cell number (RCN) is plotted against pStat1 and Stat1 (inset) for unstimulated (time 0, dotted line) and stimulated (5 minutes, bold line) pre-B cells. (B) Normalization of the MFI of pStat1 versus Stat1 obtained by flow cytometry was obtained for each B cell/stromal cell supernatant stimulation condition. Data are provided as pStat1/Stat1 ratio ± SEM. *P < .02. (C) Leukemic pre-B–cell lines ABE 8.1/2 and 70Z/3 were cultured 24 hours in the presence of either TC-PTP+/+ or TC-PTP−/− bone marrow stromal cell culture supernatant (stroma +/+ or stroma −/−). Cells were stained with annexin V and propidium iodide (PI) for flow cytometry analysis. The combined percentage of preapoptotic (annexin V+, PI−) and apoptotic (annexin V+, PI+) cells is provided. Representative data are shown for each subset. (D) Quantitation of annexin V+ in leukemic cells; □, leukemic cells incubated with TC-PTP+/+ stroma supernatant (n = 4); ▪, leukemic cells incubated with TC-PTP−/− stroma supernatant (n = 4). Fold increase in the number of apoptotic annexin V+ cells ± SEM is provided relative to time 0 leukemic culture. **P < .005. All experiments were repeated at least 3 times.
The flow cytometry MFI of pStat1 versus Stat1 for each experimental group was expressed as pStat1/Stat1 ratio to normalize all results according to total Stat1 proteins (Figure 6B). When TC-PTP+/+ stromal cell culture supernatant was used as stimulus, no difference was seen between the pStat1/Stat1 ratio of unstimulated and stimulated samples for all 4 experimental groups (ratio ∼1). An augmentation of pStat1/Stat1 ratio (4.7 ± 1) was obtained by stimulation of TC-PTP+/+ B cells with TC-PTP−/− stromal cell culture supernatant. A further increase in the pStat1/Stat1 ratio (8.3 ± 2) was calculated when TC-PTP−/− B cells were stimulated with TC-PTP−/− stromal cell culture supernatant. Equivalent results were obtained by treating either B+/+ cells or B−/− cells with exogenous IFN-γ (500 pg/mL) and pStat1/Stat1 ratios were back to unstimulated levels when experiments were done in the presence of IFN-γ–capturing antibodies (Figure 6B). These results indicate that a soluble factor, identified as IFN-γ, secreted by bone marrow stromal cells of TC-PTP−/− mice is directly responsible for the Stat1 hyperphosphorylation of B cells.
The presence of IFN-γ within the bone marrow microenvironment can have an impact on multiple hematopoietic cell lineages as well as on the development of malignant cells. To investigate the relative importance of bone marrow environment on the growth of cancer cells, we studied 2 established murine pre-B lymphoblast cell lines. The ABE 8.1/2, an early pre-B leukemic cell line, and the 70Z/3, a more advance stage of pre-B leukemic cells, were compared for early apoptotic event after 24 hours of incubation with TC-PTP+/+ or TC-PTP−/− stromal cell culture supernatant (Figure 6C). Annexin V and propidium iodide staining of leukemic cells followed by flow cytometry analysis demonstrated that ABE 8.1/2 cells have increased total annexin V+ cells when cultured in the presence of supernatant from TC-PTP−/− stromal cell cultures compared to TC-PTP+/+ stroma supernatant (annexin V+ 13.4% versus 31.1%). Similar results, respectively, were obtained when exogenous IFN-γ (500 pg/mL) was added to the leukemic cell culture (data not shown). However, the 70Z/3 cells responded very differently to the same culture conditions. As expected, IFN-γ did not induce apoptosis in these cells because they are from a later pre-B stage of development (annexin V+, 5.3% versus 6.5%; Figure 6C) as described.14 Quantification of our results determined a significant 1.6-fold ± 0.2 increase in apoptosis after 24 hours of incubation with culture supernatant containing IFN-γ in ABE-8.1/2 cells, whereas no difference was obtained with the 70Z/3 cells (Figure 6D). Our results indicate that TC-PTP–deficient bone marrow stromal cells have the natural ability to induce apoptosis in B-cell leukemia derived from early B-cell developmental stages.
Discussion
In this study, we report that the absence of TC-PTP within the bone marrow microenvironment results in abnormal B lymphopoiesis leading to an hindered progression of the B-cell progenitors from the pre-B–cell to the immature B-cell stage. We demonstrated that cell autonomous but more importantly bone marrow stroma defects contribute to impaired B-cell development by the abnormal secretion of IFN-γ.
Proper development of hematopoietic lineages necessitates intercellular interactions between hematopoietic precursor cells and bone marrow stromal cells, and the secretion of key cytokines by the latter (for a review, see Wilson and Trumpp15 ). The inability of TC-PTP+/+ bone marrow cells to rescue TC-PTP−/− recipients together with the rescue of irradiated TC-PTP+/+ recipient mice by TC-PTP−/− marrow pointed to a defective bone marrow microenvironment in TC-PTP−/− mice.2 Previous work revealed that IFN-γ can be detected in the liver of TC-PTP−/− mice as early as 3 days of age and, at 20 days of age, the serum concentration of IFN-γ reaches 4 ng/mL.3 However, the cells secreting this cytokine were not identified. We now show that bone marrow stroma from TC-PTP−/− mice secrete large amounts of IFN-γ. Bone marrow stromal cells are not a major source of this cytokine under normal conditions. Indeed, cell types usually associated with IFN-γ secretion include T, NK, and subsets of dendritic cells (reviewed by Walzer et al16 and Glimcher et al17 ).
Bone marrow stromal cells are known to express cytokines and growth factors such as IL-6, IL-8, IL-11, IL-12, IL-14, IL-15, LIF, G-CSF, GM-CSF, M-SCF, FL, and SCF.18 Because TC-PTP is a negative regulator of several cytokine and growth factor signaling pathways through the Jaks and Stats,8–10 its absence could provide an abnormal cytokine milieu. Certain cytokines normally secreted by bone marrow stroma such as IL-12 and IL-15 are known to be required for IFN-γ response.19,20 Their up-regulation due to the absence of TC-PTP on bone marrow stromal cells could explain the abnormal secretion of IFN-γ, which further affects B-cell development. Another possibility is that the lack of TC-PTP creates an imbalance between proinflammatory and anti-inflammatory cytokines favoring proinflammatory virallike response.21 A recent study demonstrated that viral infection can induce the production of IFN-γ by bone marrow stromal cells, and this can mediate partial cytotoxic T lymphocyte (CTL) responses.22
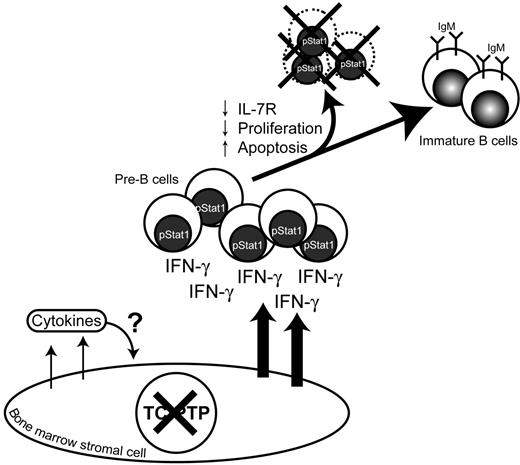
As depicted in Figure 7, secretion of IFN-γ was detected in primary bone marrow stroma cultures from TC-PTP−/− mice and was associated with hyperphosphorylation of Stat1 in pre-B cells isolated from the bone marrow of these animals. IFN-γ secretion leads to apoptosis, decreased number of pre-B cells expressing surface IL-7R, decreased proliferation in response to IL-7, which was associated with hypophosphorylation of downstream effectors Jak1 and Stat5. It has been reported that stimulation of pre-B–cell lines with IFN-γ leads to decreased responsiveness to IL-7 and increased apoptosis in these cells.23 Furthermore, studies involving primary Balb/c bone marrow B cells showed that IFN-γ can suppress IL-7–dependent colony formation and can induce apoptosis in pre-B cells.13 In addition, transgenic mice expressing high levels of IFN-γ in the bone marrow were shown to generate significantly lower pre-B and B cells.24 Consequently, anomalous production of IFN-γ by the bone marrow microenvironment may be the cause of B-cell developmental delay at the pre-B stage in TC-PTP−/− mice leading to decreased number of immature B cells leaving the bone marrow.
B lymphopoiesis is modulated by TC-PTP. In the absence of TC-PTP, bone marrow stromal cells provide an abnormal cytokine environment. IFN-γ secretion by bone marrow stromal cells induces Stat1 phosphorylation of pre-B cells leading to decreased number of pre-B cells expressing surface IL-7R thus reducing IL-7 proliferation response. IFN-γ also induces apoptosis in those pre-B cells leading to a decreased immature IgM+ B-cell population.
B lymphopoiesis is modulated by TC-PTP. In the absence of TC-PTP, bone marrow stromal cells provide an abnormal cytokine environment. IFN-γ secretion by bone marrow stromal cells induces Stat1 phosphorylation of pre-B cells leading to decreased number of pre-B cells expressing surface IL-7R thus reducing IL-7 proliferation response. IFN-γ also induces apoptosis in those pre-B cells leading to a decreased immature IgM+ B-cell population.
Bone marrow immature B cells are also known to secrete low levels of IFN-γ. By secreting this cytokine, immature B cells down-regulate their integrin receptor allowing their migration to the spleen for further maturation.25–27 Our study has not measured IFN-γ mRNA in bone marrow immature B cells; therefore, we cannot exclude this additional source of IFN-γ. The transition from pre-B to immature B cell is predicated on successful immunoglobulin heavy chain (IgH) gene rearrangement, through VDJ recombination. B-cell progenitors that fail to express IgH undergo apoptosis (reviewed by Dudley et al28 ). Therefore, a cell autonomous defect in DNA repair or induced by IFN-γ secretion during the recombination process could be another cause of impaired B-cell development in TC-PTP−/− mice.
The natural ability of TC-PTP–deficient stroma to secrete IFN-γ outlines the potential usefulness of modulating bone marrow microenvironment for the treatment of early B-cell stage leukemia. IFN-γ can induce proliferation arrest in stroma/IL-7–dependent normal bone marrow B cells as well as in Burkitt lymphoma B cells and B-cell acute lymphoblastic leukemia (pre-B–ALL) and cause apoptosis.23,29 This specific cell death is known to require Stat1 and is mediated by CD95.30,31 Our results presented with the leukemic cell lines further support this therapeutic approach. Other applications for modulation of TC-PTP activity may be considered. It could augment protection of the host against viral infection, which may be of particular importance if immunosuppressive treatment were used. IFN-γ has been approved for treatment of chronic granulomatous disease; however, because de novo secretion of IFN-γ has more profound effects on target cells than exogenous IFN-γ,32 use of a TC-PTP–specific inhibitor to enhance endogenous secretion of IFN-γ could lead to improved clinical outcomes. Constitutive activation of Stat1 in absence of TC-PTP may find practical application in the treatment of cancer. In contrast to other Stat, Stat1 acts as a tumor suppressor (reviewed by Haura et al33 ). Thus, increasing Stat1 activity may help reduce tumor burden and metastasis. TC-PTP–specific blocking agents might provide a useful pharmacologic approach toward this goal.
Authorship
Contribution: A.B. designed research, performed research, collected data, analyzed data, and wrote the paper; N.D. performed research, collected data, and analyzed data; K.M.H. performed research, collected data, and analyzed data; J.-F.T. performed research, collected data, and analyzed data; K.M.D. performed research; and M.L.T. designed research and supervised all experiments and results.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michel L. Tremblay, McGill Cancer Centre, McGill University, 3655 Promenade Sir-William-Osler, Rm 701, Montréal, Québec, H3G 1Y6, Canada; e-mail: michel.tremblay@mcgill.ca.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by a grant from the National Cancer Institute of Canada (NCIC; no. 010427; M.L.T.).
We thank Ailsa Lee Loy and Alexander Normandin for technical assistance; Ken MacDonald, Eric Massicotte, and Martine Dupuis for expert help in flow cytometry; and Dr Sébastien Trop, Dr Yves R. Boisclair, and Maxime Hallé for critical review of this manuscript.
A.B. is a cancer immunology fellow of the Cancer Research Institute of New York (New York, NY). N.D. is a recipient of a doctoral research award from the Canadian Institutes of Health Research (CIHR). K.M.H. was supported by a Canada Graduate Scholarship Doctoral Award from CIHR. M.L.T. is a recipient of the Jeanne and Jean-Louis Lévesque Chair in Cancer Research.

![Figure 6. Stat1 response analysis of pre-B cells stimulated with bone marrow stromal cell supernatants. (A) Cultured pre-B cells (pool of either WT [B+/+] or KO [B−/−]) were stimulated 5 minutes with the supernatant of P7 TC-PTP+/+ or TC-PTP−/− bone marrow stromal cells cultures (with or without the presence of anti–IFN-γ antibody [α-IFN-γ]; 5 WT, 5 KO stroma). Intracellular expression of Stat1 and phosphorylated Stat1 (pStat1) was then analyzed by flow cytometry. The relative cell number (RCN) is plotted against pStat1 and Stat1 (inset) for unstimulated (time 0, dotted line) and stimulated (5 minutes, bold line) pre-B cells. (B) Normalization of the MFI of pStat1 versus Stat1 obtained by flow cytometry was obtained for each B cell/stromal cell supernatant stimulation condition. Data are provided as pStat1/Stat1 ratio ± SEM. *P < .02. (C) Leukemic pre-B–cell lines ABE 8.1/2 and 70Z/3 were cultured 24 hours in the presence of either TC-PTP+/+ or TC-PTP−/− bone marrow stromal cell culture supernatant (stroma +/+ or stroma −/−). Cells were stained with annexin V and propidium iodide (PI) for flow cytometry analysis. The combined percentage of preapoptotic (annexin V+, PI−) and apoptotic (annexin V+, PI+) cells is provided. Representative data are shown for each subset. (D) Quantitation of annexin V+ in leukemic cells; □, leukemic cells incubated with TC-PTP+/+ stroma supernatant (n = 4); ▪, leukemic cells incubated with TC-PTP−/− stroma supernatant (n = 4). Fold increase in the number of apoptotic annexin V+ cells ± SEM is provided relative to time 0 leukemic culture. **P < .005. All experiments were repeated at least 3 times.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-08-044370/4/m_zh80100701030006.jpeg?Expires=1769302680&Signature=mt43ekDf8PbxuM5Eu-Xn9X2rj-uSxzeJo1aanSSRYhF5uEk3ZxNhVmssRo85XyRlTLwSkl5QJsdHA4XcL3aYNBiYeFEcz575cl-5pkj4GTGtzvHDV0STuXOEb7tw3J0XyNuwRsnmTIWiyhuVMVTBNsrThf~ysq2bPUOdRWcIcVYjrzqNHC9pyimVQu5NKx7qARMFL5RRjKx3wQ8g88YKUaoqYFgoo5IP3ZM7quN7XsxqKvbNZjS59aPZ9~hNuo4SBp95I~zruP0TX-o0tE3Aod6Y4A-I3KM6nmEqDlNJy-Dn0aZ6-j4TxwiFcKQF3ohNzzlh5ajDsLESYTlVFear-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal