Abstract
Despite advances in treatment, there was little evidence until recently that this led to improvement in the survival of patients with indolent lymphoma, with patients continuing to have an unremitting course of relapse of disease. There appears to have been a change in the natural history of these diseases with the introduction of chemoimmunotherapy that may finally result in improvements in survival. With so many agents available for the treatment of indolent lymphomas, questions that have to be addressed include the following: is there still a role for a “watch-and-wait” approach in asymptomatic patients or should they be treated at diagnosis, what are the optimal first-line and salvage treatments, what is the role of maintenance therapy, and is there any role for stem cell transplantation in these diseases? No established treatment of choice has yet emerged, and many of these questions remain unresolved. It is highly likely that our treatment approaches will continue to evolve as the results of ongoing clinical trials are released and that improvement in outcome will result from identification of therapies that target the underlying pathophysiology of the diseases.
Introduction
Almost 60 000 new cases of non-Hodgkin lymphoma (NHL) were diagnosed in the United States in 2006.1 NHL is extremely heterogeneous in its molecular pathophysiology, histology, and clinical course, and there are major differences in the incidence of subtypes in different geographical locations and among different racial and ethnic populations. The World Health Organization (WHO) lymphoma classification2 is based on cell of origin and pathophysiology of the lymphoma and does not include the terminology “indolent lymphoma,” which is defined as one which “tends to grow and spread slowly and has few symptoms” (http://www.nih.gov/). The indolent lymphomas encompass the low-grade and some categories of intermediate-grade NHL in the working formulation
For indolent lymphomas the goal of therapy has been to maintain the best quality of life and treat only when patients develop symptoms. Any alteration to this approach requires demonstration of improved survival with early institution of therapy, or identification of criteria that define patients sufficiently at “high risk” to merit early therapy. There are many available therapies and no consensus on an optimal first-line or relapse treatment. The following discussion presents my approach for the management of indolent lymphoma based on 25 years of clinical practice in oncology, research, and review of the work of distinguished colleagues. Literature is cited where available to support treatment practice and recommendations.
Indolent lymphoma
The frequency of the indolent lymphomas by the WHO classification is shown in Table 1.3 By far the most common is follicular lymphoma (FL), the second most common subtype of lymphoma worldwide, accounting for approximately 20% of malignant lymphomas in adults, but 40% of all lymphomas diagnosed in the United States and in Western Europe.4 FL is derived from germinal center B cells and maintains the gene expression profile of this stage of differentiation.5 Morphologically, the disease is composed of a mixture of centrocytes and centroblasts and is graded from I to III, depending on the proportion of large cells per high-power field. Grades I and II are indolent disease. The rare subtype grade IIIb is more aggressive, and I manage this entity like diffuse large B-cell lymphoma. FL cells express CD19, CD20, CD22, and surface immunoglobulin, and 60% express CD10. A hallmark of the disease is t(14;18), contributing to overexpression of the antiapoptotic protein BCL2. Median age at presentation is 60 years, and men and women are equally affected. Patients usually present with asymptomatic lymphadenopathy, most cases are advanced stage, and 50% have bone marrow involvement at presentation. Lymphadenopathy may wax and wane, and spontaneous remissions can occur, albeit rarely.6 Disease transformation to a more aggressive histologic type is a common terminal event.7 Until recently there was little evidence that the natural history of FL had changed over the past 30 years from the median survival of 10 years from diagnosis,8 but this may be changing with the introduction of monoclonal antibodies in combination with chemotherapy, with more recent data suggesting that with improvements in treatment the median survival is now 12 to 14 years.9,10 The clinical course is extremely variable, with some patients having an extremely aggressive course and death within 1 year, whereas others may live for more than 20 years and never require therapy. It is vital that samples from patients are studied in the laboratory to determine the biologic characteristics associated with progression, with response to therapy, to identify surrogate markers of response, and to identify targets for new drug development, which can then be tested in subsequent clinical trials. My approach is shown in Figure 1.
Frequencies of indolent lymphomas according to the WHO Classification System
| . | Frequency, % . |
|---|---|
| Follicular lymphoma | 22 |
| Small lymphocytic lymphoma | 6 |
| Marginal zone B-cell lymphoma, mucosa-associated lymphoid tissue type | 5 |
| Marginal zone B-cell lymphoma, nodal type | 1 |
| Lymphoplasmacytic lymphoma | 1 |
| . | Frequency, % . |
|---|---|
| Follicular lymphoma | 22 |
| Small lymphocytic lymphoma | 6 |
| Marginal zone B-cell lymphoma, mucosa-associated lymphoid tissue type | 5 |
| Marginal zone B-cell lymphoma, nodal type | 1 |
| Lymphoplasmacytic lymphoma | 1 |
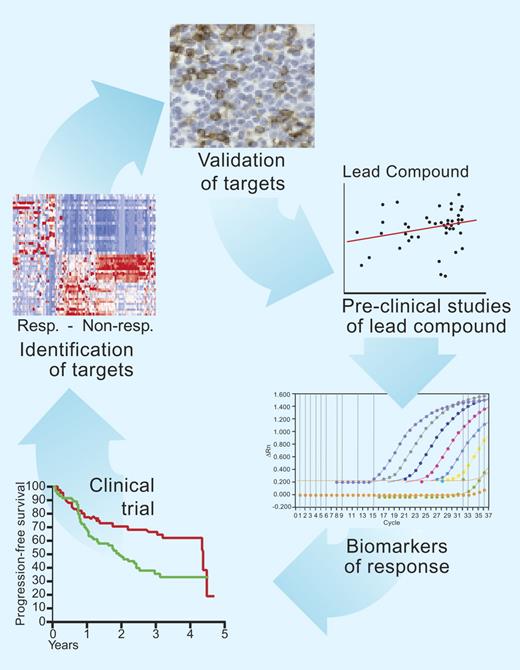
Laboratory and clinical studies in indolent lymphoma. Improvements in outcome in indolent lymphoma will come from study of the molecular determinants of outcome to treatment. Once these determinants are identified and validated, new drug treatments can be tested in subsequent clinical trials. Surrogate markers are useful to identify more rapidly whether the novel agent is targeting the lymphoma cells. Illustration by Paulette Dennis.
Laboratory and clinical studies in indolent lymphoma. Improvements in outcome in indolent lymphoma will come from study of the molecular determinants of outcome to treatment. Once these determinants are identified and validated, new drug treatments can be tested in subsequent clinical trials. Surrogate markers are useful to identify more rapidly whether the novel agent is targeting the lymphoma cells. Illustration by Paulette Dennis.
How I diagnose indolent lymphoma
Suggested guidelines for the diagnosis of indolent lymphomas have been outlined by the National Comprehensive Cancer Network (guidelines available at http://www.nccn.org/) and by the European Society for Medical Oncology.11 In all cases possible, I confirm diagnosis by excisional biopsy of an accessible lymph node (LN) with review by an expert hematopathologist with expertise in lymphoma diagnosis. Fine-needle aspiration is not appropriate for diagnosis in these conditions. I obtain informed consent for use of excess tissue from LN biopsies at the time of presentation and at each subsequent relapse of disease for research purposes to investigate the molecular biology of these diseases. Initial staging workup includes a computed tomography scan of the chest, abdomen, and pelvis and a bone marrow aspirate and biopsy. I pay particular attention to sites of bulk disease. I use the Follicular Lymphoma International Prognostic Index (FLIPI) score to assess risk of progression.12
How I follow patients
I follow patients remaining on expectant course every 3 months for history, physical examination, and blood counts, including LDH. Special attention is paid to any change in symptoms that might be suggestive of transformation. I have a very low threshold to recommend repeat biopsy to examine for histologic evidence of transformation. I do not routinely perform repeat scanning unless this is indicated by symptoms or signs.
Depending on the treatment approach used, I usually restage after 2 to 3 cycles of therapy to ensure responsiveness and fully restage after completion of therapy. Staging of response in indolent lymphomas is by the revised response criteria.13 Although we now seek curative approaches in indolent lymphomas, the failure to achieve complete remission (CR) or complete eradication of disease does not have the same implication in indolent lymphomas as in aggressive lymphomas and a partial remission (PR) may be a sufficient response to therapy to alleviate symptoms. The impact of newer technologies such as positron emission tomography (PET), which are included in the revised guidelines for aggressive lymphomas,13 have been much less studied in the indolent lymphomas. There is considerable heterogeneity in uptake of fluorine-18 fluoro-deoxyglucose based on histology, but PET demonstrates 94% sensitivity and 100% specificity for staging in FL.14 I do not use PET scans routinely in patients with indolent lymphomas, but I have found them useful to direct biopsy in cases where transformation is suspected.
When I institute therapy
My current practice is to offer all eligible patients inclusion in clinical trials. This ensures delivery of optimal care and helps inform design of subsequent trials, hopefully, leading to cure. Information on available clinical trials can be found at http://www.clinicaltrials.gov/. I continue to use expectant management for asymptomatic patients with low-bulk disease until clear indications for initiation of treatment are seen, except for those patients enrolled in clinical trial assessing the impact of early therapy. This approach is based on the demonstration of no survival advantage for institution of immediate compared with deferred treatment until time of progression.15 In addition, 3 randomized trials, performed in the era before rituximab, confirmed no survival benefit for early therapy.16–18 In the National Cancer Institute study in 104 patients with newly diagnosed FL, deferred treatment was compared with immediate treatment with cyclophosphamide 650 mg/m2 intravenously, doxorubicin 25 mg/m2 intravenously, etoposide 120 mg/m2 intravenously on day 1, mechlorethamine 6 mg/m2 intravenously, vincristine 1.4 mg/m2 intravenously on day 8, prednisone 60 mg/m2 orally daily on days 1 to 14, procarbazine 100 mg/m2 orally daily on days 8 to 14, methotrexate 500 mg/m2 intravenously on day 15 with leucovorin 50 mg/m2 orally every 6 hours for 4 doses beginning 24 hours after methotrexate with cycles repeated every 28 days (ProMACE-MOPP) followed by total nodal irradiation. An updated analysis of this data is long overdue, but there was no difference in overall survival (OS) between the 2 arms at the time of the last analysis.16 The Groupe pour l'Etude de Lymphome Folliculaire (GELF) used defined criteria for patients in whom immediate therapy was not felt to be indicated (Table 2) and randomly assigned 193 patients to deferred treatment or to receive prednimustine 200 mg/(m2/ · day) for 5 days per month for 18 months or IFN-α 5 MU/day for 3 months then 5 MU 3 times per week for 15 months.17 The median OS time was not reached and was the same in all 3 arms of the study. The British National Lymphoma Investigation (BNLI)18 compared treatment in 309 patients with asymptomatic advanced-stage, indolent lymphoma in whom 158 patients were randomly assigned to receive immediate therapy with oral chlorambucil 10 mg/day continuously, and 151 patients were randomly assigned to deferred treatment until disease progression (Table 2). In both arms, local radiotherapy to symptomatic nodes was allowed. There was no difference in OS or cause-specific survival between the 2 groups with 16 years median follow-up. A meta-analysis of more than 2000 patients with early-stage chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) showed no difference in survival between early versus deferred therapy using alkylating agents.19
Criteria for delaying treatment in follicular lymphoma
| GELF17 |
| All of the following: |
| Maximum diameter of disease < 7 cm |
| Fewer than 3 nodal sites |
| No systemic symptoms |
| Spleen < 16 cm on CT |
| No significant effusions |
| No risk of local compressive symptoms |
| No circulating lymphoma cells |
| No marrow compromise (Hb < 10 g/dL, WBC count < 1.5 × 109/L, platelet count < 100 × 109/L) |
| BNLI18 |
| None of the following: |
| B symptoms or pruritus |
| Rapid generalized disease progression |
| Marrow compromise (Hb ≤ 10 g/dL, WBC count < 3.0 × 109/L, or platelet count < 100 × 109/L) |
| Life-threatening organ involvement |
| Renal infiltration |
| Bone lesions |
| GELF17 |
| All of the following: |
| Maximum diameter of disease < 7 cm |
| Fewer than 3 nodal sites |
| No systemic symptoms |
| Spleen < 16 cm on CT |
| No significant effusions |
| No risk of local compressive symptoms |
| No circulating lymphoma cells |
| No marrow compromise (Hb < 10 g/dL, WBC count < 1.5 × 109/L, platelet count < 100 × 109/L) |
| BNLI18 |
| None of the following: |
| B symptoms or pruritus |
| Rapid generalized disease progression |
| Marrow compromise (Hb ≤ 10 g/dL, WBC count < 3.0 × 109/L, or platelet count < 100 × 109/L) |
| Life-threatening organ involvement |
| Renal infiltration |
| Bone lesions |
To convert Hb from grams per deciliter to grams per liter, multiply grams per deciliter by 10.
GELF indicates Groupe pour l'Etude de Lymphome Folliculaire; Hb, hemoglobin level; WBC, white blood cell; BNLI, British National Lymphoma Investigation.
A major clinical trial question is whether identification of clinical or molecular risk factors can identify which patients are candidates for early therapy. The FLIPI is a 5-factor prognostic index based on the clinical characteristics, age, stage, number of nodal sites, and hemoglobin and LDH levels, and it defines 3 prognostic risk groups of almost equal numbers of patients.12 This tool is useful in assessing the likely need for early treatment of patients and potential outcome, as well as in comparing the outcomes of different clinical trials. A survival predictor score has also been developed from gene expression profiling studies.5 The results from that study suggest that the molecular determinants of biologic heterogeneity are already present in the diagnostic LN biopsies rather than by the later acquisition of secondary genetic changes. A major component of the gene expression prognostic signature is related to immune cells in the tumor microenvironment.20–22 Future guidelines for treatment will likely be based on clinical staging systems, genetic profiles and immune response signatures, but these factors do not yet help us to decide who should have immediate therapy.
From available data there is little to suggest that we should change our practice of watch and wait for asymptomatic patients with low-bulk disease, but data demonstrate that this practice is becoming much less common in the United States.23 The National LymphoCare study is a prospective observational study designed to assess presentation, prognosis, treatment, and clinical outcomes in newly diagnosed FL. The treating physician determines management according to clinical judgment with no prescribed treatment regimen, and data regarding histology, stage, therapy, response, relapse, and death are recorded. Among 1493 patients enrolled at 237 centers, 26% of initially observed patients had switched to active therapy after a median of 2.8 months on observation since diagnosis, and by the first follow-up visit only 19% of patients continued on watch and wait at 6 months (Table 3). This observation is in stark contrast to the data from the BLNI study demonstrating that censored for nonlymphoma death 19% of patients and 40% for those older than 70 years randomly assigned to expectant management still did not require therapy at 10 years.18
National LymphoCare Study survey of current practice for FL in the United States
| Treatment . | Frequency, % . |
|---|---|
| Watch and wait | 19 |
| Rituximab monotherapy | 13 |
| Chemoimmunotherapy | 51 |
| R-CHOP | 59 |
| R-CVP | 19 |
| R-fludarabine based | 11 |
| R-other | 11 |
| Chemotherapy alone | 4 |
| Radiation alone | 5 |
| Treatment . | Frequency, % . |
|---|---|
| Watch and wait | 19 |
| Rituximab monotherapy | 13 |
| Chemoimmunotherapy | 51 |
| R-CHOP | 59 |
| R-CVP | 19 |
| R-fludarabine based | 11 |
| R-other | 11 |
| Chemotherapy alone | 4 |
| Radiation alone | 5 |
R-CHOP indicates rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-CVP, rituximab, cyclophosphamide, vincristine, and prednisone.
How I treat indolent lymphoma
I initiate treatment in patients with symptomatic disease, bulky lymphadenopathy or splenomegaly or both, risk of local compressive disease, marrow compromise, or rapid disease progression. Once treatment is indicated, many treatment approaches are available (Table 4). The concept that the approach can be to “do nothing” or discuss an approach with considerable rates of morbidity and mortality such as stem cell transplantation (SCT) is a confusing one for the patient with newly diagnosed disease (as well as for the physician), and considerable consultation time is required to review available treatment approaches.
Treatment strategies for indolent lymphomas
| Advanced-stage disease |
| Watchful waiting |
| Alkylating agents |
| Purine analogs |
| Combination chemotherapy |
| Monoclonal antibodies |
| Unconjugated |
| Conjugated radioimmunoconjugates and immunotoxins |
| Chemotherapy + monoclonal antibodies (chemoimmunotherapy) |
| High-dose chemotherapy + autologous/allogeneic stem cell transplantation |
| Reduced intensity conditioning allogeneic stem cell transplantation |
| Palliative radiotherapy |
| Localized disease |
| Radiotherapy |
| Watchful waiting |
| Advanced-stage disease |
| Watchful waiting |
| Alkylating agents |
| Purine analogs |
| Combination chemotherapy |
| Monoclonal antibodies |
| Unconjugated |
| Conjugated radioimmunoconjugates and immunotoxins |
| Chemotherapy + monoclonal antibodies (chemoimmunotherapy) |
| High-dose chemotherapy + autologous/allogeneic stem cell transplantation |
| Reduced intensity conditioning allogeneic stem cell transplantation |
| Palliative radiotherapy |
| Localized disease |
| Radiotherapy |
| Watchful waiting |
Optimal first-line treatment is enrollment in randomized clinical trials. In the National LymphoCare study23 academic sites are more likely than community sites to treat patients on clinical trials (12% versus 4%), but it is lamentable that such a small proportion of these patients are enrolled in clinical trials. For patients who are not eligible for or who refuse entry into clinical trials, my preferred first-line therapy is chemoimmunotherapy, based on data demonstrating higher response rates, longer duration of responses, and perhaps improved survival with this approach. I favor alkylator- over fludarabine-based regimens for FL, based on concerns about the ability to obtain stem cells for later use for autologous SCT in patients treated with fludarabine.24 I offer more aggressive first-line therapy for patients who progress within 1 year of presentation, because these patients have a worse outcome.17 Elderly patients or those with poor performance status remain candidates for single-agent chlorambucil. Single-agent monoclonal antibody therapy is appropriate for patients who chose to avoid chemotherapy, and I discuss the results of clinical trials of prolonged or maintenance therapy with rituximab. Although data suggest a survival advantage with the use of interferon α (IFN-α) in combination with chemotherapy, this is associated with a significant side effect profile, and I rarely use this agent. Although optimal results are seen when radioimmunoconjugates are used earlier in the disease course, I reserve these agents for later in the disease course. I do not consider the use of SCT in first remission in FL unless in a randomized clinical trial. I also enroll all possible patients with CLL/SLL in randomized clinical trials. If patients refuse trial entry, my current treatment of choice is fludarabine, cyclophosphamide, and rituximab (FCR), based on the high response rates and long duration of responses observed in phase 2 studies.25 Fludarabine or chlorambucil immunotherapy may be indicated when there is impaired performance status. I consider patients with CLL/SLL for allogeneic SCT in first remission if they had particularly poor prognostic features and had not achieved CR with optimal front-line therapy.26
Data from the National LymphoCare study demonstrate that chemoimmunotherapy is now the treatment of choice of physicians in the United States (Table 3).23 No randomized trials demonstrate a benefit for the addition of anthracyclines, but CHOP-R is heavily favored over CVP-R or fludarabine-based regimens. Choice to initiate therapy was associated with FLIPI, stage, and grade, but FLIPI was not associated with decision to use a specific treatment approach. Significant regional and center differences were observed, strongly suggesting that physician preference is the predominant factor that drives initial therapy. For example, initial watch and wait was used in 31% in the Northeast but in 13% in the Southeast, whereas fludarabine-based chemoimmunotherapy was used in 18% of patients in the Southwest and only 3% in the Northeast.
Alkylating agents
The alkylating agents chlorambucil and cyclophophamide with or without prednisone and CVP or CHOP, and other alkylator-based combination chemotherapy regimens have been the standard of therapy for decades. Single-agent alkylators at different doses and schedules produce overall response (OR) rates of 50% to 75% in FL.27,28 Comparable response rates, but higher CR rates with longer progression-free survival (PFS) are seen with CVP compared with chlorambucil but no survival advantage.29,30 The addition of anthracyclines has not improved the response rate or duration of the response,31,32 but its use may be associated with a lower risk of histologic transformation.16,33 This finding has to be confirmed, particularly in the era of chemoimmunotherapy.
Purine analogues
The purine analogues have been studied extensively in various types of indolent lymphoma. Fludarabine monotherapy produces response rates of 65% to 84%, with 37% to 47% CR in patients with previously untreated FL.34 In a randomized trial of 381 patients with previously untreated indolent lymphoma CR rates were higher with fludarabine than with CVP.35 Fludarabine combinations result in increased response rates, with 89% CR rate in a Eastern Cooperative Oncology Group (ECOG) trial combining fludarabine and cyclophosphamide (FC),36 whereas fludarabine and mitoxantrone (FM) produced a 91% overall response rate (ORR), 43% CR, and 2-year disease-free survival (DFS) of 63%.37 A higher CR rare was seen with FM (68%) compared with CHOP (42%) in a randomized trial.38 The use of alkylator-based regimens or purine analog–based regimens appears to vary geographically, suggesting personal preference for the use of regimens in which the clinician has experience, rather than alterations of practice based on the results of the published studies. In CLL/SLL, fludarabine is associated with a higher response rate and longer duration of response than chlorambucil39 but no OS advantage. The use of fludarabine in combination with cyclophosphamide is associated with a higher response rate and longer duration of response compared with fludarabine alone in randomized trials.40 The highest response rates have been with FCR.25
Biologic therapy
IFN-α is approved by the Food and Drug Administration (FDA) for the treatment of advanced-stage FL in combination with anthracycline-based chemotherapy, based on improved survival in a clinical trials12,41,42 and meta-analysis of phase 3 trial data.43 IFN-α has been widely used in Europe but not in the United States, where it is felt that its toxicity profile outweighs any potential benefit. In the Southwest Oncology Group (SWOG) study,44 571 patients with stage III and IV indolent lymphoma were treated with ProMACE-MOPP, and 279 responding patients were randomly assigned to 24 months of observation versus treatment with IFN-α. No statistically significant difference in PFS or OS was observed between observation and IFN-α groups at 4 years.
Monoclonal antibody therapy
I consider the monoclonal antibodies to be the most exciting agents to emerge in the treatment of indolent lymphomas, and recent data suggest their use may finally be leading to improvement in patient survival.9,10 The most widely used monoclonal antibody is rituximab, a chimeric unconjugated antibody against the CD20 antigen licensed by the FDA45 and the European Agency for the Evaluation of Medicinal Products46 for treatment of patients with relapsed or refractory CD20+ low-grade FL; for the first-line treatment of CD20+ FL in combination with CVP chemotherapy, and for the treatment of CD20+ low-grade NHL in patients with stable disease or who achieve a PR or CR following first-line treatment with CVP chemotherapy.
Following phase 1 studies,47 rituximab at a dose of 375 mg/m2 weekly for 4 weeks was selected for the pivotal phase 2 trial, and this remains the standard dose.48 In patients with relapsed indolent lymphoma OR was 48% and 60% in FL. Median PFS for responders was 13 months. Factors associated with lower response rates include chemo-resistant disease,48 bulky disease,49 and treatment late in the disease course.50 OR was 73% in previously untreated patients with low-bulk disease,51 and some of these patients have needed no further treatment and have no evidence of polymerase chain reaction (PCR)–detectable minimal residual disease (MRD) after 7 years.52 Extended use with 8 weeks instead of 4 weeks is associated with improvement in OR and duration of response.53 Comparable or even longer durations of response have been observed with retreatment.54
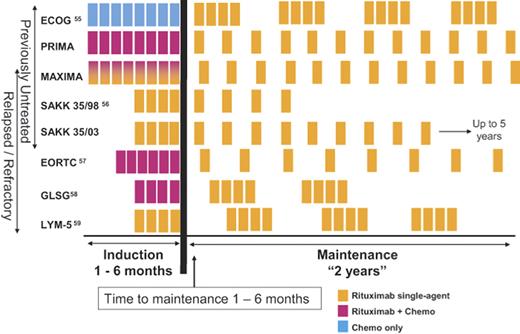
A number of trials in front-line therapy and in relapsed or refractory patients have investigated the potential benefits of extended or maintenance rituximab treatment,55–59 and all demonstrated prolonged time to progression in patients receiving maintenance rituximab (Table 5). The results from the E1496 randomized trial from ECOG and Cancer and Leukemia Group B comparing CVP alone with CVP followed by rituximab in patients with advanced-stage FL demonstrated that the addition of rituximab maintenance significantly improved OS55 and led to FDA approval for rituximab therapy in patients responding to CVP chemotherapy. A problem with interpretation of the role of maintenance therapy or in recommending a specific regimen is that there is no standard schedule, and trials have been performed in rituximab naive patients, as well as in patients treated with previous rituximab monotherapy or combination chemoimmunotherapy as shown in Figure 2.
Studies of rituximab maintenance therapy in indolent lymphomas
| Trial . | Disease setting . | Diseases included . | Previous therapy . |
|---|---|---|---|
| ECOG55 | First line | Follicular, small lymphocytic | CVP |
| SAKK56 | First line, relapsed/refractory | Follicular, mantle cell | Rituximab |
| EORTC57 | Relapsed/refractory | Follicular | CHOP vs R-CHOP |
| GLSG58 | Relapsed/refractory | Follicular, mantle cell | FCM vs R-FCM |
| LYM-559 | Relapsed/refractory | Follicular, small lymphocytic | Rituximab |
| Trial . | Disease setting . | Diseases included . | Previous therapy . |
|---|---|---|---|
| ECOG55 | First line | Follicular, small lymphocytic | CVP |
| SAKK56 | First line, relapsed/refractory | Follicular, mantle cell | Rituximab |
| EORTC57 | Relapsed/refractory | Follicular | CHOP vs R-CHOP |
| GLSG58 | Relapsed/refractory | Follicular, mantle cell | FCM vs R-FCM |
| LYM-559 | Relapsed/refractory | Follicular, small lymphocytic | Rituximab |
Clinical trials examining rituximab maintenance therapy. The induction phase has included the use of chemotherapy alone, rituximab alone, or chemoimmunotherapy. The PRIMA and MAXIMA studies are ongoing randomized studies examining the use of chemoimmunotherapy followed by rituximab maintenance.
Clinical trials examining rituximab maintenance therapy. The induction phase has included the use of chemotherapy alone, rituximab alone, or chemoimmunotherapy. The PRIMA and MAXIMA studies are ongoing randomized studies examining the use of chemoimmunotherapy followed by rituximab maintenance.
Chemoimmunotherapy
In a phase 2 study 40 patients with indolent lymphoma were treated with 6 infusions of rituximab (375 mg/m2 per dose) in combination with 6 doses of CHOP chemotherapy (R-CHOP),60 OR was 95% with 55% CR, with 45% OR in patients with bulky disease. In a phase 2 study of 40 patients with indolent lymphomas rituximab in combination with fludarabine produced OR of 90% with 80% CR, with similar response rates in treatment nave and previously treated patients.61
A number of randomized trials show a benefit for the use of rituximab with chemotherapy compared with chemotherapy alone (Table 6).57,62–65 Each study showed an improvement in TTF, and more recent follow-up data suggests improved OS in patients treated with chemoimmunotherapy compared with chemotherapy alone. A meta-analysis of these trials demonstrates that OS, OR, and disease control are significantly better in those on chemoimmunotherapy compared with chemotherapy for FL and mantle cell lymphoma.66 Data from the German Low-grade Study Group (GLSG) suggest that it is the addition of rituximab that has led to the recent improvement in survival of patients with FL.10 A recent independently assessed analysis of the clinical benefits provided by rituximab in relation to cost concluded that it is highly cost effective.67
Randomized trials of chemotherapy versus chemoimmunotherapy
| Study and treatment . | No. of patients . | Median FU, mo . | OR, % . | CR, % . | Median TTF, mo* . | OS, % . | P . |
|---|---|---|---|---|---|---|---|
| M3902162 | 53 | ||||||
| CVP | 159 | 57 | 10 | 15 | 77 | .029 | |
| R-CVP | 162 | 81 | 41 | 34 | 83 | ||
| GLSG63 | 18 | ||||||
| CHOP | 205 | 90 | 17 | 29 | 90 | .016 | |
| R-CHOP | 223 | 96 | 20 | NR | 95 | ||
| M3902364 | 47 | ||||||
| MCP | 96 | 75 | 25 | 26 | 74 | .010 | |
| R-MCP | 105 | 92 | 50 | NR | 87 | ||
| FL200065 | 42 | ||||||
| CHVP-IFN | 183 | 73 | 63 | 46 | 84 | .010 | |
| R-CHVP-IFN | 175 | 84 | 79 | 67 | 91 |
| Study and treatment . | No. of patients . | Median FU, mo . | OR, % . | CR, % . | Median TTF, mo* . | OS, % . | P . |
|---|---|---|---|---|---|---|---|
| M3902162 | 53 | ||||||
| CVP | 159 | 57 | 10 | 15 | 77 | .029 | |
| R-CVP | 162 | 81 | 41 | 34 | 83 | ||
| GLSG63 | 18 | ||||||
| CHOP | 205 | 90 | 17 | 29 | 90 | .016 | |
| R-CHOP | 223 | 96 | 20 | NR | 95 | ||
| M3902364 | 47 | ||||||
| MCP | 96 | 75 | 25 | 26 | 74 | .010 | |
| R-MCP | 105 | 92 | 50 | NR | 87 | ||
| FL200065 | 42 | ||||||
| CHVP-IFN | 183 | 73 | 63 | 46 | 84 | .010 | |
| R-CHVP-IFN | 175 | 84 | 79 | 67 | 91 |
FU indicates follow-up; OR, odds ratio, CR, complete remission; TTF, time to treatment failure; OS, overall survival; MCP, mitoxantrone, chlorambucil and prednisolone; and CHVP, cyclophosphamide, doxorubicin, teniposide, and prednisone.
*P values for TTF in all studies were less than .001.
Conjugated-radiolabeled monoclonal antibody therapy
Complexing a radioisotope to a monoclonal antibody (radioimmunoconjugate) might be expected to improve efficacy over antibody therapy alone. Tositumomab complexes 131I to the anti-B1 antibody and has been studied extensively in the treatment of heavily pretreated,68 untreated,69 and for retreatment of indolent lymphomas.70 Best responses are seen in patients with previously untreated FL with a 95% OR, 75% CR, and 80% of assessable patients achieving eradication of PCR-detectable MRD for patients treated with a single treatment course with tositumomab.69 Median PFS was 6.1 years, with 40 patients remaining in remission for 4.3 to 7.7 years, and no cases of myelodysplastic syndrome were observed. A SWOG study investigated chemoimmunotherapy with 6 cycles of CHOP chemotherapy followed 4 to 8 weeks later by tositumomab in 90 patients with previously untreated, advanced-stage FL.71 The OR was 91%, including 69% CR, and at median follow-up time of 5.1 years the estimated 5-year OS was 87% and PFS was 67%, 23% better than CHOP alone on previous SWOG protocols. Ibritumomab tiuxetan is a 90 Y-labeled anti-CD20 antibody and produced an OR of 74% and 15% CR in 57 patients with FL refractory to rituximab.72 Toxicity is primarily hematologic, with nadir counts occurring at 7 to 9 weeks and lasting approximately 1 to 4 weeks. The risk of hematologic toxicity increased with dose delivered and with degree of baseline bone marrow lymphoma involvement.73 An acceptable safety profile was observed in relapsed patients with less than 25% lymphoma marrow involvement, adequate marrow reserve, platelet counts greater than 100 × 109/L, and neutrophil counts greater than 1.5 × 109/L.
High-dose therapy as consolidation of first remission
The role of high-dose therapy and autologous SCT in patients with FL during first remission was explored in phase 2 trials74,75 and in 3 phase 3 randomized trials.76–78 The GLSG trial recruited 307 previously untreated patients up to 60 years of age, and patients who responded after induction chemotherapy with 2 cycles of CHOP or MCP were randomly assigned to autologous SCT or IFN-α maintenance.76 Among 240 evaluable patients, the 5-year PFS was 64.7% for ASCT and 33.3% in the IFN-α arm (P < .001). Acute toxicity was higher in the ASCT group, but early mortality was below 2.5% in both study arms. Longer follow-up is necessary to determine the effect of ASCT on OS. In the Groupe Ouest Est des Leucemies Aigues et des Maladies du Sang study, 172 patients with newly diagnosed advanced FL were randomly assigned either to cyclophosphamide, doxorubicin, teniposide, prednisone (CHVP) and IFN-α or to high-dose therapy followed by purged ASCT.77 Patients treated with high-dose therapy had a higher response rate than patients who received chemotherapy and IFN-α (81% versus 69%, P = .045) and a longer median PFS (not reached versus 45 months), but this did not translate into a better OS because of an excess of secondary malignancies after transplantation. A subgroup of patients with a significantly higher event-free survival rate with autologous SCT could be identified using the FLIPI. The GELF94 study enrolled 401 patients with previously untreated advanced-stage FL who were randomly assigned to receive CHVP plus IFN-α compared with 4 courses of CHOP followed by high-dose therapy (HDT) with total body irradiation (TBI) and autologous SCT, and OR rates were similar in both groups (79% and 78%, respectively) and 87% of eligible patients underwent ASCT. Intent-to-treat analysis after a median follow-up of 7.5 years showed no difference between the 2 arms for OS (P = .53) or PFS (P = .11). Long-term follow-up demonstrated no statistically significant benefit in favor of first-line ASCT in patients with follicular lymphoma, which they conclude should be reserved for relapsed patients. In view of these results, my opinion is that autologous SCT should be used in first remission only in the setting of clinical trials.
How I treat relapsed indolent lymphoma
The treatment options after relapse remain the same as for first-line therapy (Table 4), and relapsed patients should ideally be treated in clinical trials. Relapsed asymptomatic disease is not necessarily an indication for treatment, and patients can again be managed expectantly. A number of factors must be taken into account in planning therapy, and it is not possible to define treatment at relapse without considering the goal of therapy (palliative versus potentially curative), performance status, previous therapy, response, and duration of response. Single-agent rituximab is approved for relapsed lymphoma and is widely used in this setting. A multicenter randomized trial in relapsed patients has demonstrated a survival advantage for chemoimmunotherapy with CHOP-R or CHOP followed by R compared with CHOP alone and a further benefit for rituximab maintenance therapy.57 For younger patients who are suitable candidates for either HDT and autologous SCT or reduced intensity conditioning (RIC) allogeneic transplantation, I discuss the potential role and timing of transplantation. Best results are seen when transplantation is considered early in the course of disease before patients become chemorefractory, and I continue to offer high-dose therapy and autologous SCT for younger patients with chemoresponsive relapsed disease. SCT approaches must be considered in the context of the improving results that are being seen with salvage therapy alone. I no longer consider autologous SCT for CLL/SLL,79 but I continue to explore RIC allogeneic transplantations, which appear promising in selected patients.80
Role of transplantation in relapsed indolent lymphomas
Unlike aggressive lymphomas, the use of high-dose chemotherapy with autologous SCT in the treatment of indolent lymphomas has not yet been fully established. The rationale for considering transplantation is that the disease is incurable using standard approaches, and young patients with indolent lymphomas will die of their disease, and promising results have been observed in a number of phase 2 studies.81–83 Detection of MRD has been a useful surrogate marker for tracking long-term PFS in patients examining the autologous stem cells or serial samples after transplantation.83–87 A major concern relates to the risk of development of secondary myelodysplasia/acute myeloid leukemia.88 The European Bone Marrow Transplant Registry–sponsored CUP (conventional chemotherapy, unpurged, purged autograft) study is the only prospective randomized trial to assess the role of autologous SCT in patients with relapsed FL.89 The results of the study suggest a PFS and OS advantage of ASCT over conventional chemotherapy, with 4-year OS of 46% for the chemotherapy arm versus 71% for the unpurged and 77% for the purged ASCT arms. The study was closed early because of slow accrual with 140 of the planned 250 patients accrued and only 89 randomly assigned to treatment. In CLL/SLL, the use of HDT and autologous SCT was not associated with improved outcome in patients who received a transplant in first remission compared with those who received a transplant later in their disease course.79
Allogeneic BMT
There is increasing use of allogeneic SCT in the management of indolent lymphomas. In a report of the International Bone Marrow Transplant Registry (IBMTR), results after SCT are described for 904 patients with FL.90 Allogeneic SCT has increased transplant-related mortality (TRM) but lower risk of relapse, and long-term PFS has been observed after allogeneic SCT even in patients with refractory FL.91 Outcomes are improving, and TRM decreased with RIC regimens. RIC-incorporating alemtuzumab therapy was associated with a low incidence of graft-versus-host disease, TRM was decreased in patients with indolent compared with higher grade histology, and PFS at 3 years was 65% for patients with indolent lymphoma.92 The effectiveness of donor lymphocyte infusion to treat relapse after allogeneic SCT provides strong evidence for a graft-versus-lymphoma effect that can be exploited in indolent lymphomas.79,92
How I treat patients with limited-stage disease
It appears paradoxical that I continue to follow an expectant treatment approach for patients with advanced-stage disease, yet patients with localized (stage I or II) disease for immediate radiation therapy (XRT). This decision is based on the curative potential of this approach in patients with localized disease.93–96 No significant survival differences have been observed using involved field versus extended field versus total lymphoid irradiation.94 Long-term follow-up studies from Stanford of 177 patients with localized FL treated with XRT demonstrated a median survival of 13.8 years, with 20-year survival of 35%.97 A retrospective study in Stanford in 43 patients with localized disease in whom a watch-and-wait approach was initially followed suggested similar outcome, and more than half of these patients remained untreated for 6 years.98 The outcome was not worsened by delayed treatment compared with previously observed results. The role of additional chemotherapy or rituximab therapy to XRT in limited-stage disease is being addressed in ongoing clinical trials.
Special circumstances
Mucosa-associated lymphoid tissue (MALT) lymphomas deserve very brief special mention because they behave differently than most other indolent lymphomas and require such different treatment approaches. Bacterial infection with the Gram-negative rod, Helicobacter pylori, is associated with 92% of gastric MALT lymphomas,99 suggesting that gastric MALT lymphoma is “driven” by H pylori. Combination antibiotics and histamine blocker for eradication of H pylori produces 70% CR in patients with localized gastric MALT lymphomas, independent of local or disseminated disease, with median PFS of 5.6 years with 80% OS at 10 years.100 The importance of this findings is the suggestion that outcome can be improved with eradication of a factor which drives proliferation of the malignant cells, although putative factors driving other indolent lymphomas have yet to be identified.
Conclusions
Despite any data demonstrating any benefit for early therapy, patients are being treated earlier in their disease course. There is no clear-cut treatment pathway for patients with indolent lymphomas, and, although we have a good evidence base to decide on a particular treatment, there is little or no data regarding the optimal sequencing of treatment approaches in these diseases. In the absence of such data, treatment choices remain empiric and should always involve discussion regarding patient choice and goal of therapy. This is likely to become even more complicated because many novel agents are currently being investigated in preclinical and clinical studies, particularly, novel monoclonal antibodies and agents that alter the antiapoptotic pathways. The impact of these new agents on practice will depend on the results of the ongoing clinical trials, which should always be the treatment of choice in these diseases until we have a clear-cut established treatment approach which leads to cure for the majority of patients.
Authorship
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: John G. Gribben, Institute of Cancer, Barts and The London Queen Mary School of Medicine, London, United Kingdom; e-mail: john.gribben@cancer.org.uk
References
National Institutes of Health