Abstract
The efficacy and safety of zanolimumab in patients with refractory cutaneous T-cell lymphoma (CTCL) have been assessed in two phase 2, multicenter, prospective, open-label, uncontrolled clinical studies. Patients with treatment refractory CD4+ CTCL (mycosis fungoides [MF], n = 38; Sézary syndrome [SS], n = 9) received 17 weekly infusions of zanolimumab (early-stage patients, 280 and 560 mg; advanced-stage patients, 280 and 980 mg). The primary end point was objective response (OR) as assessed by composite assessment of index lesion disease activity score. Secondary end points included physician's global assessment (PGA), time to response, response duration, and time to progression. ORs were recorded for patients in both CTCL types (MF, 13 ORs; SS, 2 ORs). In the high-dose groups (560 and 980 mg dose groups), a response rate of 56% was obtained with a median response of 81 weeks. Adverse events reported most frequently included low-grade infections and eczematous dermatitis. Zanolimumab showed marked clinical efficacy in the treatment of patients with refractory MF, with early onset of response, high response rate, and durable responses. The treatment was well tolerated with no dose-related toxicity other than the targeted depletion of peripheral T cells. A pivotal study has been initiated based on these findings.
Introduction
Cutaneous T-cell lymphomas (CTCLs) represent lymphoproliferative disorders characterized by primary cutaneous infiltration of malignant T cells. According to the World Health Organization–European Organisation for Research and Treatment of Cancer (WHO-EORTC) classification,1 CTCL belongs to mature extranodal non-Hodgkin lymphomas. The neoplastic T cells show clonal expansion, and in most cases a CD4+ phenotype is apparent. CTCL encompasses a number of distinct clinical entities, including mycosis fungoides (MF), Sézary syndrome (SS), and more rare varieties of peripheral T-cell lymphomas, which are all characterized by infiltration of the skin by malignant T cells. MF, the most common type of CTCL, can manifest with patch/plaque, skin tumors, and erythroderma, and in the most advanced stage visceral involvement is seen. Many patients remain at the same stage for prolonged periods; however, some progress from patch/plaque to skin tumors.2,3 Large cell transformation associated with marked worsening of prognosis can also occur.
A histologic hallmark of MF is the presence of Pautrier microabscesses where accumulating malignant T cells are seen in the proximity of antigen-presenting cells (APCs) comprising Langerhans cells and non-Langerhans APCs with unique capacity to activate autologous T cells.4–6 Interactions between APCs and malignant T cells may result in chronic stimulation and growth promotion of the malignant cells.
SS represents a distinct entity and is regarded a leukemic variant of CTCL characterized by skin erythroderma, lymph node enlargement, and the presence of Sézary cells in peripheral blood. In both MF and SS, the malignant cells express CD4 in most cases. The CD4 expression does not seem to be down-regulated during disease progression and is present in all stages of the diseases. Thus, targeting CD4 seems an attractive treatment modality in these diseases.
In the literature, clinical efficacy has been reported in 2 small open studies with chimeric anti-CD4 monoclonal antibodies (mAbs). In one study, a nondepleting anti-CD4 mAb was given as a single administration, and dose escalation was performed in 7 patients. Two objective responses were observed in the highest dose group.7 In another study, 8 patients were treated twice weekly for 3 weeks with a chimeric, depleting anti-CD4 mAb. In this study, 5 patients achieved objective responses.8 .
Zanolimumab (HuMax-CD4; Merck Serono, Geneva, Switzerland) is a fully human anti-CD4 mAb isolated from transgenic mice as a hybridoma clone and subsequently expressed in Chinese hamster ovary (CHO) cells. The antibody is specific for the CD4 receptor expressed on most T lymphocytes and to a lesser extent on macrophages. The antibody prevents interaction between the CD4 receptor and the major histocompatibility complex class II molecule and thereby interferes with T-cell activation. Further, in vitro data demonstrate cell killing via antibody-dependent cellular cytotoxicity (ADCC). Zanolimumab does not induce complement-dependent cytotoxicity (CDC) when bound to cell surface in vitro.
Due to the capacity to inhibit activation of CD4+ T cells and to deplete CD4+ T cells via ADCC, we performed 2 open-label, clinical phase 2 studies with zanolimumab in patients with therapy-resistant CTCL of CD4+ MF and SS subtypes. We found marked efficacy both in early- and advanced-stage MF in the highest dose groups (56% overall response rate). Zanolimumab was well tolerated with no dose-related toxicity.
Patients, materials, and methods
Objectives
The primary objective was to assess the effect of zanolimumab on response rates and duration in patients with treatment-refractory and persistent early-stage and advanced-stage CTCL. The secondary objective included assessment of time to and duration of response, serum concentrations, and safety of zanolimumab in this population.
Study design and drug administration
Two essentially identical prospective, multicenter, open-label, uncontrolled phase 2 studies (Hx-CD4-007 and Hx-CD4-008) of zanolimumab were conducted—one including patients with treatment-refractory and persistent early-stage CTCL (MF stages IB-IIA; doses of 280 or 560 mg weekly) and one including treatment-refractory and persistent advanced-stage CTCL (MF stages IIB-IVB and SS; doses of 280 or 980 mg weekly). The studies were conducted at 10 departments of oncology, hematology, and dermatology in the United States, Germany, the United Kingdom, Sweden, and Denmark. Ethical approval was obtained from the independent ethics committees and institutional review boards of each site before study initiation. The studies were performed in accordance with the Declaration of Helsinki and its amendments in force at the initiation of the studies. All patients gave written informed consent before inclusion in the studies, which were monitored by an independent data safety monitoring board.
Study duration was 20 weeks, including 17 weekly infusions over a period of 16 weeks and a follow-up visit 4 weeks later. The studies were designed as single-arm studies applying the same dose level, 280 mg weekly, for all patients. During data review the observed CD4+ T-cell depletion was considered inadequate. Consequently, the protocols were amended, increasing the dose from 280 to 560 mg in the early-stage patient protocol and from 280 to 980 mg in the advanced-stage patient protocol. Patients enrolled at 280 mg dose stayed on that dose level during the entire study period.
Zanolimumab was administered as an intravenous infusion over 15 minutes for the 280 mg dose and 40 minutes for the 560 and 980 mg doses. To minimize the risk of infusion-related adverse events (AEs), patients enrolled after the first protocol amendment were pretreated with 1 g paracetamol (acetaminophen) or a similar antipyretic agent not later than half an hour prior to the first treatment. The patients were kept for observation approximately 30 minutes after the infusion.
Responders were followed until disease progression. For patients with stable disease (SD) the protocol did not specify follow-up beyond 20 weeks. For patients with a CD4+ T-cell count below 0.350 ×109/L (350/μL), sampling for CD4+ T-cell count and registration of AEs and concomitant medication took place every 3 months until CD4+ T cell counts were more than 0.350 ×109/L (350/μL). Results include 2.5 years of poststudy safety information.
Patient population
Adult patients with treatment-refractory and persistent early-stage or advanced-stage CTCL (MF/SS) were included in the studies. Staging was based on the tumor-nodes-metastasis-blood classification and overall staging system described at the National Cancer Institute (NCI) workshop by the Committee on Staging and Classification of Cutaneous T-Cell Lymphomas.9 A biopsy compatible with the diagnosis of CTCL and a CD4+ phenotype within 6 months of study entry was required for inclusion. Early-stage patients were to be refractory to, to be intolerant to, or to have reached a response plateau for at least 6 months on at least 2 prior therapies, with at least one being phototherapy (PUVA or UV-B) or total body skin electron beam irradiation therapy or topical chemotherapy. Advanced-stage patients were to be refractory to, to be intolerant to, or to have reached a response plateau for at least 6 months on at least one prior systemic anticancer therapy.
Patients with histopathological evidence of large cell transformation or poorly differentiated tumors or prior treatment with anti-CD4 monoclonal antibodies were excluded from the studies. In addition, the following key exclusion criteria applied: more than 2 conventional systemic chemotherapies, antipsoriatic and systemic anticancer therapies within 4 weeks of inclusion, treatment with topical glucocorticosteroids within 2 weeks of inclusion, additional prior or current malignancies, chronic infectious disease requiring medication, and significant concurrent, uncontrolled, or active medical condition, known positive serology for HIV, or clinically significant laboratory abnormalities.
During the studies, systemic or skin-directed anticancer drugs and therapies were prohibited with the exception of topical steroids, which were allowed as rescue treatment for patients developing eczematous dermatitis not involving target lesions.
Response criteria
Assessment of clinical efficacy was performed at weeks 0, 2, 4, 6, 10, 12, 16, and 20 and for responders every 4 weeks until progression. The primary efficacy assessment tool was composite assessment of index lesion disease severity (CA) score.10 When calculating the CA score, a maximum of 5 index lesions were assessed with regard to the following 5 signs or symptoms: erythema, scaling, plaque elevation, hypopigmentation/hyperpigmentation reflective of active disease, and surface area. Each of the first 4 signs or symptoms was graded 0 to 8 (Table 1), whereas surface area was graded 0 to 18. The CA score is the sum of all grades at a specific visit. The CA ratio is the ratio of the score at a postbaseline study visit to that at the baseline visit. Efficacy as measured by physician's global assessment (PGA) was also evaluated (Table 2).
Composite assessment grading scale for each index lesion sign or symptom
| Grade . | Sign or symptom . |
|---|---|
| 0 | No evidence of sign/symptom |
| 2 | Mild: less than average presentation of sign/symptom |
| 4 | Moderate: average disease presentation of sign/symptom |
| 6 | Severe: more than 25% worse than average severity of sign/symptom |
| 8 | Very severe: the near worst severity of sign/symptom |
| Grade . | Sign or symptom . |
|---|---|
| 0 | No evidence of sign/symptom |
| 2 | Mild: less than average presentation of sign/symptom |
| 4 | Moderate: average disease presentation of sign/symptom |
| 6 | Severe: more than 25% worse than average severity of sign/symptom |
| 8 | Very severe: the near worst severity of sign/symptom |
Intermediate grades can be applied as midpoints between the defined grades 0, 2, 4, 6, and 8.
PGA of clinical condition: grades and response criteria
| Grade . | Response* . | Comparison with baseline . |
|---|---|---|
| 0: completely clear | CCR/CR† | No evidence of disease (100% improvement) |
| 1: almost clear | PR | Very significant clearance (≥ 90% to < 100%) |
| 2: marked improvement | PR | Significant improvement (≥ 75% to < 90%); some evidence of disease remains |
| 3: moderate improvement | PR | Intermediate between slight and marked improvement (≥ 50% to < 75%) |
| 4: slight improvement | SD | Some improvement (≥ 25% to < 50%), but significant evidence of disease has not changed |
| 5: no change | SD | Disease has not changed (± < 25%) |
| 6: worse | PD | Disease is worse by 25% or more |
| Grade . | Response* . | Comparison with baseline . |
|---|---|---|
| 0: completely clear | CCR/CR† | No evidence of disease (100% improvement) |
| 1: almost clear | PR | Very significant clearance (≥ 90% to < 100%) |
| 2: marked improvement | PR | Significant improvement (≥ 75% to < 90%); some evidence of disease remains |
| 3: moderate improvement | PR | Intermediate between slight and marked improvement (≥ 50% to < 75%) |
| 4: slight improvement | SD | Some improvement (≥ 25% to < 50%), but significant evidence of disease has not changed |
| 5: no change | SD | Disease has not changed (± < 25%) |
| 6: worse | PD | Disease is worse by 25% or more |
CCR indicates clinical complete response; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
*All responses had to be confirmed by a second assessment after at least 4 weeks.
†CR is CCR plus a cutaneous biopsy from a cleared lesion documenting absence of histologic signs of MF.
The primary end point was objective response according to the CA score across 16 weeks of therapy and 4 weeks of follow-up. Objective response was defined as complete response (CR), clinical complete response (CCR), or partial response (PR) with a confirmation at least 4 weeks later. CCR required a CA ratio to baseline of 0 and no evidence of nonindex lesion disease such as nodes, tumors, or visceral involvement. CR required CCR plus a biopsy from a cleared lesion documenting absence of histologic signs of CTCL. PR required a CA ratio to baseline of 0.5 or less, no new clinically abnormal lymph nodes, less than 25% progression of clinically abnormal lymph nodes, no new cutaneous tumors, and no pathologically positive lymph node or visceral disease in an area previously documented to be negative. Progressive disease (PD) required a CA ratio to baseline of 1.25 or higher, 25% or greater increase in the number or area of clinically abnormal lymph nodes, new cutaneous tumors, or new pathologically positive lymph nodes or visceral disease. If the criteria for CCR, CR, PR, or PD were not met, the response was defined as SD.
Secondary end points included PGA, pruritus assessed by the patients, time to response, response duration, host immune response, and serum concentrations of zanolimumab (7-day postdose trough concentrations).
Time to response was defined as the number of days from first dose administration to the time of the first observation of objective response. Response duration was defined as the number of days from the first observation where the patient meets the criteria for response (partial or clinical complete response with a confirmation 4 weeks later) to the time where the patient's disease progresses.
Time to event history was censored if prohibited concomitant medication was used or the patient was withdrawn for reasons other than progressive disease. AEs were coded using MedDRA version 6.0 (MedDRA MSSO, Reston, VA).
Safety assessment
Safety assessment included adverse events, vital signs, standard clinical hematology and biochemistry every other week, and urinalysis at baseline and end of study. Leukocyte differential counts by fluorescence-activated cell sorter (FACS) scan flow cytometry were carried out at baseline, week 2, and every 4 weeks until end of study. Patients with low CD4+ T-cell counts were followed every 3 months until the count was above 0.350 ×109/L (350/μL). Immunogenicity and host immune response was determined by an enzyme-linked immunosorbent assay (ELISA) at baseline and end of study. Adverse events (AEs) were recorded weekly until week 16 and followed up according to standard regulatory requirements. For responders, AEs were recorded every 4 weeks until PD. Serious adverse events (SAEs) were reported on an ongoing basis throughout the study period and the poststudy follow-up period. AE toxicity grading was according to NCI Common Toxicity Criteria.
Statistical methods
No statistical hypothesis testing was carried out with the exception of CD4-cell decline and recovery. End points are presented by descriptive statistics. All data presentations include the full analysis population/intent-to-treat population (all patients who received at least one dose of study medication irrespective of their compliance to the planned course of treatment). The presentation of efficacy results is split by CTCL type (MF/SS). The analysis of CD4+ T-cell decline was based on 7-day postdose assessments from first dose administration until and including values 7 days after the last drug administration, and a log-linear random coefficient model was applied to describe an exponential decay rate from the patients' baseline CD4+ T-cell count. The analysis of CD4+ T-cell recovery was based on assessments later than 7 days after the last drug administration. The CD4+ T-cell recovery was modeled using a linear random coefficient model.
Results
Patients
The patient demographics are summarized in Table 3. Thirty-eight patients were diagnosed with MF and 9 with SS. Table 4 summarizes the patients' prior exposure to CTCL therapies. Patients were heavily pretreated. All patients had received and failed/relapsed at least 2 prior treatment regimens. For advanced-stage patients at least one prior treatment regimen was systemic. Eleven of 25 early-stage patients had received more than 4 prior CTCL therapies—for 7 of the 25 patients, 2 or more therapies were systemic. Fifteen of 22 advanced-stage patients had received more than 4 prior CTCL therapies—for 16 of the 22 patients, 2 or more therapies were systemic. Skin-directed therapies included PUVA, UVB, topical mechlorethamine HCl, and topical steroid (clobetasol), and systemic therapies included interferon, bexarotene, methotrexate, chlorambucil, and CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone).
Demographics of all patients by CTCL stage
| . | 280 mg zanolimumab . | 560 mg zanolimumab . | 980 mg zanolimumab . | |
|---|---|---|---|---|
| IB-IIA . | IIB-IVB . | IB-IIA . | IIB-IVB . | |
| No. of patients in each stage | 11 | 13 | 14 | 9 |
| CTCL subtype, no. MF/no. SS | 11/0 | 9/4 | 14/0 | 4/5 |
| Sex, no. male/no. female | 9/2 | 11/2 | 10/4 | 6/3 |
| Race, no. white/no. black | 11/0 | 13/0 | 14/0 | 8/1 |
| Median age, y (range) | 70 (53-81) | 57 (42-79) | 57.5 (36-80) | 58 (35-78) |
| Median duration of CTCL, y (range) | 2.2 (0.1-7.4) | 2.2 (0.5-13.7) | 5.3 (0.4-46.9) | 2.8 (0.8-7.5) |
| . | 280 mg zanolimumab . | 560 mg zanolimumab . | 980 mg zanolimumab . | |
|---|---|---|---|---|
| IB-IIA . | IIB-IVB . | IB-IIA . | IIB-IVB . | |
| No. of patients in each stage | 11 | 13 | 14 | 9 |
| CTCL subtype, no. MF/no. SS | 11/0 | 9/4 | 14/0 | 4/5 |
| Sex, no. male/no. female | 9/2 | 11/2 | 10/4 | 6/3 |
| Race, no. white/no. black | 11/0 | 13/0 | 14/0 | 8/1 |
| Median age, y (range) | 70 (53-81) | 57 (42-79) | 57.5 (36-80) | 58 (35-78) |
| Median duration of CTCL, y (range) | 2.2 (0.1-7.4) | 2.2 (0.5-13.7) | 5.3 (0.4-46.9) | 2.8 (0.8-7.5) |
CTCL indicates cutaneous T-cell lymphoma; MF/SS, mycosis fungoides/Śezary syndrome.
Prior CTCL therapies
| Type and no. of treatments . | No. of patients . | |
|---|---|---|
| Early stage . | Advanced stage . | |
| Systemic and skin-directed | ||
| 0 | 0 | 0 |
| 1 | 0 | 0 |
| 2 | 8 | 2 |
| 3 | 6 | 5 |
| 4 or more | 11 | 15 |
| Systemic only | ||
| 0 | 13 | 0 |
| 1 | 5 | 6 |
| 2 | 3 | 3 |
| 3 | 2 | 8 |
| 4 or more | 2 | 5 |
| Type and no. of treatments . | No. of patients . | |
|---|---|---|
| Early stage . | Advanced stage . | |
| Systemic and skin-directed | ||
| 0 | 0 | 0 |
| 1 | 0 | 0 |
| 2 | 8 | 2 |
| 3 | 6 | 5 |
| 4 or more | 11 | 15 |
| Systemic only | ||
| 0 | 13 | 0 |
| 1 | 5 | 6 |
| 2 | 3 | 3 |
| 3 | 2 | 8 |
| 4 or more | 2 | 5 |
Early-stage patients (n = 25) were eligible if they had failed 2 or more prior therapies. Advanced-stage patients (n = 22) were eligible if they had failed one or more prior systemic therapies.
Efficacy
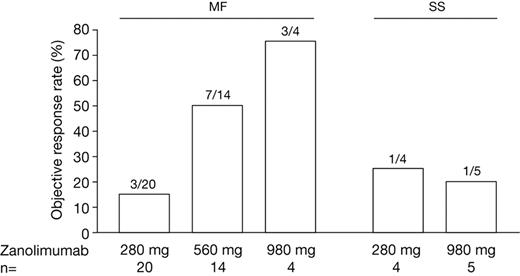
Evaluated by CA, Figure 1 illustrates response rates (for patients with MF, the 280 mg group contains both early- and advanced-stage patients). Overall, 13 of 38 patients with MF (1 CR, 3 CCRs, and 9 PRs) and 2 of 9 patients with SS (2 PRs) obtained an objective response to zanolimumab. A higher proportion of patients with MF (56%) at the high-dose levels (7 of 14 at 560 mg and 3 of 4 at 980 mg) achieved objective response compared with 15% at the 280 mg dose (3 of 20). In patients with SS, the response rate was 1 in 4 at the 280 mg dose and 1 in 5 at the 980 mg dose.
Objective response rates assessed by CA score (all patients). MF (mycosis fungoides): 280 mg encompasses both early-stage (n = 11) and advanced-stage (n = 9), 560 mg encompasses early-stage (n = 14), and 980 mg encompasses advanced-stage patients (n = 4); SS (Sézary syndrome): all advanced stage.
Objective response rates assessed by CA score (all patients). MF (mycosis fungoides): 280 mg encompasses both early-stage (n = 11) and advanced-stage (n = 9), 560 mg encompasses early-stage (n = 14), and 980 mg encompasses advanced-stage patients (n = 4); SS (Sézary syndrome): all advanced stage.
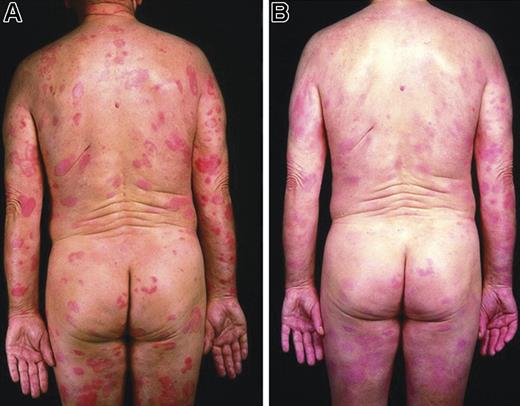
Evaluated by PGA, 10 of 18 patients with MF at the higher dose (560 mg and 980 mg) levels and 5 of 20 at the 280 mg dose level achieved objective response. Figure 2 demonstrates a marked clinical improvement in an early-stage (stage IB) patient at week 4, and Figure 3 demonstrates complete clearance of a tumor in a patient with advanced-stage MF (stage IVA) at week 8. Furthermore, using the PGA scoring system, 3 of 9 patients with SS obtained objective responses (1 patient in the 280 mg dose group and 2 patients in the 980 mg dose group).
Clinical improvement in a patient with early-stage (IB) mycosis fungoides. (A) Baseline. (B) Week 4.
Clinical improvement in a patient with early-stage (IB) mycosis fungoides. (A) Baseline. (B) Week 4.
Complete clearance of a tumor in a patient with advanced-stage mycosis fungoides (stage IVA). (A-B) Baseline. (C-D) Cleared tumor at week 8.
Complete clearance of a tumor in a patient with advanced-stage mycosis fungoides (stage IVA). (A-B) Baseline. (C-D) Cleared tumor at week 8.
Among responders, time to response ranged from 2 to 12 weeks. In patients with MF at the high dose levels, the median time to response was 8 weeks, and 9 of 10 responses were achieved within 8 weeks. In patients with MF at the low dose, the 3 responses were obtained at weeks 4, 8, and 12. The 2 SS responses occurred at weeks 4 (280 mg) and 12 (980 mg).
In patients with MF, responses in the low-dose group are documented with duration of 12, 13, and 24 weeks. At these time points the patients discontinued the study before they had PD. For the high-dose group, response duration was between 8 and 91 weeks (median, 81 weeks). Nine of 13 MF responders had responses lasting more than 20 weeks. In patients with SS, the 2 responses lasted 8 weeks (280 mg) and 61 weeks (980 mg). Thus, in patients with MF, high-dose treatment resulted in earlier and more durable responses than treatment with low dose.
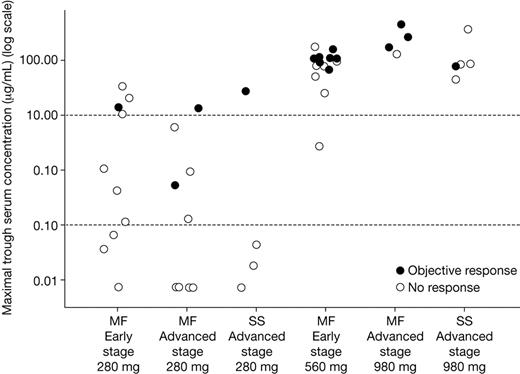
Among patients receiving the higher doses, maximum trough concentrations of zanolimumab (the highest of the 7-day postinfusion values recorded) ranged from 3 to 459 μg/mL. Among patients receiving the low dose, concentrations ranged from below level of quantification ([BLQ] 0.015 μg/mL) to 34 μg/mL. The relationship between objective response and maximum trough zanolimumab concentration is illustrated in Figure 4. Twelve of 13 MF responders (92%), including 2 of the 3 responders in the low-dose group and both of the SS responders, had a maximal trough value of at least 10 μg/mL. The overall response rate in patients achieving concentrations of at least 10 μg/mL was 50% (14 of 28) compared with 5% (1 of 19) in patients achieving concentrations of less than 10 μg/mL.
Relationship between maximal trough serum zanolimumab concentrations and response (all patients).
Relationship between maximal trough serum zanolimumab concentrations and response (all patients).
Nine of 38 patients with MF (24%) developed inflammatory skin reactions (dermatitis, eczema). Seven of these 9 patients (78%) obtained an objective response, indicating an association between response and the occurrence of eczema or dermatitis.
Among the 13 patients with MF responding to zanolimumab treatment, 11 patients (85%) reported improvement in severity of pruritus during the study period, while among 25 nonresponders 52% of patients had some improvement in pruritus.
Safety
Zanolimumab caused profound depletion of circulating CD4+ T cells in patients with MF. The decrease in the number of CD4+ T cells during treatment is characterized by an exponential decay. The depletion was significantly different between the 4 treatment groups (Tables 5 and 6). Compared with the 280 mg dose level, a higher decline was observed with 560 and 980 mg (P < .05). The weekly rates of decline were 12% and 14% in the 280 mg earlyand advanced-stage groups, respectively, and 24% and 22% in the 560 and 980 mg dose groups, respectively (Tables 5 and 6). The CD4+ T-cell counts (median and range) 1 week after the last infusion of zanolimumab are shown in Table 7. Hereafter, recovery of CD4+ T cells is characterized by a linear increase. Of interest, no difference in recovery of the CD4+ T cells was observed between the 4 groups, with a recovery rate of 0.137}109/L (137/μmL) per year (95% confidence interval [CI], 106 to 168; Table 6). CD4+ T-cell counts in patients with SS were high, characteristic for the leukemic state of the patients, and only partial depletion of peripheral blood CD4+ T cells was observed (data not shown).
CD4+ T-cell decline in patients with mycosis fungoides
| Dose, mg (stage) . | Reduction per week, % . | 95% CI, % . |
|---|---|---|
| 280 (early) | 12 | 6-18 |
| 280 (advanced) | 14 | 8-20 |
| 560 (early) | 24 | 19-28 |
| 980 (advanced) | 22 | 13-30 |
| Dose, mg (stage) . | Reduction per week, % . | 95% CI, % . |
|---|---|---|
| 280 (early) | 12 | 6-18 |
| 280 (advanced) | 14 | 8-20 |
| 560 (early) | 24 | 19-28 |
| 980 (advanced) | 22 | 13-30 |
CD4+ T cell recovery in patients with mycosis fungoides
| . | All doses and stages . |
|---|---|
| Increase per year, cell/μL | 137 |
| 95% CI, cells/μL | 106-168 |
| . | All doses and stages . |
|---|---|
| Increase per year, cell/μL | 137 |
| 95% CI, cells/μL | 106-168 |
CD4+ T-cell count 1 week after last infusion in patients with mycosis fungoides
| Dose, mg (stage) . | Median, cells/μL . | Range . |
|---|---|---|
| 280 (early) | 264 | 53-670 |
| 280 (advanced) | 195 | 16-935 |
| 560 (early) | 81 | 13-327 |
| 980 (advanced) | 42 | 15-51 |
| Dose, mg (stage) . | Median, cells/μL . | Range . |
|---|---|---|
| 280 (early) | 264 | 53-670 |
| 280 (advanced) | 195 | 16-935 |
| 560 (early) | 81 | 13-327 |
| 980 (advanced) | 42 | 15-51 |
AEs are summarized in Tables 8-9. The 47 patients reported 276 AEs during the study period. Six nonserious grade 3 AEs were considered related to zanolimumab (1 case of dermatitis, 2 cases of aggravated pruritus, 1 case of eczema, 1 case of flulike symptoms, and 1 muscle fiber rupture), as shown in Table 9. Three patients had SAEs considered related to the treatment. During infusion, dizziness and shivering developed in 2 patients with known insulin-dependent diabetes mellitus. One of the patients also developed low-grade fever. Both patients recovered, and the events were recorded as grade 1 cytokine release syndrome. Over a period of 2 months a patient in the 980 mg group had a grade 3 perioral herpes infection (duration 9 days) and a grade 3 groin infection (staphylococcal and herpes, duration 10 days). Although fever, bacterial skin infections, and herpes simplex reinfections are commonly seen in patients with CTCL, a causal relationship to zanolimumab cannot be excluded. All patients with related SAEs recovered. AEs of low-toxicity grading qualified for SAEs due to overnight stay at the clinic. Following introduction of premedication with paracetamol, no further cases of cytokine release syndrome were reported. No SAEs of grade 4 or 5 were reported during the study period.
Adverse events (AEs) and serious AEs (SAEs) reported during the study period, excluding decreased CD4+ T-cell counts, by zanolimumab dosage and CTCL stage
| . | No. patients (no. events) . | |||
|---|---|---|---|---|
| 280 mg zanolimumab . | 560 mg zanolimumab . | 980 mg zanolimumab . | ||
| IB-IIA . | IIB-IVB . | IB-IIA . | IIB-IVB . | |
| All AEs | 11 (48) | 13 (112) | 14 (62) | 9 (54) |
| Non-SAEs | 11 (46) | 13 (106) | 14 (62) | 9 (47) |
| Nonserious related AEs | 8 (30) | 12 (55) | 13 (46) | 8 (19) |
| Grade 1 | 7 (22) | 12 (51) | 10 (24) | 6 (12) |
| Grade 2 | 4 (7) | 3 (4) | 9 (18) | 4 (6) |
| Grade 3 | 1 (1) | 0 (0) | 4 (4) | 1 (1) |
| All SAEs | 2 (2) | 3 (6) | 0 (0) | 5 (7) |
| Related SAEs | 0 (0) | 2 (3) | 0 (0) | 1 (2) |
| Grade 1 | 0 (0) | 2 (2) | 0 (0) | 0 (0) |
| Grade 2 | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Grade 3 | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| . | No. patients (no. events) . | |||
|---|---|---|---|---|
| 280 mg zanolimumab . | 560 mg zanolimumab . | 980 mg zanolimumab . | ||
| IB-IIA . | IIB-IVB . | IB-IIA . | IIB-IVB . | |
| All AEs | 11 (48) | 13 (112) | 14 (62) | 9 (54) |
| Non-SAEs | 11 (46) | 13 (106) | 14 (62) | 9 (47) |
| Nonserious related AEs | 8 (30) | 12 (55) | 13 (46) | 8 (19) |
| Grade 1 | 7 (22) | 12 (51) | 10 (24) | 6 (12) |
| Grade 2 | 4 (7) | 3 (4) | 9 (18) | 4 (6) |
| Grade 3 | 1 (1) | 0 (0) | 4 (4) | 1 (1) |
| All SAEs | 2 (2) | 3 (6) | 0 (0) | 5 (7) |
| Related SAEs | 0 (0) | 2 (3) | 0 (0) | 1 (2) |
| Grade 1 | 0 (0) | 2 (2) | 0 (0) | 0 (0) |
| Grade 2 | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Grade 3 | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
Grades 4 and 5 did not include any patients.
Three related SAEs were reported during poststudy follow-up. One year after successful completion of 11 infusions and after obtaining a CCR, a case of a fatal malignant hepatic tumor was reported. This patient had a CD4+ T-cell count of 0.323 × 109/L (323/μL) 3 months after the last infusion. Ten months after measuring the recovered T-cell count, the patient developed abdominal pain and ascites and was diagnosed with a tumor in the liver. Ten months after the last dose a case of peripheral neuropathy was reported in a patient who had complained of symptoms compatible with neuropathy 4 years before entering the study. Finally, a patient died of pneumonia/disease progression 11 months after the last dose. After zanolimumab treatment was discontinued, the patient was treated with both interferon-α and bexarotene due to disease progression. Following this, pneumonia developed while the patient's CD4+ T-cell count was not fully recovered (0.131 × 109/L [131/μL]). Subsequent polymerase chain reaction for cytomegalovirus was found positive. The patient was treated with ganciclovir and showed slow clinical improvement. Later the patient developed pneumothorax and recurrent infections and became progressively worse with decreasing renal, liver, and lung function. Bone marrow biopsy revealed increased number of T cells indicative of lymphoma.
Infections and infestations.
During the studies, 23 patients reported 34 infections. Most were localized to the skin and upper respiratory tract. Sixty-five percent of infectious events were low-grade skin infections. Twenty-six percent of infections were localized to the upper respiratory tract, all of which were nonserious, with 6 events grade 2 and the remaining events grade 1. Only 9 infections were considered related to the zanolimumab treatment and, in general, no dose relation was apparent for infections (Table 9). The related infections were 6 cases of skin infections, 1 upper respiratory tract infection, 1 case of oral candidiasis, and 1 case of pneumonia.
Nonserious related adverse events (AEs) reported during the study period, excluding decreased CD4+ T-cell counts
| . | No. patients (no. events) . | ||
|---|---|---|---|
| Grade 1 . | Grade 2 . | Grade 3 . | |
| Skin and subcutaneous tissue disorders | |||
| Eczema and dermatitis | 4 (4) | 3 (3) | 2 (2) |
| Urticaria | 1 (1) | 2 (2) | 0 (0) |
| Pruritus | 2 (2) | 3 (3) | 2 (2) |
| Other skin and subcutaneous tissue disorders | 6 (6) | 2 (2) | 0 (0) |
| General disorders and administration-site conditions | |||
| Fatigue | 9 (11) | 0 (0) | 0 (0) |
| Flulike symptoms | 4 (8) | 3 (3) | 1 (1) |
| Other general disorders and administration-site conditions | 13 (25) | 4 (4) | 0 (0) |
| Infections and infestations | |||
| Skin-related infections | 3 (3) | 2 (3) | 0 (0) |
| Upper respiratory tract infections | 1 (1) | 0 (0) | 0 (0) |
| Pneumonia | 0 (0) | 1 (1) | 0 (0) |
| Oral candidiasis | 0 (0) | 1 (1) | 0 (0) |
| Other system organ classes | |||
| Muscle fiber rupture | 0 (0) | 0 (0) | 1 (1) |
| Other | 20 (48) | 10 (13) | 0 (0) |
| . | No. patients (no. events) . | ||
|---|---|---|---|
| Grade 1 . | Grade 2 . | Grade 3 . | |
| Skin and subcutaneous tissue disorders | |||
| Eczema and dermatitis | 4 (4) | 3 (3) | 2 (2) |
| Urticaria | 1 (1) | 2 (2) | 0 (0) |
| Pruritus | 2 (2) | 3 (3) | 2 (2) |
| Other skin and subcutaneous tissue disorders | 6 (6) | 2 (2) | 0 (0) |
| General disorders and administration-site conditions | |||
| Fatigue | 9 (11) | 0 (0) | 0 (0) |
| Flulike symptoms | 4 (8) | 3 (3) | 1 (1) |
| Other general disorders and administration-site conditions | 13 (25) | 4 (4) | 0 (0) |
| Infections and infestations | |||
| Skin-related infections | 3 (3) | 2 (3) | 0 (0) |
| Upper respiratory tract infections | 1 (1) | 0 (0) | 0 (0) |
| Pneumonia | 0 (0) | 1 (1) | 0 (0) |
| Oral candidiasis | 0 (0) | 1 (1) | 0 (0) |
| Other system organ classes | |||
| Muscle fiber rupture | 0 (0) | 0 (0) | 1 (1) |
| Other | 20 (48) | 10 (13) | 0 (0) |
Nine patients reported eczema or dermatitis during the study; 4 cases were grade 1, 3 were grade 2, and 2 were grade 3 (Table 9).
Safety laboratory parameters.
Apart from the CD4+ T-cell depletion, which is attributed to the desired dynamic effect of zanolimumab, changes in clinical chemistry and hematology parameters were sporadic and transient, and there was no pattern suggesting any impact of zanolimumab. Specifically, zanolimumab did not reduce the number of peripheral blood monocytes. The hematology findings were eosinophilia, leukocytosis, lymphocytosis, lymphopenia, neutropenia, neutrophilia, and monocytosis. Clinical chemistry findings were increased blood creatinine, hyponatremia, hyperglycemia, increased alanine aminotransferase, increased aspartate aminotransferase, and increased lactate dehydrogenase. None of the lab findings were grade 3.
Immunogenicity.
Only one patient had a borderline (8-fold) increase in titer of antibodies against zanolimumab. The patient's CD4+ T-cell count decreased from a baseline value of 1.311 × 109/L (1311/μL) to 0.068 × 109/L (68/μL) at week 16, indicating no functional effect of these antibodies on CD4+ T-cell depletion.
Discussion
Zanolimumab is a biologically active drug selectively targeting a surface molecule present on cancer cells in most patients with MF and SS. Targeting the CD4 molecule has several potential advantages in that CD4 is expressed only on a subset of T cells and weakly on monocytes/macrophages/dendritic cells, is highly expressed on the malignant cells in most CTCL patients, is present in all stages of the disease and thus not down-regulated during disease progression, and is involved in cell signaling. Binding of zanolimumab to CD4+ T cells has been shown to block T-cell activation and induction of inhibitory signaling pathways, including phosphorylation of SH2-containing inositol 5-phosphatase (SHIP-1) and the adaptor molecule DOK-1 (David A. Rider, Carin E. G. Havenith, Ruby de Ridder, Janine Schuurman, Cedric Favre, Joanne C. Cooper, Simon Walker, John Voorhees, Ole Baadsgaard, Susanne Marschner, Jan G. J. van de Winkel, John Cambier, Paul W.H.I. Parren, and Denis R. Alexande, manuscript submitted). Zanolimumab therefore may be able to effectively block chronic stimulatory signals to tumor cells by macrophages (APCs) in the Pautrier microabscesses. Furthermore, in vitro data demonstrate that zanolimumab is capable of depleting CD4+ T cells via ADCC. Finally, predominantly CD4+CD45RO+ “memory” T cells are depleted in vivo following treatment with zanolimumab11 —a phenotype present on most malignant cells in CD4+ CTCL.
In these open-label, clinical phase 2 studies, 38 patients with MF and 9 patients with SS were treated with zanolimumab, a fully human anti-CD4 mAb. In patients with MF, objective responses were seen in 3 patients (15%) receiving low-dose zanolimumab (280 mg). All 3 patients (1 CR and 2 PRs) discontinued the study before disease progression at 12, 13, and 24 weeks. With high-dose treatment (560 mg or 980 mg), an increased response rate of 56% was observed. In the high-dose groups, 90% of responses were already present 8 weeks following treatment initiation, and median response duration of 81 weeks was observed. Thus, zanolimumab induced fast onset of durable responses in many patients with refractory MF. Other drugs recently accepted for treatment of CTCL include bexarotene and denileukin diftitox. In two phase 2/3 trials of bexarotene in early-stage (including stage IA) and late-stage patients, a median disease control in the 300 mg/m2/d dose groups, measured from first dosing to clinical relapse, was reported as 30 weeks and 42.7 weeks, respectively.10,12 In these trials, bexarotene demonstrated a median time to response of 8.1 and 25.7 weeks, respectively. In a pivotal phase 3 study, denileukin diftitox showed a median disease control of 6.9 months (measured from first dose) with a median time to response of 6 weeks.13
Measuring serum trough levels of zanolimumab (dose measured immediately before next administration—7 days following last infusion), a clear correlation between dose and trough levels was observed in patients with MF. In the low-dose group, almost no free antibody was present in circulation 1 week after drug administration. In the high-dose groups, 17 of 18 patients obtained maximal trough values above 10 μg/mL. Of interest, in 12 of 13 objective responders, including 2 of the 3 responders in the low-dose group, a maximal trough value of more than 10 μg/mL was observed.
In SS, 2 objective responses were seen in the 9 patients (22%). In SS, treatment with zanolimumab only leads to partial depletion of peripheral blood CD4+ T cells (data not shown). These data indicate greater efficacy of zanolimumab in MF compared with the more malignant SS. However, future use of zanolimumab in SS could depend on increased dose, prolonged treatment period, or combination with chemotherapy, interferon, or extracorporal photopheresis.
Zanolimumab demonstrated an overall favorable safety profile. The most frequently encountered AEs included inflammatory skin reactions and infections of the skin and upper respiratory tract.
Skin inflammation reported as dermatitis or eczema was observed in some patients during treatment with zanolimumab. Development of skin inflammation including rash or dermatitis has also been described with other T-cell–targeting therapies. Examples include denileukin diftitox, where 35% of CTCL patients developed skin rash13 ; 4162W94, a depleting anti-CD4 mAb, where 62% of rheumatoid patients developed rash14 ; and anti-CD3 treatment of insulin-dependant diabetes mellitus, where 73% of treated patients developed rash.15 Thus, it is known from the literature that targeting T cells and in particular the CD4+ subpopulation can result in inflammatory skin reactions.
One explanation could be that within the CD4+ T-cell population, inhibitory T-cell subsets such as regulatory T cells (Tregs) exist. Tregs are characterized by a CD4+CD25+ phenotype16 and are demonstrated to potently suppress immune reactions. In a recent paper it has been suggested that activation of malignant cells in CTCL converts the tumor cells to a Treg phenotype (up-regulation of CTLA-4, FoxP3, and CD25), which may explain the anergic, immunosuppressive nature of CTCL.17 Depletion of CD4+ T cells in CTCL thus might unmask inflammatory signals resulting in development of skin reactions. In these zanolimumab studies most patients with dermatitis or eczema (7 of 9) obtained an objective response. Thus, depletion of CD4+ T cells might unmask immune response against tumor cells including lymphomas, as previously suggested.18 The significance of depleting CD4+CD25+ Tregs has been addressed in animal models, resulting in decreased tumor growth and enhanced tumor rejection.19,20
Twenty-three patients reported infections. No differences in incidences were observed between the dose groups. Only 9 infections were considered related to the zanolimumab treatment. Patients with MF have a predisposition to infections, in particular skin-associated infections but also systemic infections, due to widespread disruption of the normal protective skin barrier21 and in more advanced stages potential immunosuppression.22 In our studies, 65% of infectious events were skin infections, thus reflecting comorbidity in this patient population.21 About 26% of infections were related to the upper respiratory tract; all of these were nonserious and 6 events were grade 2 and the remaining events grade 1. Only one patient reported a related serious infection (pneumonia), occurring 11 months after leaving the study. Due to progression of CTCL the patient was treated with bexarotene and interferon-α after leaving the study. While the patient's CD4+ T-cell count was not fully recovered (0.131 × 109/L [131/μL]) a pneumonia was diagnosed. Subsequently CTCL further progressed, the patient developed pneumothorax, and died.
Despite a more profound decrease in CD4+ T-cell counts in the high-dose groups, no dose-related increase in morbidity related to infections was observed. Thus, no unexpected findings related to infections were observed in this patient population treated with zanolimumab. This observation parallels findings in other patient populations treated with depleting anti-CD4 mAbs. In a long-term follow-up study in rheumatoid arthritis, no opportunistic infections were reported in 23 of 25 patients available for follow-up at 18 and 30 months.23 Furthermore, using cM-T412 in a placebo-controlled setting in rheumatoid arthritis, no increased frequency of infections was reported.24 In contrast to findings following CD4 depletion, more profound depletion of immune cells (eg, via an anti-CD52 monoclonal antibody) has resulted in occurrence of atypical infections, including reactivation of CMV in 18% of patients with advanced CTCL.25
Zanolimumab showed minimal immunogenicity with only one patient developing borderline antibodies to zanolimumab (human-antihuman antibodies [HAHAs]) that did not appear to inhibit depletion of peripheral blood CD4+ T cells.
Comparing low-dose (280 mg) and high-dose (560 and 980 mg) regimens, a significantly increased decline in absolute CD4+ T-cell counts was observed (P < .005) with a more pronounced depletion in high-dose groups. Of interest, no difference in recovery of the CD4+ T cells was observed between the groups and, based on observed cell counts, a recovery rate of 0.137 × 109/L (range, 0.106 × 109/L to 0.167 × 109/L) (137/μL; range, 106/μL to 167/μ L) per year or 0.00037 × 109/L (0.37/μL) per day was estimated. With an average blood volume of 5 L in adults and estimating that 2% of T cells are in the circulation,26 0.00037 × 109/L (0.37/μL) per day corresponds to approximately 108 CD4+ T cells per day during the observed recovery phase. Our observation is similar to findings by Mclean et al,27 who used CD45RA expression on CD4+ T cells as a measure of the production of new lymphocytes following radiotherapy estimated by chromosome damage, and also correlates to reconstitution rates observed in young cancer patients treated with chemotherapy where recovery rates of 0.00054 × 109/L, 0.00026 × 109/L, and 0.00062 × 109/L (0.54/μL, 0.26/μL, and 0.62/μL, respectively) CD4+ T cells per day were observed in 3 non-Hodgkin lymphoma patients above 20 years of age.28 Thus, we find the observed recovery rate of CD4+ T cells following treatment with zanolimumab in line with expected values, and our finding also parallels findings from patients with rheumatoid arthritis treated with cM-T412, a depleting anti-CD4 mAb.23 Other cells than T cells are known to express the CD4 molecule, although to a lesser extent. These cells include blood monocytes. In these 2 trials, we did not observe changes in peripheral blood monocytes. We did not investigate changes in other CD4 antigen-expressing cells, but we would consider it unlikely that macrophages or dendritic cells were affected because these cells, like monocytes, have low CD4 expression. This may contribute to preserved immune system activity in these patients.
In conclusion, in these clinical phase 2 studies, zanolimumab showed marked clinical efficacy in treatment of patients with refractory MF with high response rate, early onset, and durable responses. The treatment was well tolerated, and no dose-related toxicities (excluding the targeted depletion of peripheral blood CD4+ T cells) were observed. Based on these findings zanolimumab is currently being tested in a pivotal study in the United States in patients failing bexarotene and at least one more systemic treatment modality.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by research funding from Genmab (Y.H.K., M.D., E.O., R.G., L.I., A.Ö., S.W., T.M.I., T.S., R.K., K.C., S.J.K.). We thank Genmab for supplying study material, including zanolimumab; contributing to the design and conduct of the study; collecting, managing, analyzing, and interpreting the data; and contributing to the preparation of the manuscript. We also thank Marc Andersen, Genmab, for statistical analysis of the CD4+ T-cell data and Jan Harry Petersen, Genmab, for editorial assistance.
Authorship
Contribution: Y.H.K., M.D., E.O., R.G., A.Ö., K.M.K., S.L., O.B., and S.J.K. designed the research; Y.H.K., M.D., E.O., R.G., L.I.,A.Ö., S.W., T.M.I., T.S., R.K., and K.C. performed the research and collected the data; Y.H.K., M.D., E.O., A.Ö., T.M.I., K.M.K., S.L., O.B., and S.J.K. analyzed and interpreted the data; K.M.K. performed the statistical analysis; Y.H.K., A.Ö., T.M.I., and S.L. prepared the manuscript; and M.D., E.O., R.G., L.I., A.Ö., S.W., T.M.I., T.S., R.K., K.M.K., S.L., O.B., and S.J.K. critically reviewed the manuscript.
Conflict-of-interest disclosure: Several of the authors (S.L., O.B., K.M.K.) are employed by Genmab A/S, whose potential product was studied in the present work. E.O., R.G., S.W., and K.C have received payment from Genmab A/S for consultancy services. The other authors declare no conflicting financial interests.
Correspondence: Youn H. Kim, Multidisciplinary Cutaneous Lymphoma Program, Stanford Comprehensive Cancer Center, 875 Blake Wilbur Dr, Stanford, CA 94305; e-mail: younkim@stanford.edu


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal