Abstract
The T-cell receptor ζ (TCRζ) chain is a master sensor and regulator of lymphocyte responses. Loss of TCRζ expression has been documented in infectious, inflammatory, and malignant diseases, suggesting that it may serve to limit T-cell reactivity and effector responses at sites of tissue damage. These observations prompted us to explore the relationship between TCRζ expression and effector function in T cells. We report here that TCRζdim lymphocytes are enriched for antigen-experienced cells refractory to TCR-induced proliferation. Compared to their TCRζbright counterparts, TCRζdim cells share characteristics of differentiated effector T cells but use accessory pathways for transducing signals for inflammatory cytokine gene expression and cell contact-dependent pathways to activate monocytes. TCRζdim T cells accumulate in inflamed tissues in vivo and have intrinsic migratory activity in vitro. Whilst blocking leukocyte trafficking with anti-TNF therapy in vivo is associated with the accumulation of TCRζdim T cells in peripheral blood, this T-cell subset retains the capacity to migrate in vitro. Taken together, the functional properties of TCRζdim T cells make them promising cellular targets for the treatment of chronic inflammatory disease.
Introduction
The acquisition of immune responsiveness is mediated through the assembly, expression, and function of antigen receptors at the cell surface.1 In T lymphocytes, the antigen T-cell receptor (TCR) is a multiple subunit complex comprising clonotypic, disulfide-linked α and β antigen recognition chains devoid of signaling motifs associated with the invariant chain subunits CD3γ, δ, and ϵ, and TCRζ.2 These invariant chains assemble in the mature TCR/CD3 complex as noncovalently linked CD3γϵ and CD3δϵ heterodimers and disulfide-linked TCRζ–ζ homodimers and transmit signals inside the cell following TCR ligation-induced tyrosine phosphorylation of immune receptor tyrosine-based activation motifs contained within their extended intracytoplasmic tails.3–5 These phosphotyrosine motifs form docking sites for Syk family tyrosine kinases, such as ζ-associated protein of 70 kDa (ZAP-70), which in turn couple the antigen receptor complex to transmembrane adaptor proteins and downstream signaling pathways.6
The TCRζ subunit differs in its genetic organization, chromosomal localization, and domain structure from the CD3γ, δ, and ϵ invariant chains. Thus, in CD4+ and CD8+ T cells the 16-kDa TCRζ chain subserves a unique role in TCR/CD3 complex assembly by associating with newly synthesized hexameric αβγϵδϵ complexes resulting in the transport and expression of mature αβγϵδϵζ2 complexes to the cell surface.7 Furthermore, metabolic labeling and pulse chase experiments have demonstrated that de novo synthesis of TCRζ is rate-limiting, thereby regulating the amount of TCR/CD3 complex expressed at the cell surface.8,9 In NK cells, the TCRζ chain may form homodimers or heterodimers with the FcϵR common γ chain signaling subunit, which in turn may form signaling complexes with several activating receptors, including NK cell protein 46 (NKp46), NKp30, and the low-affinity IgG receptor FcγRIII (CD16).10,11 In light of these crucial functions the TCRζ chain could be viewed as a master regulator and sensor of innate as well as adaptive immune responses. It follows from this that aberrations in its expression or function should have profound effects on immune function.
The events leading to the down-regulation of TCR/CD3 complex expression on serial ligation by peptide/MHC complexes are well documented.12 Less well understood are the molecular mechanisms for the sustained down-regulation of TCRζ observed in chronic bacterial, HIV, and mycobacterial infections, autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus, and in solid tumor and hematologic malignancies (reviewed by Baniyash13 ). That this phenomenon is associated with so many diverse pathologies has raised the possibility that down-regulation of TCRζ may serve to attenuate T-cell activation at sites of tissue damage, thereby limiting the effects of unbridled T-cell reactivity and pathologic effector responses.14,15 Whilst this model is entirely consistent with a central role for antigen in driving autoimmune inflammatory activity, it implies that chronically activated T cells rendered hyporesponsive to TCR engagement through loss of TCRζ expression should play a less prominent role in the established phase of disease.13,14
To gain further insight into the immunobiology of T cells expressing low levels of TCRζ (hereafter, TCRζdim), we explored the relationship between loss of TCRζ expression and T-cell function. Our results shed light on the nature of persistent effector responses of T cells that become refractory to antigenic restimulation during the evolution of immune responses. They also have implications for cell-based therapies aimed at achieving long-term remission in patients with chronic inflammatory disease.
Patients, materials, and methods
Healthy donors and patients
Laboratory staff members, aged 22 to 45 years of age, were enrolled as healthy donors. All patients were recruited from the Hammersmith Hospitals NHS Trust (London, United Kingdom). CMV reactive lymphocytes were obtained from HLA-B*0702+ patients with chronic myeloid leukemia selected by CMV seropositivity. Single donor plateletpheresis residues were obtained from the North London Blood Transfusion Service (Colindale, United Kingdom). Patients with severe, active seropositive rheumatoid arthritis (RA) meeting the American College of Rheumatology revised classification criteria for RA,16 and requiring anti-TNF therapy, were recruited to the study. Prior to anti-TNF therapy, all patients were treated with nonsteroidal anti-inflammatory drugs in addition to methotrexate and sulfasalazine, with or without hydroxychloroquine and low-dose prednisolone. Peripheral blood (PB) and synovial fluid (SF) lymphocytes were obtained from patients with RA, psoriatic arthritis, or reactive arthritis who attended the clinic with a flare of disease requiring therapeutic arthrocentesis. Synovial mononuclear cell suspensions were prepared from RA synovial tissue obtained at joint replacement surgery as described.17 Written, informed consent was obtained from all participating donors and patients in accordance with the Declaration of Helsinki, and all protocols were approved by the Riverside Research Ethics Committee, Hammersmith Hospitals NHS Trust.
Human lymphocytes
PB lymphocytes (PBLs) from heparinized peripheral venous blood (or mononuclear cells from SF) were obtained by Ficoll-Hypaque density gradient centrifugation (specific density 1.077 g/mL; Nycomed Pharma, Oslo, Norway) and used immediately for flow cytometric analysis. Where relevant, additional aliquots were prepared and frozen until required. PB monocytes and T cells were purified by elutriation (JE6; Beckman Coulter, Fullerton, CA) in 1% heat-inactivated fetal calf serum (FCS), as described,17 and purity assessed by flow cytometry. Single-cell suspensions of synovial membrane mononuclear cells were prepared as described.17 Cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 100 U/mL penicillin/streptomycin, 50 μM 2-ME, 1 mM sodium pyruvate, and 25 mM HEPES (complete medium).
Antibodies and recombinant cytokines
The following antibodies were purchased from BD Biosciences PharMingen (San Diego, CA): anti–CD8-APC, anti–CD28-FITC, anti–CD45RA-APC, anti–TNFα-FITC, anti–IFNγ-FITC, and anti–IL-10–APC. Anti–CD45RO-FITC was obtained from Serotec (Oxford, United Kingdom), mouse IgG1-FITC and anti–CD3-FITC from Sigma (St Louis, MO), mouse IgG1-PE and anti–TCRζ-PE (TIA2, which recognizes a cytoplasmic domain epitope) from Immunotech (Coulter, Hialeah, FL), and mouse IgG–PerCP, anti–CD3-PerCP, anti–CD4-PerCP, anti–CD8-PerCP, anti–CD16-FITC, and anti–CD56-FITC from Becton Dickinson (San Jose, CA). Anti–TCRζ-FITC (clone 6B10.2, which recognizes a transmembrane epitope of TCRζ) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-CD3ϵ antibody used for stimulation (clone OKT3) was obtained from the American Type Culture Collection (Rockville, MD). Human recombinant TNF-α and IL-2 were generous gifts of Prof W. Stec (Centre of Molecular Studies, Lodz, Poland) and Dr P. Lomedico (Hoffman-La Roche, Nutley, NJ), respectively. All other cytokines were purchased from PeproTech EC (London, United Kingdom) or R&D Systems (Wiesbaden, Germany).
Flow cytometry
Cells were stained by standard methods and analyzed using an LSR BD FACScan analyzer and CellQuest software (Becton Dickinson). For analysis of TCRζ and intracellular cytokine expression, cells were fixed with 2% formaldehyde and permeabilized in buffer containing 10 μg/mL saponin, according to established protocols.18 The efficiency of permeabilization was determined by uptake of trypan blue (always > 99%).
T-cell stimulation and effector responses
For proliferation, unfractionated PBLs were labeled with 5 μM CFSE (Molecular Probes, Eugene, OR) prior to stimulation in complete medium with plate bound OKT3 (2 μg/mL) with or without soluble anti-CD28 (2 μg/mL). Cells were harvested at the indicated times, stained with specific antibodies to TCRζ (TIA2-PE), together with anti-CD3, anti-CD4, or anti-CD8 conjugated to PerCP and analyzed by flow cytometry. CMV-reactive PBLs were stimulated with specific pp65 CMV peptide (amino acid sequence: TPRVTGGGAM, from Proimmune, Oxford, United Kingdom) in the presence of 50 U/mL IL-2 and 10 ng/mL IL-7 for up to 8 days prior to staining with anti-TCRζ (6B10.2-FITC), HLA-B7-tetramer-PE (Proimmune), and anti–CD8-APC and analysis by flow cytometry. Cytokine-activated T cells were generated from elutriated T cells over 8 days using IL-2 (25 ng/mL), TNF-α (25 ng/mL), and IL-6 (100 ng/mL) as described.17 Cytokine expression was determined by staining fixed and permeabilized PBL with directly conjugated anticytokine antibodies (all from BD Biosciences PharMingen), after stimulation for 6 hours at 37°C with 20 ng/mL phorbol myristate acetate (PMA) and 200 ng/mL ionomycin (both from Sigma). Golgistop (containing monensin, from BD Biosciences PharMingen) was added for the last hour of stimulation. Specificity of staining was confirmed using isotype-specific control antibodies and by ligand-blocking experiments using the corresponding recombinant cytokine. For CD28 stimulation of TCRζdim cells, T cells were cocultured with Chinese hamster ovary (CHO) cells transfected with CD80 or CD86 (generously provided by Dr D. Sansom, University of Birmingham, Birmingham, United Kingdom) for 24 hours, and supernatants harvested and assayed for IFN-γ or IL-10 expression by enzyme-linked immunoadsorbent assay (ELISA; BD Biosciences PharMingen). Cell contact-dependent activation of monocytes by TCRζbright or TCRζdim T cells fixed in PBS containing 0.05% glutaraldehyde and neutralized with an equal volume of buffer containing 0.2 M glycine was performed as described.17 Stimulation of monocytes with LPS (10 ng/mL) in the absence of T cells was used as a positive control. Culture supernatants were harvested after 24 hours and assayed for TNF-α levels by ELISA (BD PharMingen). Transendothelial migration of PBLs or elutriated T cells 24 hours after plating over monolayers of human umbilical vein endothelial cells (HUVECs) previously stimulated with 10 ng/mL TNF-α for 48 hours was performed essentially as described.19 Numbers of migrating TCRζbright or TCRζdim T cells were determined by flow cytometry.
Assessment of clinical response
Patients with active RA requiring anti-TNF treatment as part of their standard care were identified according to national guidelines. All patients were treated with intravenous infusions of infliximab (3 mg/kg) at week 0, 2, 6, and then 8 weekly thereafter, while continuing existing disease-modifying drug therapy. At baseline and at the indicated times disease activity and response to therapy were determined using the disease activity score (DAS), which includes the 28 joint count (DAS28).20 At baseline, all patients had DAS28 scores of more than 5.1. Good, moderate, and poor responses were defined according to European League Against Rheumatism (EULAR) DAS response criteria at 14 and 30 weeks.21 PBLs were obtained at the indicated times after starting therapy and frozen prior to analysis.
Statistical analysis
Differences in phenotype between TCRζbright and TCRζdim subsets were determined by pair-wise analyses using the Wilcoxon signed rank test or Student t test, according to sample distribution. Correlations between changes in TCRζ expression and disease activity were determined by linear regression analysis. The limit for statistical significance was set at .05.
Results
To study TCRζ expression at the single-cell level in mixed populations of PBLs from healthy donors we adapted quantitative flow cytometric assays first developed to study deficient ζ-chain expression in T cells from patients with cancer.22 The TCRζ chain has a short 9 amino acid extracellular domain, so we used mouse monoclonal antibodies (clones TIA-2 or 6B10) that detect cytoplasmic domain epitopes of the ζ chain in T cells after fixing and permeabilization. Both antibodies recognize phosphorylated as well as unphosphorylated forms of the TCRζ chain, implying that any loss of signal is not due to activation-induced phosphorylation of the ζ-chain polypeptide (data not shown). As expected, staining of PBLs from healthy donors defined distinct lymphocyte subsets, including populations of CD3+TCRζ+ cells, CD3−TCRζ+ cells, and a double-negative population of monocytes and B cells that do not express TCRζ (Figure 1A and data not shown). We documented a relationship between TCRζ and CD3ϵ expression, with TCRζbright T cells expressing the highest levels of CD3ϵ, and TCRζintermediate or TCRζdim cells expressing levels of surface CD3ϵ that for some donors were at least an order of magnitude lower (Figure 1A). This relationship varied considerably between donors but was not related to cell viability based on annexin-V staining (data not shown). Analysis of CD3−TCRζ+ cells confirmed expression of TCRζ in both CD56+ and CD16+ NK cell subsets; CD3−CD56bright cells were found to be uniformly TCRζ−, whereas the CD3−CD16+ NK cell subset was TCRζbright (data not shown; Nicola Dalbeth and Margaret Callan, unpublished data, May 2005). This would be consistent with the physical association between CD16 and the ζ chain.10
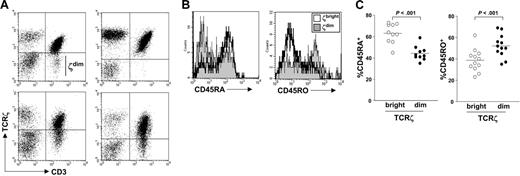
TCRζ expression in PBLs from healthy donors. (A) PBLs were analyzed for expression of TCRζ and CD3 by flow cytometry. Four donors illustrating the heterogeneity of TCRζ expression are shown. (B) PBLs were stained for TCRζ and CD3 expression. CD45RA and CD45RO expression on TCRζbright and TCRζdim subsets were then determined by flow cytometry. (C) The percent CD45RA+ or CD45RO+ cells residing within TCRζbright and TCRζdim subsets were analyzed for 10 or 12 healthy donors, respectively. Horizontal lines represent mean values.
TCRζ expression in PBLs from healthy donors. (A) PBLs were analyzed for expression of TCRζ and CD3 by flow cytometry. Four donors illustrating the heterogeneity of TCRζ expression are shown. (B) PBLs were stained for TCRζ and CD3 expression. CD45RA and CD45RO expression on TCRζbright and TCRζdim subsets were then determined by flow cytometry. (C) The percent CD45RA+ or CD45RO+ cells residing within TCRζbright and TCRζdim subsets were analyzed for 10 or 12 healthy donors, respectively. Horizontal lines represent mean values.
The relationship between antigen engagement and reduced levels of TCRζ reported previously suggested that TCRζ expression should discriminate between subsets of naïve and memory T-cell subsets defined by conventional cell surface markers. To this end, we stained healthy donor PBLs with antibodies specific for CD45RA or CD45RO to define subsets of naïve and memory T cells, respectively, and then compared the expression of these markers on TCRζbright and TCRζdim cell subsets. Histogram plots from a representative experiment, shown in Figure 1B, demonstrated enrichment of CD45RA+ T cells in the TCRζbright population and many CD45RO+ T cells residing within the TCRζdim subset. Nevertheless, there was considerable overlap between subsets, findings supported by analysis of multiple donors (Figure 1C). Indeed, as many as 40% of TCRζbright T cells expressed CD45RO, whereas a similar proportion of TCRζdim T cells expressed CD45RA. More extensive phenotyping failed to demonstrate uniform loss of CD62L or CCR7 expression on all TCRζdim T cells when compared to the TCRζbright subset, indicating that low expression of TCRζ does not define differentiating effector memory T cells per se, but rather analysis of TCRζ expression might provide additional functional information besides that provided by conventional cell surface phenotyping.
To examine the functional consequences of TCRζ expression on TCR responsiveness, we stained fresh PBLs from healthy donors with carboxy-fluorescein diacetate succinimidyl ester (CFSE) and tracked the proliferative capacity of CD4+ and CD8+ T-cell subsets in response to stimulation with OKT3, or OKT3 and anti-CD28 in combination. At the indicated times cells were harvested, stained for TCRζ expression, and analyzed by CFSE dye dilution. Although little proliferation was detected within the first few days of TCR stimulation, dividing T cells could be detected at subsequent time points, and these were uniformly TCRζbright (Figure 2A arrows). In contrast, the majority of TCRζintermediate and TCRζdim T cells failed to proliferate throughout the period of stimulation. Note that populations of nonproliferating TCRζdim CD4+ and CD8+ T cells, representing less than 10% of the total, were observed at day 4 but not at subsequent time points. The reasons for this are unknown, although it is conceivable that these T cells undergo programmed cell death after nonproductive TCR engagement. By day 6, expression of TCRζ in proliferating cells declined progressively with each cycle of cell division. Although this result was predicted, the extent to which proliferation was restricted to a subset of CD4+ or CD8+ T cells expressing high levels of TCRζ was unexpected. These findings could not be explained by suboptimal levels of TCR stimulation, because higher concentrations of OKT3 and anti-CD28 antibodies induced similar patterns of proliferation with respect to TCRζ expression.
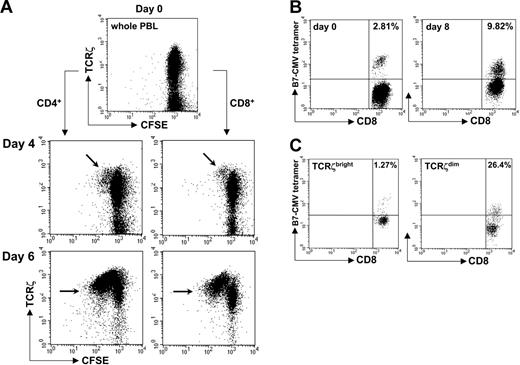
The TCRζdim cell subset is enriched for antigen experienced T cells. (A) Healthy donor PBLs were stained with CFSE prior to stimulation with OKT3. At the indicated times, cells were harvested, stained for TCRζ together with CD3, CD4, or CD8, and dilution of CFSE fluorescence determined by flow cytometry. Proliferating TCRζbright cells are indicated (arrows). (B) Fresh PBLs from HLA-B*0702+ CMV-reactive donors were stimulated in vitro with p65 CMV peptide, IL-2, and IL-7. Eight days later, cells were stained for the presence of peptide-reactive CD8+ T cells using fluorescent HLA-B7/p65 MHC-peptide tetramers and anti-CD8. (C) Cells were stained for TCRζ expression and the percent CD8+ tetramer-positive T cells within TCRζbright and TCRζdim subsets determined. The percent tetramer-positive cells is indicated. Data are representative of multiple experiments.
The TCRζdim cell subset is enriched for antigen experienced T cells. (A) Healthy donor PBLs were stained with CFSE prior to stimulation with OKT3. At the indicated times, cells were harvested, stained for TCRζ together with CD3, CD4, or CD8, and dilution of CFSE fluorescence determined by flow cytometry. Proliferating TCRζbright cells are indicated (arrows). (B) Fresh PBLs from HLA-B*0702+ CMV-reactive donors were stimulated in vitro with p65 CMV peptide, IL-2, and IL-7. Eight days later, cells were stained for the presence of peptide-reactive CD8+ T cells using fluorescent HLA-B7/p65 MHC-peptide tetramers and anti-CD8. (C) Cells were stained for TCRζ expression and the percent CD8+ tetramer-positive T cells within TCRζbright and TCRζdim subsets determined. The percent tetramer-positive cells is indicated. Data are representative of multiple experiments.
It followed from this that after TCR stimulation in vitro, antigen-reactive T cells should reside in the TCRζdim compartment. To test this directly, we analyzed fresh PBLs from HLA-B*0702+ CMV-reactive donors after stimulation with specific CMV peptide and tracked antigen-specific CD8+ T cells with fluorescent HLA-B7/p65 MHC-peptide tetramers. In a representative experiment, the proportion of tetramer-positive T cells increased from less than 3% prior to stimulation to almost 10% after 8 days of culture (Figure 2B). By gating on TCRζbright and TCRζdim T-cell subsets, we confirmed that the majority of peptide-specific CD8+ T cells reside within the TCRζdim population after 8 days of stimulation (1.27% tetramer-positive TCRζbright cells versus 26.4% tetramer-positive TCRζdim cells, Figure 2C); note the downward shift in tetramer staining consequent on down-regulation of the TCR complex after stimulation (Figure 2B-C right panels). Together these experiments demonstrated that TCRζ staining can be used to identify populations of tetramer-positive T cells that have undergone productive engagement with specific antigen and suggest that circulating ζdim cells represent polyclonal populations of antigen experienced lymphocytes in vivo.
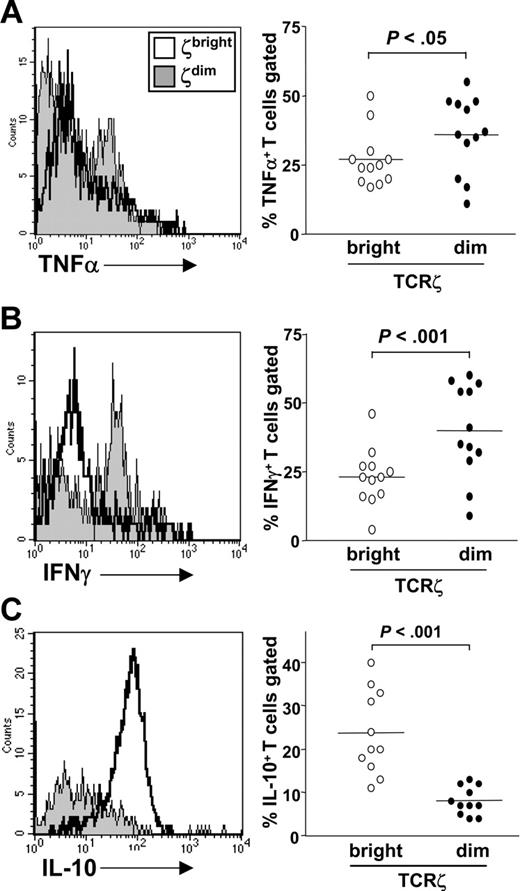
A characteristic feature of effector T cells is their capacity to produce cytokines. Current paradigms of T-cell differentiation suggest that T cells undergo a complex series of epigenetic events to acquire stable transcriptional competence for cytokine gene expression.23 These events depend on signals integrated from both the antigen T-cell receptor and cytokine receptors that induce the expression and activation of lineage-specific transcription factors.24 We therefore explored whether TCRζdim T cells that had already engaged antigen in vivo were competent for cytokine gene expression when compared to TCRζbright cells from the same donor. To test this, fresh PBLs were stimulated in vitro for 6 hours with phorbol ester and calcium ionophore. This stimulus, in contrast to anti-TCR antibodies, allowed us to assess competence for cytokine gene expression by activating T cells through pathways independent of TCRζ expression. Monensin was added to cultures for the last hour of stimulation prior to harvesting and staining using specific antibodies for CD3ϵ and TCRζ in combination with the indicated anticytokine antibody. Representative histogram plots are shown for each cytokine (Figure 3 left panel), alongside data derived from 12 healthy donors (Figure 3 right panel). The TCRζdim subset (shaded histograms) was enriched for cells expressing TNF-α and IFN-γ when compared to the TCRζbright population from the same donor (Figure 3A-B). By contrast, the dominant IL-10–producing cell subset was found to reside within the TCRζbright subset (Figure 3C), indicating that cytokine expression in general was not confined to the TCRζdim subset. A similar trend was seen for IL-4, but levels of expression were too low for a meaningful statistical analysis.
TCRζdim cells are enriched for cytokine-producing effector T cells. Fresh PBLs were stimulated with PMA and ionomycin for 6 hours prior to staining for CD3, TCRζ, and intracellular expression of (A) TNF-α, (B) IFN-γ, and (C) IL-10. Data are expressed as percent cytokine-expressing cells within TCRζbright or TCRζdim subsets for 12 healthy donors. Horizontal lines represent mean values.
TCRζdim cells are enriched for cytokine-producing effector T cells. Fresh PBLs were stimulated with PMA and ionomycin for 6 hours prior to staining for CD3, TCRζ, and intracellular expression of (A) TNF-α, (B) IFN-γ, and (C) IL-10. Data are expressed as percent cytokine-expressing cells within TCRζbright or TCRζdim subsets for 12 healthy donors. Horizontal lines represent mean values.
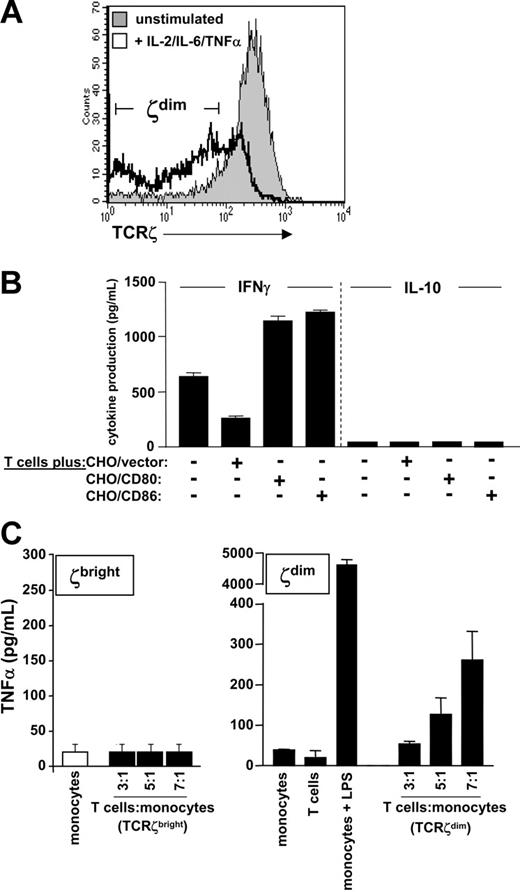
The results described thus far indicated that circulating TCRζdim T cells were enriched for cells that had previously engaged antigen and that were primed for cytokine production. However, the proliferative responses of T cells to TCR engagement shown in Figure 2 indicated that the TCRζdim subset was refractory to membrane proximal TCR signals. To define alternative signaling pathways of effector function, we prepared bulk populations of TCRζdim T cells by stimulating elutriated T cells with inflammatory cytokines such as TNF-α, which down-regulates TCRζ in murine T cells,25 using a combination of IL-2, IL-6, and TNF-α sufficient to support T-cell differentiation in vitro in the absence of specific antigen and accessory cells.22,26 After 7 days of cytokine stimulation a significant proportion of T cells were TCRζdim (67.3% compared to 12.2% for unstimulated T cells; Figure 4A). We then tested the capacity of TCRζdim T cells to produce IFN-γ or IL-10 either in the absence of stimulation or following costimulation by CHO cell transfectants expressing CD80 or CD86; CHO cells expressing empty vector were used as a negative control. In the absence of CHO cells, stimulation with the cytokine cocktail alone was sufficient to induce IFN-γ production (Figure 4B). Whilst costimulation with CHO/vector cells reduced IFN-γ production by TCRζdim T cells, stimulation with CHO cells expressing either CD80 or CD86 was equally efficient in augmenting IFN-γ production. Neither cytokine nor costimulatory signals were sufficient to induce T-cell IL-10 production.
Antigen-independent effector function of TCRζdim T cells. (A) TCRζdim T cells were generated from purified T cells following stimulation for 7 days with IL-2, IL-6, and TNF-α in the absence of antigen or antigen-presenting cells. TCRζ expression is shown compared to that of unstimulated T cells. (B) TCRζdim T cells were cocultured with CHO cells expressing empty vector, CD80, or CD86 for 24 hours. Supernatants were harvested and IFN-γ and IL-10 levels determined by ELISA. (C) Resting (TCRζbright) or activated TCRζdim T cells were fixed prior to coculture at the indicated ratios with purified monocytes. Supernatants were harvested 24 hours later and TNF-α levels determined by ELISA. Supernatants derived from cultures of monocytes or T cells alone or LPS-stimulated monocytes were used as negative and positive controls, respectively. Data are representative of multiple experiments, and represent mean cytokine levels ± SD in panels B and C.
Antigen-independent effector function of TCRζdim T cells. (A) TCRζdim T cells were generated from purified T cells following stimulation for 7 days with IL-2, IL-6, and TNF-α in the absence of antigen or antigen-presenting cells. TCRζ expression is shown compared to that of unstimulated T cells. (B) TCRζdim T cells were cocultured with CHO cells expressing empty vector, CD80, or CD86 for 24 hours. Supernatants were harvested and IFN-γ and IL-10 levels determined by ELISA. (C) Resting (TCRζbright) or activated TCRζdim T cells were fixed prior to coculture at the indicated ratios with purified monocytes. Supernatants were harvested 24 hours later and TNF-α levels determined by ELISA. Supernatants derived from cultures of monocytes or T cells alone or LPS-stimulated monocytes were used as negative and positive controls, respectively. Data are representative of multiple experiments, and represent mean cytokine levels ± SD in panels B and C.
To test whether TCRζdim T cells could reciprocally activate monocytes, TCRζbright or TCRζdim T cells were fixed prior to incubation with elutriated monocytes and supernatants harvested 24 hours later and assayed for monocyte-derived TNF-α production. Monocytes or T cells alone produced little or no TNF-α, whereas LPS stimulation induced abundant TNF-α (Figure 4C right panel). Although coculture of fixed TCRζ+ cells with monocytes failed to elicit TNF-α production (Figure 4C left panel), cytokine-stimulated TCRζdim T cells induced monocytes to produce TNF-α in a dose-dependent fashion when T cell–monocyte ratios were increased from 3:1 to 7:1. TNF-α production was attenuated when T-cell–monocyte contact was blocked with a Transwell insert (data not shown). These data demonstrated that although TCRζdim cells are relatively refractory to TCR-induced proliferation, they are by no means senescent or inert, being capable of inflammatory cytokine expression in response to cytokine receptor or costimulatory signals. TCRζdim cells are also potent activators of monocytes through cell contact-dependent pathways.
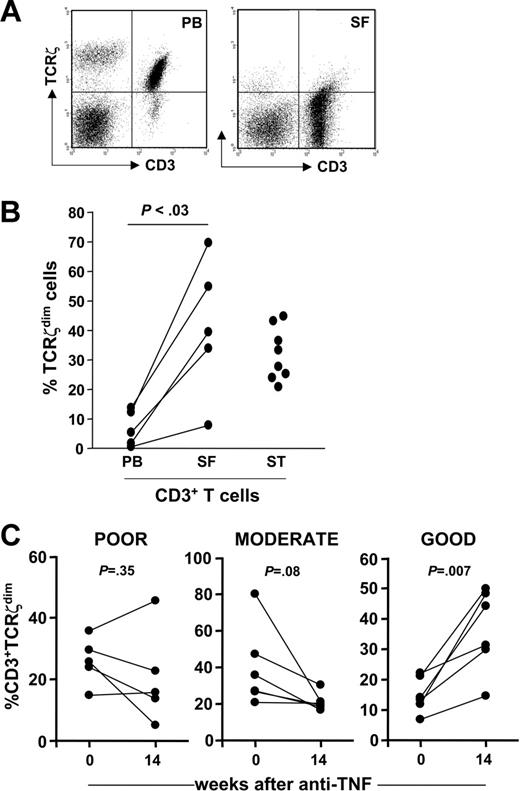
This effector phenotype, being reminiscent of RA synovial T cells,17 prompted us to compare expression of TCRζ in paired PB and SF T cells from patients with an acute flare of inflammatory arthritis who attended a clinic. TCRζ expression profiles were notable in several respects. First, a significant proportion of SF T cells had negligible levels of TCRζ expression, equivalent to that detected in the CD3ϵ−TCRζ− double-negative cell population, and yet surface expression of CD3ϵ was spared (Figure 5A). Second, although the numbers of CD3−TCRζ+ NK cells were lower in SF than in PB, expression of TCRζ was also reduced. Furthermore, lower numbers of TCRζdim T cells were detected in PB of patients with an acute disease flare (Figure 5B). This was associated with accumulation of the TCRζdim subset in SF when compared to PB from the same patient (P < .03), suggesting selective migration of these effector cells to inflamed joints and their exit from the circulating pool; increased numbers of TCRζdim T cells in RA synovial tissue (ST) was consistent (Figure 5B). If circulating TCRζdim T cells represented populations of antigen-experienced effector cells captured prior to their migration to inflamed joints, then blocking cell migration should lead to accumulation of this subset in blood. We took advantage of the fact that TNF blockade inhibits leukocyte trafficking27,28 by tracking PB TCRζdim cells in 17 patients with chronic, active RA (DAS28 ≥ 5.1) before and after treatment with anti-TNF. We documented considerable variability in expression of PB TCRζdim T-cell numbers following anti-TNF therapy. However, when data were stratified according to validated EULAR clinical response criteria we found that at 30 weeks the best clinical outcomes were associated with an accumulation in the percent CD3+TCRζdim T cells at 14 weeks (Figure 5C); this relationship held true for CD4+ but not CD8+ T cells, whereas no such association was found between clinical response to anti-TNF and other cellular parameters, such as total lymphocyte counts, or the percent CD3+ or CD45RO+ T cells (data not shown).
TCRζdim T cells are enriched at sites of inflammation and accumulate in PB after treatment with anti-TNF in a subset of patients. (A) A dot plot of TCRζ versus CD3ϵ expression in PB and SF T cells from a patient with RA, as determined by flow cytometry. (B) Paired PB and SF mononuclear cells from patients with active inflammatory synovitis were stained for TCRζ and analyzed by flow cytometry. Mononuclear cell suspensions were prepared from synovial tissue (ST) specimens obtained from patients with RA at joint arthroplasty. Data are expressed as percent CD3+TCRζdim T cells in each compartment. (C) The percent CD3+TCRζdim cells was determined by flow cytometry in PBLs from 17 patients with RA at baseline and 14 weeks after treatment with anti-TNF. Data were stratified according to EULAR clinical response criteria (poor, moderate, or good), defined at 30 weeks.
TCRζdim T cells are enriched at sites of inflammation and accumulate in PB after treatment with anti-TNF in a subset of patients. (A) A dot plot of TCRζ versus CD3ϵ expression in PB and SF T cells from a patient with RA, as determined by flow cytometry. (B) Paired PB and SF mononuclear cells from patients with active inflammatory synovitis were stained for TCRζ and analyzed by flow cytometry. Mononuclear cell suspensions were prepared from synovial tissue (ST) specimens obtained from patients with RA at joint arthroplasty. Data are expressed as percent CD3+TCRζdim T cells in each compartment. (C) The percent CD3+TCRζdim cells was determined by flow cytometry in PBLs from 17 patients with RA at baseline and 14 weeks after treatment with anti-TNF. Data were stratified according to EULAR clinical response criteria (poor, moderate, or good), defined at 30 weeks.
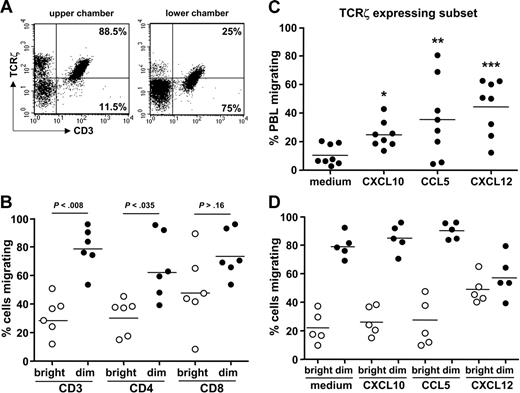
We reasoned that accumulation and persistence of PB TCRζdim effector T cells in anti-TNF responders might explain why disease flares occur on withdrawing anti-TNF therapy, after which TCRζdim T cells would migrate back to the synovial joint compartment and reinitiate a local inflammatory response. Ethics precluded further investigation in patients, and so we opted to study transendothelial migration of TCRζdim T-cell subsets in vitro. Preliminary experiments demonstrated that by 24 hours about 20% to 25% of PBLs from healthy donors migrate across TNF-stimulated endothelial monolayers cultured in the Transwell. Flow cytometric analysis demonstrated that cells migrating to the lower chamber expressed lower levels TCRζ. This was true for both T-cell and NK cell subsets (Figure 6A), and the expression profiles for upper and lower chambers were quite comparable to those we had observed for PB and SF (Figure 5A). Transmigration did not perturb TCRζ expression because the proportions of TCRζbright and TCRζdim T cells were similar to those in PB cultured for the same period in the absence of endothelial cells or Transwell, nor was it dependent on proliferation because CFSE fluorescence of migrating and nonmigrating T cells was comparable (data not shown). Even though there are many more TCRζbright than TCRζdim T cells in the population added to the upper chamber, a much higher proportion of TCRζdim cells migrated when compared to their TCRζbright counterparts (∼70% versus 30%), differences more striking for CD4+ than CD8+ subsets (Figure 6B). Migration of PBLs could be further enhanced by the addition of CXCL-10, CCL5, or CXCL-12 to the lower chamber, chemokines known to be up-regulated in RA synovial joints (Figure 6C).29 Subset analysis revealed enhanced migration of TCRζbright T cells in the presence of CXCL-12 (Figure 6D), whereas the migratory profiles of TCRζdim cells were substantially higher and barely augmented in the presence of exogenous chemokine (Figure 6D). Indeed, for the TCRζdim subset transmigration in response to CXCL-12 was reduced when compared to cells migrating in the absence of exogenous chemokine. Taken together, these results suggest that antigen-experienced TCRζdim T cells have likely acquired in vivo the propensity to migrate in part through expression of chemokines and their cognate receptors at the cell surface.
TCRζdim T cells exhibit enhanced migratory capacity in vitro. (A) Healthy donor PBLs (5 × 106) were applied to the gelatin-coated Transwell containing a monolayer of TNF-α–stimulated HUVECs and cells in upper and lower chambers harvested at 24 hours prior to staining for expression of CD3ϵ and TCRζ and analysis by flow cytometry. Representative dot plots are shown. (B) Subset analysis of migrating cells. After 24 hours cells were harvested and stained for TCRζ and CD3, CD4, or CD8, as in panel A. Data are expressed as the percentage of cells migrating relative to the total number of each cell subset added to the Transwell. The significance of differences between migration of TCRζbright and TCRζdim cells is shown. (C) The chemokines CXCL10, CCL5, or CXCL12 were added to each lower chamber of the Transwell at 50 ng/mL and the total number of PBLs migrating determined after 24 hours; *P < .013; **P = .063; ***P < .004 compared to cells migrating in the absence of exogenous chemokine. (D) The effects of each chemokine on the migration of TCRζbright and TCRζdim cells were determined by flow cytometry, as described. Differences in migration between subsets was highly significant (P < .002), with the exception of migration in response to CXCL12. Within subset differences between “medium” and CXCL12-stimulated cells were also significant (P < .023).
TCRζdim T cells exhibit enhanced migratory capacity in vitro. (A) Healthy donor PBLs (5 × 106) were applied to the gelatin-coated Transwell containing a monolayer of TNF-α–stimulated HUVECs and cells in upper and lower chambers harvested at 24 hours prior to staining for expression of CD3ϵ and TCRζ and analysis by flow cytometry. Representative dot plots are shown. (B) Subset analysis of migrating cells. After 24 hours cells were harvested and stained for TCRζ and CD3, CD4, or CD8, as in panel A. Data are expressed as the percentage of cells migrating relative to the total number of each cell subset added to the Transwell. The significance of differences between migration of TCRζbright and TCRζdim cells is shown. (C) The chemokines CXCL10, CCL5, or CXCL12 were added to each lower chamber of the Transwell at 50 ng/mL and the total number of PBLs migrating determined after 24 hours; *P < .013; **P = .063; ***P < .004 compared to cells migrating in the absence of exogenous chemokine. (D) The effects of each chemokine on the migration of TCRζbright and TCRζdim cells were determined by flow cytometry, as described. Differences in migration between subsets was highly significant (P < .002), with the exception of migration in response to CXCL12. Within subset differences between “medium” and CXCL12-stimulated cells were also significant (P < .023).
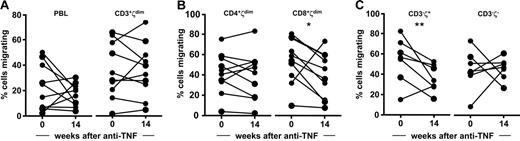
Finally, we asked whether TCRζdim cells accumulating in PB retain the capacity to migrate despite anti-TNF therapy. To this end, the migratory competence of PB cells obtained from RA patients with active disease at baseline was compared with cells acquired after 14 weeks of anti-TNF treatment. Although the results demonstrated a trend toward reduced migration of CD8+TCRζdim T cells and a significant reduction in migration of the CD3−TCRζ+ NK cell subsets, the capacity of CD3+ and CD4+TCRζdim T-cell subsets to migrate across activated endothelium in vitro was not influenced by anti-TNF treatment in vivo (Figure 7). We believe that these results provide a rational explanation for disease flares associated with treatment withdrawal and have implications for both the design and monitoring of therapeutic regimens aimed at prolonged periods of biologic drug-free remission.
PB CD4+TCRζdim T cells retain the capacity to migrate in vitro despite anti-TNF treatment in vivo. PBLs acquired from patients with RA at baseline and 14 weeks after treatment with anti-TNF were analyzed for their capacity to migrate in vitro using the transendothelial migration assay. Migrating cell subsets were determined by flow cytometry. Data are expressed as percent cells migrating for (A) PBLs and CD3+TCRζdim T cells, (B) CD4+TCRζdim and CD8+TCRζdim T cells, and (C) the CD3−TCRζ+ NK and the nonlymphocyte CD3−TCRζ− cell subsets; *P = .066; **P < .047.
PB CD4+TCRζdim T cells retain the capacity to migrate in vitro despite anti-TNF treatment in vivo. PBLs acquired from patients with RA at baseline and 14 weeks after treatment with anti-TNF were analyzed for their capacity to migrate in vitro using the transendothelial migration assay. Migrating cell subsets were determined by flow cytometry. Data are expressed as percent cells migrating for (A) PBLs and CD3+TCRζdim T cells, (B) CD4+TCRζdim and CD8+TCRζdim T cells, and (C) the CD3−TCRζ+ NK and the nonlymphocyte CD3−TCRζ− cell subsets; *P = .066; **P < .047.
Discussion
It seems likely that T cells expressing low levels of TCRζ define polyclonal populations of antigen-experienced T cells in vivo based on their cell surface phenotype, tetramer staining, cytokine expression, proliferative responses, and migratory competence. Even though their accumulation in inflamed tissues appears consistent with this, we cannot yet account for the heterogeneity of this TCRζdim population. This subset will include populations of T cells that have very recently engaged antigen, perhaps explaining their refractoriness to TCR restimulation, but also T cells in which loss of TCRζ expression persists over time. Our data seem consistent with this because during an acute inflammatory response TCRζdim cells are depleted from the PB compartment and enriched in inflamed tissue; TNF blockade reverses this. Furthermore, transendothelial migration experiments demonstrated an enhanced propensity for circulating TCRζdim T cells to migrate when compared to their TCRζbright counterparts, implying that migration is associated with, and perhaps even facilitated by, loss of TCRζ expression in peripheral lymphoid organs. We suggest that on migration to tissues in vivo this subset would be exposed to an inflammatory milieu where hypoxia, reactive oxygen intermediates, depletion of essential nutrients, inflammatory cytokines, and activated cells of myeloid lineage each contribute to chronic down-regulation of TCRζ expression.25,30–33 Therein, aberrant TCR signals and their functional sequelae may be perpetuated.
We still do not fully understand which extracellular cues sustain the effector function of TCRζdim T cells and how these apparently dysfunctional cells contribute actively to pathogenic processes, besides the apparent imbalance of TCR-dependent arrest of cell motility and competing chemokinetic “go” signals promoting adhesion and migration. One possibility is that TCRζ-deficient TCR/CD3 complexes are capable of transmitting signals to induce and sustain inflammatory cytokine gene expression through compensatory up-regulation of the FcϵRIγ signaling subunit, as first described in intraepithelial lymphocytes of TCRζ-deficient mice.34,35 Indeed, T cells from TCRζ-deficient mice are fully competent to differentiate into IFN-γ–producing Th1 cells,36 a finding consistent with the enrichment for IFN-γ and TNF-α expression in human TCRζdim T cells (Figure 3). On the other hand, the relative absence of IL-10–expressing T cells in the same population could point to an intrinsic requirement for intact TCR signaling to maintain immune homeostasis through the generation or function of regulatory T-cell subsets, as suggested in studies of patients with diabetes treated with anti-CD3.37 Appropriate antigen-dependent expansion of adaptive CD4+CD25highCD62L−Foxp3+ regulatory T cells from a pool of differentiating effector T cells might fail for the same reason. An alternative possibility is that the chronic phase of effector function is maintained independently of antigen stimulation, as suggested by experiments in which TCRζdim T cells produce IFN-γ following engagement of costimulatory pathways, while at the same time stimulating monocytes to produce inflammatory cytokines such as TNF through cell contact-dependent pathways. Moreover, attenuation of TCRζ-dependent signaling will perturb pathways of antigen-dependent activation-induced cell death.38 It follows from this that TCRζdim T cells would persist in tissues over extended periods of time, further amplifying effector pathways mediated through cell contact with infiltrating macrophages, B cells, and resident stromal cells. Although such sustained T-cell effector responses might usefully serve to sustain immunity to foreign pathogens, they are clearly harmful in autoimmunity.
A direct link between the loss of integrity of signaling from the TCR/CD3 complex and the generation of a repertoire of arthritogenic T cells has recently been reported in SKG mice.39 A spontaneous mutation in ZAP-70, which uncouples membrane proximal TCR signal transduction, impairs thymic selection leading to a peripheral repertoire of autoreactive T cells, which, among other tissues, targets synovial joints manifesting as a symmetrical and destructive polyarthritis.39 There are striking similarities between CD4+ T cells from SKG mice and TCRζdim T cells, not least of which being their profound hyporesponsiveness to TCR engagement, skewed T-helper cell differentiation favoring expression of TNF-α, IFN-γ, and (in our hands) IL-17, in combination with a robust capacity to activate monocytes. These T-cell–monocyte interactions likely play a major role in promoting the chronic, excessive cytokine drive characteristic of both autoimmune arthritis in SKG mice and RA.17,40–42 Given the strong predilection to migrate to inflammatory tissues coupled with their effector phenotype, TCRζdim T cells should now be considered a valid cellular target for treating a wide range of chronic inflammatory diseases.
Authorship
Contribution: Z.Z., C.L.G., A.-C.V., A.F., S.O., P.I. and A.R. performed research; P.A., A.V., and C. McClinton collected data; C. Monaco, F.M.-B., F.D., T.J.V., and F.M.B analyzed data; and Z.Z., C.L.G., and A.P.C. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Z.Z. and C.L.G. contributed equally to this study.
Correspondence: Andrew P. Cope, Kennedy Institute of Rheumatology Division, Faculty of Medicine, Imperial College London, 1, Aspenlea Rd, Hammersmith, London W6 8LH, United Kingdom; e-mail: andrew.cope@imperial.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Wellcome Trust, the Arthritis Research Campaign, and the British Heart Foundation.
The authors gratefully acknowledge Eugenio Macchiarulo for help with the tetramer staining and Jane Douglas for collecting clinical data.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal