Abstract
Tumor growth promotes the expansion of myeloid suppressor cells. An inverse correlation between natural killer (NK) cell activation and myeloid suppressor cell (MSC) expansion in tumor-bearing patients and mice prompted us to investigate the role of MSCs in controlling NK antitumor cytotocixity. After adoptive transfer to naive recipients, CD11b+Gr-1+ MSCs freshly isolated from spleens of tumor-bearing mice but not naive mice were able to inhibit NK cell cytotoxicity. An in vivo imaging analysis indicates that the removal of tumors resulted in a significant increased ability (P < .05) in NK cell cytotoxicity to eliminate injected YAC-1 cells from the lungs. Fluorescence-activated cell sorter (FACS) analysis of the composition of lung leukocytes further indicates that the removal of tumors also leads to the reduction of MSCs accumulated in the lung. These data suggest that MSCs suppress NK cell cytotoxicity. The inhibition of NK cell cytotoxicity is cell-cell contact dependent. Inhibition of perforin but not granzyme B production was responsible for MSC-mediated inhibition of NK cytotoxicity. Western blot analyses further suggests that MSCs suppress IL-2–mediated NK cell cytotoxicity by affecting the activity of Stat5.
Introduction
Increased numbers of myeloid cells expressing the Gr-1 and CD11b markers have been detected in the spleen and bone marrow of mice bearing transplantable tumors and in many conditions associated with impaired immune activity.1–10 The CD11b+Gr-1+ cell population is phenotypically heterogeneous, consisting primarily of immature myeloid cells and cells expressing immature dendritic cell (DC) markers, both of which play a role in suppression of the T-cell immune response. In healthy subjects, a small number of CD11b+Gr-1+ cells (less than 4%) can be found in the blood and spleen. Immature myeloid cells isolated from human or murine bone marrow or peripheral blood can be induced to differentiate in vitro into mature antigen-presenting cells (APCs)/DCs by means of various cytokine combinations. Disturbances in cytokine homeostasis induced by tumor-derived factors result in the expansion of myeloid suppressor cells ([MSCs] CD11b+Gr-1+) in vivo.1,8,11,12 The accumulation of MSCs in the spleen and blood of tumor-bearing individuals has been observed in many different types of cancers and is associated with increased tumor burden and predicts poor survival rates.4,8,13,14 As MSCs in the spleen of tumor-bearing subjects dramatically increase, the MSCs suppress the activation of CD4+ and CD8+ T lymphocytes and thereby inhibit immune surveillance
Natural killer (NK) cell tumor cytotoxicity in cancer patients and tumor-bearing animal models is suppressed.15–22 It is unclear whether accumulation of MSCs is responsible for immunosuppression of NK cell tumor cytotoxicity. NK cells participate in the innate immune response to malignant cells. Unlike T lymphocytes, NK cells do not depend on recognition of tumor-specific antigens for antitumor cytotoxicity. In addition to their ability to recognize and eliminate altered cells by cytotoxic mechanisms, activated NK cells are potent sources of immune modulatory cytokines that directly aid in the elimination of tumor cells and also indirectly augment a developing adaptive immune response against tumor growth.
In this study, evidence is provided that in murine systems, MSCs potently suppress NK cell cytotoxicity in vitro and in vivo. The inhibitory effect requires NK-MSC direct contact, which inhibits IL-2–mediated activation of NK cells and perforin production.
Materials and methods
Mice
Adult female BALB/c mice (The Jackson Laboratory, Bar Harbor, ME) were 6 to 8 weeks old when used and were housed in the Animal Care Facility at University of Alabama at Birmingham.
Cell lines
The TS/A cell line, a major histocompatibility complex (MHC) class I–positive (H-2Dd, H-2Kd) moderately differentiated and immunogenic mammary adenocarcinoma of spontaneous origin in BALB/c mice, was maintained in vitro at 37°C in a humidified 5% CO2 atmosphere in complete medium. YAC-1 cells (American Type Culture Collection, [ATCC] Manassas, VA) were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 10 mM HEPES (pH 7.4), and antibiotics (100 units of penicillin per milliliter and 100 μg of streptomycin per milliliter).
Establishment and screening of packaging YAC-1– or TS/A-luciferase cell clones
YAC-1 or TS/A tumor cells were maintained in RPMI 1640 or DMEM medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 10 mM HEPES (pH 7.4), and antibiotics (100 units of penicillin per milliliter and 100 μg of streptomycin per milliliter).16 A lentiviral vector expressing luciferase was transfected into YAC-1 or TS/A cells at 1 plaque-forming unit (PFU) per cell in complete RPMI medium supplemented with Polybrene (4 μg/mL; Sigma-Aldrich, St Louis, MO). Infected cell populations were selected with puromycin (0.4 μg/mL) for 7 to 10 days using limiting dilution. More than 50 resistant clones of each cell type were screened using a luciferase assay as described previously.23 Ten clones of each cell type with the highest end-point titer were selected and retested. Cell clones with the highest expression of luciferase –YAC-1–Luc were selected for use in vivo and in vitro in the imaging NK cell cytotoxicity assay.
In vivo imaging of NK cell cytotocity assay
To determine NK cell cytotoxicity in naive or TS/A tumor-bearing mice, mice were injected subcutaneously with 0.1 mL of a single-cell suspension containing 3 × 105 TS/A adenocarcinoma cells or with PBS as a control in the mammary region. Four weeks after the TS/A tumor cell injection, YAC-1 cells (1 × 106 cells in 0.1 mL) expressing firefly luciferase (YAC-1–Luc) were injected into mice via the tail vein. After being anesthesized with isoflurane, mice were then injected intraperitoneally with 2.5 mg D-luciferin dissolved in 100 μL PBS for each imaging session. Whole body images were taken 15 minutes after D-luciferin injection. The animals were repeatedly imaged in the same position for 5 minutes at 0, 2, 4, and 6 hours after injection of YAC-1–Luc cells using an IVIS-100 imaging system (Xenogen, Alameda CA) to acquire the photons of light emitted from the mice. Region of interest analyses of luciferase signals using Living Image 2.50 software (Xenogen) was in units of relative photon counts per second. To determine if debulking tumor had an effect on NK cell cytotoxicity, the tumors were removed from tumor-bearing mice 2 weeks after tumor cells were implanted. Mice were first anesthetized with ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight) via intraperitoneal injection, and inhaled isoflurane was used as necessary. Debulking surgery was done via elliptic incisions centered over the subcutaneous tumors. Skin flaps were then elevated to expose adherent tumors. Once tumors were dissected clear of adjacent fascia, wounds were closed primarily using 5/0 Vicryl (polyglactin 910; Ethicon, Cornelia, GA) interrupted sutures. Mice received 2.5 mg/kg buprenorphine intraperitoneally in the recovery phase for postoperative analgesia as required. Two weeks after the removal of tumors, an in vivo measurement of NK cell cytotoxicity was conducted and cytotoxicity was calculated using identical protocols as described in “Cytotoxicity assay.”
Isolation of cells
A single-cell suspension was prepared from the spleens of control mice and mice bearing TS/A tumor. Erythrocytes were depleted using red blood cell (RBC) lysis buffer (Sigma-Aldrich), and splenocytes were washed in magnetic-activated cell sorting (MACS) buffer (1× PBS supplemented with 2 mM EDTA and 0.5% bovine serum albumin). CD11b+, Gr-1+, and NK cells were isolated using corresponding MACS microbeads (Miltenyi Biotec, Auburn, CA). The purity of cells after separation ranged between 90% and 94%.
To determine whether MSCs are increased in the lung of tumor-bearing mice, lungs from each mouse were excised, washed in PBS, minced, and digested enzymatically for 30 minutes in 15 mL (each lung) of digestion buffer (RPMI 1640, 5% FCS, 1 mg/mL collagenase [Boehringer Mannheim Biochemical, Mannheim, Germany], and 30 μg/mL DNase [Sigma-Aldrich]). Following erythrocyte lysis using NH4Cl buffer, cells were washed, resuspended in complete medium, and centrifuged for 30 minutes at 2000g in the presence of 20% Percoll (Sigma-Aldrich) to separate leukocytes from cell debris and epithelial cells. Total lung leukocyte numbers were assessed in the presence of trypan blue using a hemocytometer; viability was more than 90%.
Flow cytometry analysis
Spleen cells or leukocytes isolated from lung as described in “Isolation of cells” were washed in fluorescence-activated cell sorter (FACS) medium (1× PBS supplemented with 0.1% BSA and 0.1% NaN3) and stained with appropriately diluted antibodies according to a standard procedure followed by fixation in 2% paraformaldehyde.24 Antibodies used for FACS staining were as follows: anti–mouse CD11b, NKDX5, CD11c, TRAIL, Gr-1, F4/80, FasL, and CD8 (BD Pharmingen, San Diego, CA). All staining procedures were conducted on ice. Fluorescence was measured using a FACScan flow cytometer (BD Biosciences, San Jose, CA), and data analysis was performed using CellQuest software (BD Biosciences).
Adoptive transfer of spleen MSCs and NK cell cytotoxicity
MSCs (CD11b+Gr-1+) were isolated from the spleen of BALB/c naive and TS/A tumor-bearing mice using magnetic beads coated with Gr-1 antibody according to the manufacturer's instructions (Miltenyi Biotec). The isolated cells were washed by centrifugation, and 6 × 106 of the cells were injected into naive mice via the tail vein. One day after the adoptive transfer, in vivo cytotoxicity of NK cells was determined by measuring luciferase activity in the in vivo imaging technique for NK cell cytotoxicity. Data are representative of 3 independent in vivo experiments.
Cytotoxicity assay
YAC-1–Luc and TS/A-Luc target cells were washed once in RPMI media and plated at a concentration of 5000 per 0.1 mL in 96-well microplates. Target cells were cocultured with increasing numbers of spleen cells, red blood cell depleted, in a final volume of 0.2 mL at 5% CO2, 37°C. After a 4-hour incubation, cocultured cells were washed 3 times with PBS. The level of luciferase activity was subsequently determined by using a chemiluminescence assay according to the manufacturer's instructions (Promega, Madison, WI). For each target 3 to 6 replicates of the internal references for the 0% viability background (MIN) and the 100% viability maximal signal (MAX) were run. The 0% viability reference point was determined by plating target cells in media with a final concentration of 1% SDS (MINSDS). The 100% viability reference point (MAXmedia) was determined by plating target cells in media without effector cells. The plates were placed in a microtiter plate luminometer (ML3000; Dynatech Laboratories, Chantilly, VA) where 100 μL of the luciferase assay reagent (Promega) was injected per well and the relative light units for each sample determined. Percent viability was calculated as the mean luminescence of the experimental sample minus background (MINSDS) divided by the mean luminescence of the input number of target cells used in the assay (MAXmedia) minus background (MINSDS). Percent specific lysis is equal to (1 − percent viability) × 100 and is calculated as follows: % specific lysis = [1 − counts per 5 seconds (experimental − MINSDS)/(MAXmedia − MINSDS] × 100.24
Analysis of MSC-mediated NK cell cytotoxicity
Murine NK cells were isolated from the spleens and purified with anti-DX5 antibody–coated microbeads (Miltenyi Biotec). Purified NK cells were cocultured with spleen CD11b+Gr-1+ MSCs for different intervals in IL-2– (100 U/mL) containing media. The ability of NK cells to kill target cells (YAC-1–Luc, TS/A-Luc) was determined by the luciferase release cytotoxicity assay described in “Cytotoxicity assay.”
In a separate experiment, CD11b+Gr-1+ spleen cells and NK cells were dispensed into separate chambers of a Transwell system25–27 and the Transwells placed in a 96-well microtiter plate. NK cells were placed in the upper chamber, and CD11b+Gr-1+ cells were placed in the lower chamber. Both types of cells were cultured in the RPMI 1640 supplemented with 10% FBS and IL-2 (100 U/mL). The cells were cultured for 3 to 5 days. YAC-1–Luc cells were added to the upper chamber, and luciferase activity was measured over the last 4 hours of the culture period using a luciferase assay kit according to the manufacturer's instructions (Promega). Four to 6 replicates of all assay cultures were set up.
Western blot analysis
Western blot analysis of proteins expressed in NK cells cocultured with and without CD11b+Gr-1+ cells was carried out using the method described previously.28 In brief, after coculture, DX5+ NK cells were purified using anti-DX5–coated magnetic beads (Miltenyi Biotec). Cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were electrotransferred to nitrocellulose membranes. Membranes were saturated overnight at 4°C in PBS/0.05% Tween 20 containing 5% BSA (Sigma-Aldrich) and then probed with antibodies against perforin, granzyme B (BD Biosciences), Jak-3, Jak-1, Stat5, phosphorylated Stat5 (Cell Signaling Tech, Danvers, MA), and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at 22°C. Membranes were washed 5 times with PBS/0.05% Tween 20. Western blots were probed with goat antimouse or antirabbit secondary antibodies conjugated to Alexa Fluor 680 (Molecular Probes, Eugene, OR) or IRdye 800 (Rockland Immunochemicals, Gilbertsville, PA). Blotted proteins were detected and quantified using the Odyssey infrared imaging system (LI-COR).
Statistics
Results were expressed as means ± SEM and analyzed by 1-way analysis of variance (ANOVA) with Bonferroni correction.
Results
Decreased cytotoxicity of NK cells in tumor-bearing mice
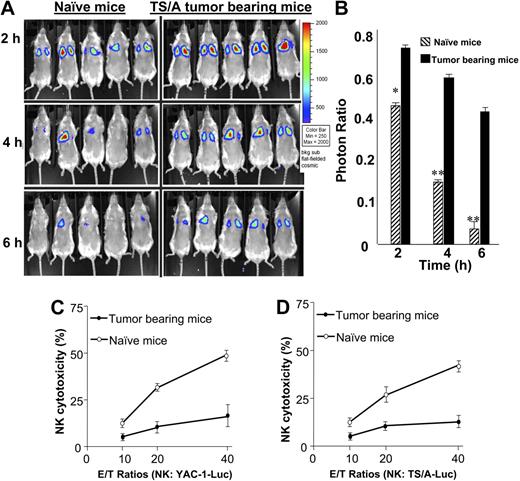
As a first step to investigate NK cell immunity in this model, the cytotoxicity of NK cells for YAC-1 cells was determined by monitoring YAC-1 clearance from the lung of BALB/c mice, a well-recognized index of NK cell activity. Thirty minutes afterinjection of YAC-1 target cells, most YAC-1–Luc target cells had migrated to the lung with the same signal intensity detected in tumor-bearing and untreated mice (data not shown). These target cells were essentially cleared within 4 hours after injection in the group of mice treated with PBS (Figure 1A; P < .001 versus luciferase activity at time 0). In contrast, after 4 hours, YAC-1 clearance was dramatically impaired in tumor-bearing animals compared with animals treated with PBS as a control (Figure 1A-B).
NK cell cytotoxicity is suppressed in tumor-bearing mice. Seven-week-old BALB/c mice were injected subcutaneously with TS/A tumor cells (3 × 105) or PBS as a control. Four weeks after tumor challenge, the mice were anesthetized and injected intravenously with YAC-1–Luc cells (1 × 106). After injection of D-luciferin the mice were imaged at hours 0, 2, 4, and 6 (A), and the total photon count per minute (photons per minute) calculated (5 animals) using Living Image software. The efficiency of NK cell killing of injected YAC-1–Luc cells was determined by measuring the numbers of photons collected at the imaging time points divided by at 0 hours collected (B). *P < .05; **P < .001. On completion of the imaging studies, NK cells (DX5+) were isolated from the spleen, and then stimulated with recombinant IL-2 (100 U/mL) for 5 days. After the incubation period, the NK cells were added to YAC-1–Luc or TS/A-Luc cells at varying effector-target (E/T) ratios (10:1, 20:1, and 40:1) as indicated in panels C and D. The cytotoxicity of NK cells to YAC-1–Luc (C) or TS/A-Luc (D) was determined using an NK cell cytotoxic assay as described in “Cytotoxicity assay.” The data represent the mean ± SEM from 5 mice from each group.
NK cell cytotoxicity is suppressed in tumor-bearing mice. Seven-week-old BALB/c mice were injected subcutaneously with TS/A tumor cells (3 × 105) or PBS as a control. Four weeks after tumor challenge, the mice were anesthetized and injected intravenously with YAC-1–Luc cells (1 × 106). After injection of D-luciferin the mice were imaged at hours 0, 2, 4, and 6 (A), and the total photon count per minute (photons per minute) calculated (5 animals) using Living Image software. The efficiency of NK cell killing of injected YAC-1–Luc cells was determined by measuring the numbers of photons collected at the imaging time points divided by at 0 hours collected (B). *P < .05; **P < .001. On completion of the imaging studies, NK cells (DX5+) were isolated from the spleen, and then stimulated with recombinant IL-2 (100 U/mL) for 5 days. After the incubation period, the NK cells were added to YAC-1–Luc or TS/A-Luc cells at varying effector-target (E/T) ratios (10:1, 20:1, and 40:1) as indicated in panels C and D. The cytotoxicity of NK cells to YAC-1–Luc (C) or TS/A-Luc (D) was determined using an NK cell cytotoxic assay as described in “Cytotoxicity assay.” The data represent the mean ± SEM from 5 mice from each group.
To determine whether tumor-bearing mice had a reduced NK cell cytotoxic activity, single-cell suspensions of the spleens of mice bearing the tumor for 4 weeks or naive mice were stimulated with IL-2 (100 U/mL) for 5 days. The NK cell–mediated killing of YAC-1–Luc target cells (Figure 1C) was dramatically impaired in tumor-bearing mice but not naive mice. At a 40:1 effector-target (E/T) ratio, there was a 8.2-fold ± 1.4-fold reduction in the cytotoxic activity of the NK cells isolated from spleens of tumor-bearing mice as compared with NK cells isolated from spleens of naive mice. Similarly, NK cell–mediated killing of TS/A tumor cells was inhibited (Figure 1D).
CD11b+Gr-1+ cells of the spleen inhibit NK cell cytotoxicity
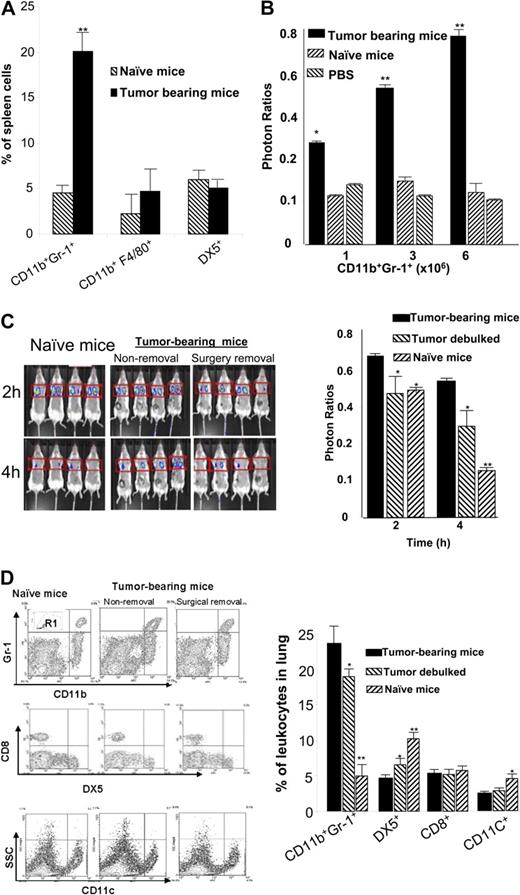
Significantly higher percentages of CD11b+Gr-1+ cells accumulated in the spleens of tumor-bearing but not naive mice (22 ± 2.4 versus 3.1 ± 0.4, respectively) (Figure 2A). Slightly higher percentages of macrophages, although not significant, were also observed in the tumor-bearing mice (Figure 2A). Further dynamic analyses of the spleen MSCs over a period of 4 weeks after tumor injection suggested that the accumulation of spleen CD11b+Gr-1+ cells was associated with tumor growth (data not shown). The percentage of spleen MSCs increased from 3.1% ± 0.4% at day 0 to 28.5% ± 2.2% at day 28. To determine if MSCs isolated from the spleen of tumor-bearing mice are responsible for the impairment of NK cell cytotoxicity in vivo, we adoptively transferred 3 doses ofCD11b+Gr-1+ MSCs isolated from tumor-bearing mice and naive mice. These doses were high (6 × 106), intermediate (3 × 106), or low (1 × 106) and have been established previously in our laboratory as the percentages of spleen CD11b+Gr-1+ MSCs that accumulated over 4 weeks of TS/A tumor growth. NK cell cytotoxcity was suppressed significantly in the group of mice subjected to adoptive transfer of MSCs isolated from tumor-bearing mice but was not suppressed with cells transferred from naive mice (Figure 2B). The degree of suppression was correlated with the dose of MSCs administered; suggesting that suppression of NK cell cytotoxicity is tumor specific. The suppressive effect was still significant even when mice received a low dose of MSCs of approximately 5% of total spleen cells.
Spleen MSCs suppress NK cell cytotoxicity in vivo. Tumor-bearing mice at 4 weeks after tumor injection were killed and spleen cells were isolated. (A) The percentages of DX5, F4/80, CD11b, and Gr-1+ cells were determined by FACS analysis as described in “Flow cytometry analysis.” The data represent the mean ± SEM from 5 mice from each group. (B) CD11b+Gr-1+ cells were isolated from the spleens of 9-week-old TS/A tumor-bearing BALB/c mice or naive mice. Increased numbers (1 × 106, 3 × 106, and 6 × 106) of sorted CD11b+Gr-1+ MSCs were transferred intravenously into 2-month-old BALB/c female mice (n = 5 per group). Twenty-four hours after adoptive transfer, the efficiency of NK cell killing of injected YAC-1–Luc cells was determined by measuring the numbers of photons collected at 6 hours divided by the photons collected at 0 hours. *P < .05; **P < .001. (C) Female BALB/c mice (n = 4): TS/A tumor-bearing mice with tumor debulked surgically (surgery removal), tumor-intact mice (nonremoval), and non–tumor-bearing PBS control mice (naive mice). Two weeks later, an in vivo measurement of NK cell cytotoxicity was determined by injection of YAC-1–Luc using an identical protocol, as described in Figure 1. The efficiency of NK cell killing of injected YAC-1–Luc cells was determined by measuring the numbers of photons collected at 2 hours and 4 hours divided by the photons collected at 0 hours. *P < .05; **P < .001. The data represent the mean ± SEM of 2 independent experiments (n = 4) (C, right panel). (D) After imaged mice were killed, the percentages of leukocytes in the lung were determined in the gated R1 region of a FACS analysis (top left). The presence of CD11b+Gr-1+, DX5+, CD8+, and CD11c+ cells was determined (left, representative plots). Results obtained from 2 independent experiments with replica 4 mice in each experiment were pooled and are presented as the mean ± SEM. *P < .05; **P < .01.
Spleen MSCs suppress NK cell cytotoxicity in vivo. Tumor-bearing mice at 4 weeks after tumor injection were killed and spleen cells were isolated. (A) The percentages of DX5, F4/80, CD11b, and Gr-1+ cells were determined by FACS analysis as described in “Flow cytometry analysis.” The data represent the mean ± SEM from 5 mice from each group. (B) CD11b+Gr-1+ cells were isolated from the spleens of 9-week-old TS/A tumor-bearing BALB/c mice or naive mice. Increased numbers (1 × 106, 3 × 106, and 6 × 106) of sorted CD11b+Gr-1+ MSCs were transferred intravenously into 2-month-old BALB/c female mice (n = 5 per group). Twenty-four hours after adoptive transfer, the efficiency of NK cell killing of injected YAC-1–Luc cells was determined by measuring the numbers of photons collected at 6 hours divided by the photons collected at 0 hours. *P < .05; **P < .001. (C) Female BALB/c mice (n = 4): TS/A tumor-bearing mice with tumor debulked surgically (surgery removal), tumor-intact mice (nonremoval), and non–tumor-bearing PBS control mice (naive mice). Two weeks later, an in vivo measurement of NK cell cytotoxicity was determined by injection of YAC-1–Luc using an identical protocol, as described in Figure 1. The efficiency of NK cell killing of injected YAC-1–Luc cells was determined by measuring the numbers of photons collected at 2 hours and 4 hours divided by the photons collected at 0 hours. *P < .05; **P < .001. The data represent the mean ± SEM of 2 independent experiments (n = 4) (C, right panel). (D) After imaged mice were killed, the percentages of leukocytes in the lung were determined in the gated R1 region of a FACS analysis (top left). The presence of CD11b+Gr-1+, DX5+, CD8+, and CD11c+ cells was determined (left, representative plots). Results obtained from 2 independent experiments with replica 4 mice in each experiment were pooled and are presented as the mean ± SEM. *P < .05; **P < .01.
To establish whether the presence of tumor was an important factor in maintaining the induction of MSCs and therefore inhibition of NK cell cytotoxicity, experiments were conducted in which the entire tumor was removed. Two weeks after the removal of tumors, an in vivo imaging analysis indicated that the removal of tumors from lungs allowed for a significantly increased ability (P < .05) in NK cell cytotoxicity to eliminate injected YAC-1–LUC cells compared with control tumor-bearing mice (Figure 2C). Further, FACS analysis of the composition of lung leukocytes indicates that the removal of tumors also leads to the reduction of MSCs that accumulate in the lung (Figure 2D) although the percentages of MSCs do not achieve the level of nontumor-bearing mice. These data suggest that decreased MSCs in the lung may be responsible for the increased NK cell cytotoxicity, because the percentages of other immune cells including CD11c+ and CD8+ cells in the lung are not altered significantly (P > .05) (Figure 2D).
MSC-mediated inhibition of NK cell cytotoxicity is cell-cell contact dependent
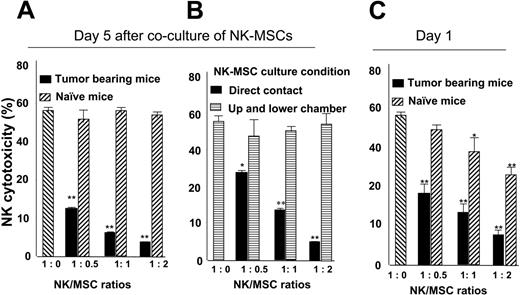
A 5-day coculture of MSCs with NK cells caused a suppression of NK cell cytotoxicity of YAC-1–Luc target cells (Figure 3A). Suppression of NK cell cytotoxicity is tumor specific because CD11b+Gr-1+ spleen cells isolated from control mice did not have a significant inhibitory effect on NK cell cytotoxicity. MSC-mediated inhibition of NK cell cytotoxicty was eliminated when NK-MSCs were cocultured for 5 days in different chambers of a Transwell system (Figure 3B), suggesting that the suppression of IL-2–mediated NK cell cytotoxicity in vitro is cell-cell contact dependent. Also, in an identical experimental setting, a 1-day coculture of NK-MSCs (Figure 3C) indicates that MSCs isolated from naive mice also have the capacity to inhibit NK cell cytotoxicity, although the inhibitory effect was much less than with MSCs isolated from tumor-bearing mice.
Spleen MSCs suppress NK cell cytotoxicity in a cell-cell contact–dependent manner. Splenic MSCs (CD11b+Gr-1+) from naive or tumor-bearing BALB/c mice were FACS sorted and cocultured with DX5+ NK cells at the ratios between 1:0 to 1:2 (NK/MSC) in a 24-well plate (A) or in a Transwell system (B). After 1 day (C) or 5 days (A-B) of NK-MSC coincubation, NK cell cytotoxicity against YAC-1–Luc cells (YAC-1–Luc/NK, 1:20) was assayed as described in “Cytotoxicity assay.” The data represent NK cell killing YAC-1–LUC activity at 4 hours after addition of YAC-1–Luc cells. Data are mean (± SEM) of triplicate wells of 3 independent experiments. *P < .05; **P < .001.
Spleen MSCs suppress NK cell cytotoxicity in a cell-cell contact–dependent manner. Splenic MSCs (CD11b+Gr-1+) from naive or tumor-bearing BALB/c mice were FACS sorted and cocultured with DX5+ NK cells at the ratios between 1:0 to 1:2 (NK/MSC) in a 24-well plate (A) or in a Transwell system (B). After 1 day (C) or 5 days (A-B) of NK-MSC coincubation, NK cell cytotoxicity against YAC-1–Luc cells (YAC-1–Luc/NK, 1:20) was assayed as described in “Cytotoxicity assay.” The data represent NK cell killing YAC-1–LUC activity at 4 hours after addition of YAC-1–Luc cells. Data are mean (± SEM) of triplicate wells of 3 independent experiments. *P < .05; **P < .001.
MSCs preferentially inhibit the production of perforin in NK cells
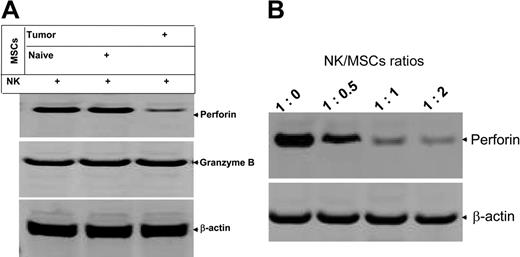
Perforin and granzyme B are 2 major effector molecules for NK cell cytotoxicity; therefore, investigations were conducted to determine whether the expression of these effector molecules are inhibited in NK cells cocultured with MSCs (NK/MSC ratio, 1:1) in the presence of IL-2 for 5 days. Immunoblot analysis indicated a selective effect; expression of perforin in IL-2–stimulated NK cells was reduced dramatically when NK cells were cocultured with MSCs isolated from the spleen of tumor-bearing mice but not naive mice (Figure 4A). The expression of granzyme B was unaffected. MSC-mediated inhibitory effects on the production of perforin in IL-2–stimulated NK cells was also MSC dose dependent (Figure 4B), suggesting that MSC-mediated inhibition is not MSC nonspecific cytotoxic. Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of expression of perforin further indicates that mRNA of perforin was not affected by coculture of NK cells with MSCs (data not shown), signifying that the effect of MSCs on the production of NK cell perforin may be regulated through posttranscriptional machinery.
MSCs preferentially inhibit the production of perforin from NK cells. Spleen DX5+ NK cells were cocultured with spleen MSCs for 5 days at the ratio of 1:1 (A) or at varying ratios as indicated in panel B. DX5+ NK cells were then isolated with DX5 antibody-coated magnetic beads using the method as described previously. NK cells (1 × 106) were lysed in protein lysis buffer, and 50 μg total protein from each lysate was resolved on a 10% SDS PAGE gel. The proteins were then transferred to a nitrocellulose membrane, and the blots were probed with the indicated antibodies. The data are representative of 3 independent experiments. β-actin served as an internal control to confirm equivalent protein loading.
MSCs preferentially inhibit the production of perforin from NK cells. Spleen DX5+ NK cells were cocultured with spleen MSCs for 5 days at the ratio of 1:1 (A) or at varying ratios as indicated in panel B. DX5+ NK cells were then isolated with DX5 antibody-coated magnetic beads using the method as described previously. NK cells (1 × 106) were lysed in protein lysis buffer, and 50 μg total protein from each lysate was resolved on a 10% SDS PAGE gel. The proteins were then transferred to a nitrocellulose membrane, and the blots were probed with the indicated antibodies. The data are representative of 3 independent experiments. β-actin served as an internal control to confirm equivalent protein loading.
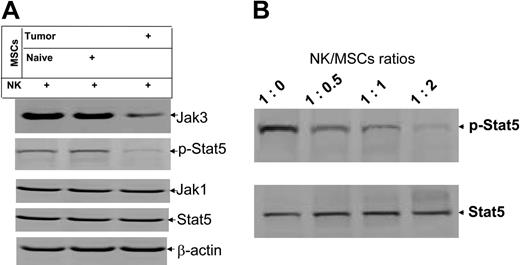
MSCs preferentially inhibit activation of Stat5 in IL-2–stimulated NK cells
Our previous data indicate that Jak3-mediated activation of the transcription factor Stat5 plays a critical role in IL-2–stimulated activation of NK cells in vitro.17 Therefore, we quantified the expression of specific proteins in IL-2–stimulated NK cells that had been cocultured with MSCs isolated from the spleen of tumor-bearing mice or naive mice. Results of Western blot analysis demonstrate that expression of the Jak3 protein (Figure 5A, top blot), but not Jak1 (Figure 5A, third blot from top), is inhibited by the MSCs isolated from the spleen of tumor-bearing mice but not naive mice. The inhibition of Jak3 expression was further demonstrated by a reduction in levels of phosphorylated Stat5, which is activated by Jak3 (Figure 5A, second blot from top). Inhibition of phosphorylation of Stat5 was not due to the differences in sample amounts, because equivalent levels of β-actin were detected in all samples (Figure 5A, bottom blot). The level of inhibition of phosphorylated Stat5 also correlated directly with the number of MSCs cocultured with NK cells. The higher the numbers of MSCs added to the coculture, the stronger the suppression of phosphorylated Stat5 (Figure 5B).
Coculture of NK-MSCs results in the inhibition of phosphorylation of NK cell Stat5. Protein lysates were produced identically to those in Figure 4. Protein lysates from NK-MSCs cocultured at 1:1 and varying ratios (B) were run in the Western blot. The data are representative of 3 independent experiments. β-Actin was used as an internal control to confirm equivalent protein loading.
Coculture of NK-MSCs results in the inhibition of phosphorylation of NK cell Stat5. Protein lysates were produced identically to those in Figure 4. Protein lysates from NK-MSCs cocultured at 1:1 and varying ratios (B) were run in the Western blot. The data are representative of 3 independent experiments. β-Actin was used as an internal control to confirm equivalent protein loading.
Discussion
The results of this investigation provide evidence that CD11b+Gr-1+ MSC suppressor cells isolated from tumor-bearing mice suppressNK cell cytotoxicity in vivo and in vitro. This conclusion is supported by the facts that NK cell cytotoxicity against its target cell, YAC-1–Luc, is significantly reduced in tumor-bearing mice; adoptive transfer of CD11b+Gr-1+ MSC suppressor cells results in a strong suppression of NK cytotoxicity in vivo; MSC-mediated suppression of NK cell cytotoxicity is cell-cell contact dependent; and inhibition of Jak3-mediated activation of Stat5 is one of the primary signaling pathways that is crucial for NK cell activation and subsequently perforin-mediated cytotoxicity.
The data presented support earlier observations of attenuated NK activity and accumulation of MSCs in cancer patients and tumor-bearing mice and represent a link between tumor immune suppression and innate immunity.3,5,7,13,29–31 NK cells have been shown previously by us17 and others29,31 to play a critical role in the elimination of implanted tumor cells, whereas tumor-derived factors inhibit the activity of NK cells and promote tumor growth. The results of debulking tumors in this study further suggest that tumor-derived factors induce MSC production, and the removal of tumor led to the reduction of MSCs in the lung. The reduction of lung MSCs is also correlated with an increase of NK cell cytotoxicity in vivo. The identity of tumor-derived factors that induce the expansion of myeloid cells and their conversion into MSCs remains unknown and will require further investigation.
The mechanisms by which MSCs mediate their suppressive effects on NK cells are not yet determined and may involve multiple events, particularly in vivo. Results from in vitro experiments indicate that the immunosuppressive properties of MSCs appear to be mediated in an NK-MSC contact–dependent manner. The cell-cell contact–dependent inhibition of NK cell cytotoxicity implies that there may be inhibitory ligand(s) expressed on the spleen MSCs and that ligand expression may be further induced by tumor derived factor(s). This speculation is supported by our data that a transient suppression of NK cell cytotoxicity was observed when myeloid precursor cells isolated from naive mice were cocultured with NK cells in the presence of IL-2. However, unlike the long-term (5-day) suppression effect of MSCs isolated from tumor-bearing mice, only a weak suppression of NK cell cytotoxicity was observed 24 hours after coculture. This transient suppressive effect may be overridden by the IL-2–mediated activation pathway in NK cells. However, in cocultures of NK cells and MSCs, where the MSCs are isolated from tumor-bearing mice, inhibitory pathway(s) may still be in dominance, suggesting that more than one inhibitory pathway may exist in MSCs isolated from tumor-bearing mice and that the pathway(s) may not be overridden by an IL-2–mediated activation pathway. Other mechanisms may also be involved, particularly in vivo. For instance, MSCs in tumor-bearing mice may indirectly suppress NK cell activity through the modulation of CD4 T-cell function by inhibiting the production of IL-2.32,33 Inhibition of production of IL-2, a potent NK stimulator, may be an indirect way that MSCs modulate NK cell activity in vivo. Our data support the involvement of MSCs in IL-2–mediated activation of NK cytotoxicity. Cell-cell contact of NK cells and MSCs causes an MSC inhibition of IL-2–mediated activation of Jak3 and a reduction/inhibition in phosphorylation of Stat5. IL-2–induced activation of JAK3 kinases is associated with tyrosine phosphorylation and activation of Stat5.34 Thus, reduction of phosphorylated Stat5 further supports the notion that MSC-mediated inhibition of NK cell cytotoxicity targets the Jak3 pathway in IL-2–stimulated NK cells. This conclusion is further supported by reduction of the expression of perforin in NK cells in contact with MSCs.
IL-2–activated Stat5 has been shown to induce the expression of a number of genes, including perforin, IFN-γ, and granzyme B.35–37 Our results indicate that MSC-mediated inhibition of NK cell cytotoxicty is correlated with a reduction in perforin production but not granzyme B or IFN-γ (data not shown). This result implies that the net effect of the MSC-NK coculture in the presence of IL-2 is a preferential down-regulation of perforin production. This may be due to MSC-mediated inhibitory pathway(s) interacting with an IL-2–mediated activation pathway in NK cells. Future study is needed to identify what inhibitory molecule(s) may be induced on the MSCs and how IL-2–mediated activation is affected.
Authorship
Contribution: C.L., J.W., S.Y., and K.R.Z. participated in designing the research; C.L. performed the research; W.G., J.K., and H.-G.Z. analyzed data; H.-G.Z. wrote the manuscript; and all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Huang-Ge Zhang, University of Alabama at Birmingham, 701 S 19th St, LHRB 473, Birmingham, AL; 35294-0007; e-mail: huang-ge.zhang@ccc.uab.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (NIH) (P30 AR48311, RO1 CA116092, RO1 CA107181, 5P30 CA013148-35) and Birmingham Veterans Administration Medical Center (VAMC) Merit Review Grants (H.-G.Z.). We thank Dr Jerald Ainsworth for editorial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal