Abstract
Vascular development is dependent on various growth factors and certain modifiers critical for providing arterial or venous identity, interaction with the surrounding stroma and tissues, hierarchic network formation, and recruitment of pericytes. Notch receptors and ligands (Jagged and Delta-like) play a critical role in this process in addition to VEGF. Dll4 is one of the Notch ligands that regulates arterial specification and maturation events. In the current study, we have shown that loss of function by either targeted allele deletion or use of a soluble form of Dll4 extracellular domain leads to inhibition of Notch signaling, resulting in increased vascular proliferation but defective maturation. Newly forming vessels have thin caliber, a markedly reduced vessel lumen, markedly reduced pericyte recruitment, and deficient vascular perfusion. sDll4 similarly induced defective vascular response in tumor implants leading to reduced tumor growth. Interference with Dll4-Notch signaling may be particularly desirable in tumors that have highly induced Dll4-Notch pathway.
Introduction
Primary vasculogenesis serves as the template from which a higher order of branching network is generated by the process defined as angiogenesis.1–3 During angiogenesis, branching of arterial and venous components is orchestrated such that the capillaries from these 2 compartments fuse in symmetry, anchored in place by interaction with matrix proteins.4 Vascular endothelial growth factor (VEGF) is indispensable for the formation of primary vascular network and secondary angiogenesis.5 VEGF however requires the presence of precise quantities of several other constituents within well-defined temporal and spatial constraints to construct and remodel the vascular system. Specifically the Notch signaling pathway is necessary to provide signals for phenotypic determination of arteries and veins, and regulated vessel migration and branching leading to the vascular morphogenesis and remodeling (Bray6 and W.J. and P.S.G., unpublished data, December 2006).
In mammals, the Notch family of proteins is composed of 4 single-pass transmembrane receptors (notch1-4) and 5 membrane-bound ligands (jagged1, 2 and Dll1, 3, and 4). Mutations of Notch receptors and ligands in mice and humans lead to abnormalities in the vascular system.7 The Notch pathway functions through cell-cell interaction such that the extracellular domain of cell membrane–bound ligand interacts with the extracellular domain of the receptor on an adjacent cell. Notch receptor activation requires cleavage of Notch intracellular domain (NICD) and translocation to the nucleus, and activation of target genes.8
Differentiation of vascular cells to arterial or venous compartments was previously thought to depend on physical factors such as blood pressure and oxygen concentration. Over the past few years, however, the differential and restricted expression of a number of genes in arterial or venous endothelial cells prior to the onset of circulation suggested the potential for genetic determination of the arterial and venous fate of primary endothelial cells. Among these genes are NOTCH1,9 NOTCH4,10 DLL4,11 and the Dll4-Notch–regulated genes EPHB4 and EFNB2 specifically expressed in venous12 and arterial endothelial cells, respectively.13,14
Vascular expression of Dll4 and its cognate receptors Notch1 and Notch4 is restricted to arterial endothelium. Dll4 is one of the earliest genes expressed in arterial endothelial cells, is induced by VEGF-VEGFR signaling, and is essential for establishment of the arterial endothelial cell fate.14–16 Haploinsufficiency of Dll4, like that of VEGF, leads to embryonic lethality due to defects in vascular development.14,17 The observed defects include loss of expression of arterial markers, reduced arterial phenotype and augmented venous phenotypes, reduced arterial lumen, and premature fusion among the arterial and venous compartment leading to short circuiting of the vascular network.14
In this study, we investigated the role of Dll4 in vascular remodeling at sites of angiogenesis, including tumor vasculature. We show that loss of Dll4 function promotes endothelial cell migration, excessive vascular network formation, and reduction in pericyte recruitment, both in embryos and adult mice. Soluble forms of Dll4 interrupt Dll4-Notch signaling and recapitulate the vascular alterations seen in the gene knock-out mice, including increased vascular network formation, decreased or absent vascular lumen, and reduced recruitment of pericytes resulting in tissue hypoxia and decreased tumor growth.
Materials and methods
Analysis of Dll4 germ-line mutant mice in embryos and adults
Dll4 knock-out mice were generated in CD1 background and described previously.14 Dll4−/− and most Dll4+/− mouse embryos have a lethal phenotype. The vasculature of Dll4+/− embryos was visualized with platelet endothelial cell adhesion molecule (PECAM) and alpha smooth muscle actin (α-SMA) staining. Dll4+/− mice that survived to adulthood were studied for alterations in the vasculature. Dll4+/− CD1 male mice and wild-type mice (6-8 weeks old) received a transplant of 5 × 106 tumor cells (S180 mouse sarcoma cell line). Tumors were harvested after 2 weeks for analysis. Dll4 expression was studied in Dll4+/− mice in the tumor tissue and adjacent normal tissue by the use of a lacZ reporter included in the targeting vector. Whole-mount embryo immunohistochemistry (PECAM antibody was from Pharmingen, San Diego, CA) and lacZ staining were carried out by standard techniques.18
Reverse-transcription–polymerase chain reaction (RT-PCR) analysis
First-strand cDNA was synthesized from total RNA using a SuperScript Preamplification System kit (GIBCOBRL, Grand Island, NY) and used (0.1 μg) for PCR with specific primers for Dll4, GAPDH, β-actin, Hey1, Hey2, Hes1, and Hes2 (primer pairs used in this study are available on request); PCR products were visualized by ethidium bromide staining.
Antibodies and other reagents
Anti-PECAM (M20) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA); anti–α-SMA (Dako, Carpinteria, CA), IgG-Fc fragment, and anti–human Fc, from Jackson Laboratories (Bar Harbor, ME); Notch1-Fc and Notch3-Fc, from R&D Systems (Minneapolis, MN); hypoxyprobe-1, from Chemicon International (Temecula, CA); rhodamine-labeled ricinus communis agglutinin I (RCA), from Vector Laboratories (Burlingame, CA); and alkaline phosphatase substrate PNPP, from Sigma Chemicals (St Louis, MO).
Cell culture
Normal human umbilical vein endothelial cells (HUVECs) and human umbilical arterial endothelial cells (HUAECs) were obtained from Cambrex (Walkersville, MD) and maintained in EGM2-supplemented medium (Invitrogen, Carlsbad, CA). For all experiments, HUVECs and HUAECs were used at passages 4 or below and collected from a confluent dish. ChoK cell line was obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured under recommended conditions.
Dll4 constructs
Full-length human Dll4 gene was cloned by PCR amplification from human cDNA (Clontech, Mountain View, CA) made from fetal lung tissues. Both full-length (amino acid residues 1-685) and C-terminally His-tagged extracellular domain (amino acid residues 1-486) proteins were expressed from pcDNA3.1 expression vector (Invitrogen). Fc fusion protein was expressed from pCXFc vector (Invitrogen). AP fusion protein was expressed from pAPtag-2 vector (GeneHunter, Nashville, TN). All proteins were transiently expressed in ChoK cells (ATCC) using Lipofectamine 2000 (Invitrogen). His-tagged and Fc-fusion Dll4 proteins were purified through nickel-NTA column and protein A–Sepharose column.19
Notch receptor binding and activation pathway
Notch1-Fc and Notch3-Fc (5 μg/mL each) were coated overnight at 4°C in PBS on 96-well plates. Dll4-AP was diluted in PBS and 0.1% Tween-20 (PBST); 50 μL of each dilution was incubated with Notch-Fc and blocked with 5% milk in PBS for one hour. Wells were washed 3 times with PBST, developed with PNPP, and read at OD405. Typically, HUVECs were grown in 100-mm dishes until 80% confluence and were cocultured with ChoK cells transiently expressing full-length Dll4 (1:1 ratio) or ChoK cells transfected with vector alone. Cocultures were treated with either rDll4-His or rDll4-Fc for a period of 24 hours, cells were harvested, and total RNA was isolated for further analysis.20
Cell sorting
For sorting transfected cells, the MACSelect 4.1 transfected cell selection kit (Miltenyi Biotech, Auburn, CA) was used as per manufacturer's instructions. In brief, cells were cotransfected with expression vector containing the plasmid of interest and pMACS 4.1 plasmid. After 36 hours, cells were harvested with 5 mM EDTA and incubated with MACSelect 4 Microbeads for 15 minutes at 4°C. The cell suspension was then passed via an MS+ column in a magnetic field. After 3 washes, the column was removed from the field and selected cells were eluted in culture medium. Selection efficiency was confirmed by fluorescence-activated cell sorter (FACS) analysis of sorted cells with fluorescent Dll4 monoclonal antibody (data not shown).
Endothelial cell (EC) tube formation assay
Matrigel (250 μL; BD Biosciences, Palo Alto, CA) was placed in each well of an ice-cold 24-well plate. The plate was allowed to sit at room temperature for 15 minutes, and at 37°C for 30 minutes for Matrigel to polymerize. HUVECs in EGM2 medium were plated at a concentration of 1 × 104 cells/well with test material at various concentrations in triplicates. After 6-hour and 24-hour incubations, pictures were taken for each concentration using a Bioquant Image Analysis system (Bioquant, Nashville, TN). Length of cords formed and number of junctions were compared among various groups using ImageJ software (NIH, Bethesda, MD). Experiments were repeated twice.19
Vessel sprouting
Endothelial cell spheroids were generated by suspending equal number of endothelial cells (1000 cells/well) in culture medium containing 0.25% (wt/vol) carboxymethylcellulose and seeded in nonadherent round-bottom 96-well plates. Endothelial cells were suspended to form a single spheroid per well. Spheroids were embedded into collagen gels and cultured for at least 24 hours. Sprouting was recorded digitally (ocular grid at 100× magnification) using the digital imaging DP-Soft (Olympus, Center Valley, PA) analyzing at least 10 spheroids per experimental group and experiment. Sprouting was also quantitated by measuring the length of the sprouts by ImageJ.19
Murine Matrigel plug angiogenesis assay
In vivo angiogenesis was assayed using the Matrigel plug assay. Matrigel rapidly forms a solid gel at body temperature, trapping the factors to allow slow release and prolonged exposure to surrounding tissues. Matrigel (8.13 mg/mL, 0.5 mL) in liquid form at 4°C was mixed with vehicle alone (PBS containing 0.25% BSA) or VEGF, or sDll4, or VEGF and sDll4 together. Matrigel (0.5 mL) was injected into the abdominal subcutaneous tissue of female Balb/C nu/nu mice (6 weeks old, 5 mice per group) along the peritoneal midline. On day 6, mice were humanely killed and plugs were recovered, weighed, and divided for hemoglobin measurement and immunohistochemical analysis. Vascular identity of the infiltrating cells was established with PECAM immunostaining. The experiment was repeated 3 times. The vascularized area in each section was calculated using ImageJ. Hemoglobin in one half of the Matrigel plug was measured using the Drabkin method (Drabkin reagent kit 525; Sigma, St Louis, MO) using the manufacturer's recommended protocol.
Immunohistochemistry and immunofluorescence
Sections (5 μm) of formalin-fixed paraffin-embedded tissues were processed using standard methods.14,19 Sections were incubated with primary antibody overnight at 4°C and appropriate secondary antibody for 1 hour at room temperature. Antibody binding was localized with ABC staining kit from Vector Laboratories according to the manufacturer's instructions and peroxidase activity detected using DAB substrate solution (Vector Laboratories). Routine negative controls were exclusion of primary and secondary antibody and substitution of normal IgG isotope for primary antibody. The positive staining area was estimated using ImageJ and analyzed by Student t test.
Fluorescent immunostaining was performed in a similar fashion to detect the expression level of EC-specific markers including PECAM. Appropriate fluorescein-conjugated secondary antibodies (Sigma-Aldrich, St Louis, MO) were used and nuclei were counterstained with 4′, 6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI). Slides were mounted with Vectashield antifade mounting solution (Vector Laboratories) and images obtained using an Olympus AX70 fluorescence microscope and Spot v2.2.2 (Diagnostic Instruments, Sterling Heights, MI) digital imaging system.
Murine tumor xenografts
Tumor cells (1.5 × 106) HT29 (human colon cancer cell line) or KS-IMM (human Kaposi sarcoma cancer cell line) were implanted subcutaneously in flanks of male athymic BalbC nu/nu mice (6-8 weeks old, 6 mice/group and repeated twice). For assessing local effects of sDll4, tumor cells were mixed with Matrigel (1:1 vol/vol; BD Biosciences) with or without 5 μg/mL sDll4. Tumor volume was measured on day 14 estimated as 0.52 × a × b2, where a and b are the largest and smallest lengths of the palpable tumor, respectively. The Student t test was used to compare tumor volumes, with P < .05 being considered significant. Animals were humanely killed, and tumor and adjacent normal tissues were harvested. Harvested tissues were divided and either fixed in formalin or frozen in OCT for analysis. Distribution and intensity of hypoxia were studied using hypoxyprobe-1 (HP1-100; Chemicon International) infused intraperitoneally at a dose of 60 mg/kg one hour prior to the tumor harvest and localized using recommended protocol. Vessel perfusion was studied using rhodamine-labeled ricinus communis agglutinin 1 (Vector Laboratories) infused 10 to 15 minutes prior to the tumor harvest and analyzed using the manufacturer's recommended protocol. All procedures were approved by our Institutional Animal Care and Use Committees and performed in accordance with the Animal Welfare Act regulations.
Results
Vascular proliferation in embryonic and adult Dll4+/− mutant mice
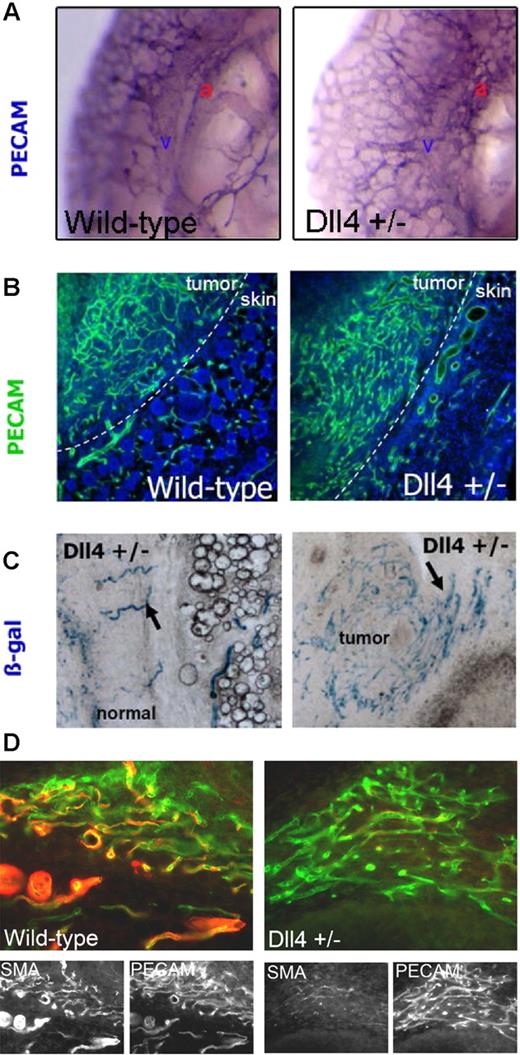
Dll4−/− and most of Dll4+/− mice die in utero due to defective vascular development.17 Close examination of the Dll4+/− embryos showed normal vasculogenesis until E8.75, when the first vascular defect became apparent. There was increased vascular proliferation appearing like honeycomb and lacking hierarchic arterial branching and maturation (Figure 1A). We next studied vascular response and remodeling in adult Dll4+/− mutant and wild-type mice. Mice (6 weeks old) received implants of S180 tumor cells. Tumor and adjacent tissue harvested after 10 days was examined for vascular response by PECAM, and α-SMA immunolocalization. Wild-type mice showed increased vascular response in the tumor (Figure 1B) and the vessels had organized network. In comparison, Dll4+/− mice showed an even greater increase in the vascular response (1.5-fold increase, P < .05). Furthermore, the vessels showed lack of architecture and loss of hierarchy. Thus vascular response was increased but maturation was lacking. Maturation of newly forming vessels accompanies the recruitment of pericytes. We hypothesized that newly forming vessels in Dll4+/− mice may be defective in pericyte recruitment. Thus localization of pericytes with α-SMA antibodies showed abundant signal in tumor vessels in wild-type mice, whereas tumor vessels in Dll4+/− mice showed a profound deficiency in pericyte coverage. Reduced recruitment of pericytes may contribute to the lack of vascular hierarchy in Dll4+/− mice tumor vessels. Furthermore, these findings reveal a novel function of Dll4 in the recruitment of pericytes to newly forming vessels. We next wished to determine if defective vascular response in adult mice leads to alteration in gene expression, in particular Dll4. To this end, we used the LacZ reporter included in the targeting vector used to generate mutant mice to observe Dll4 promoter activity. Dll4+/− mutant mice showed highly structured LacZ-expressing vessels in the normal tissue adjacent to the tumor (Figure 1C), whereas LacZ activity was markedly increased in vessels within the tumor vessels (Figure 1C), indicative of Dll4 activation in the tumor vasculature. PECAM localization in serial sections of the tumor vessels was done to determine the extent of Dll4 activation in tumor vasculature. Dll4 is expressed in the majority but not in all tumor vessels (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Dll4+/− mutant mice show defective increase in vascular proliferation. (A) The vasculature of wild-type and Dll4+/− embryos were examined using PECAM whole-mount immunostaining. Dorsal aorta and cardinal vein are labeled as a and v, respectively. Absence of large vessels and an increase in vessel branching and density was seen in Dll4+/− embryos at E10.5 compared to wild type. (B) Vascular response in Dll4+/− adult mice was examined as in panel A after tumor implantation. Wild-type mice showed organized vascular proliferation in the tumor (left half), while mutant mice showed markedly increased vascular response that lacks organization and vascular hierarchy. (C) Expression of Dll4 in tumor and normal regions in Dll4+/− mutant mice was examined by β-gal staining. Dll4 expression was observed (arrows) in a few discrete vessels in the normal tissue, while the tumor region showed many β-gal–positive vessels of similar appearance indicative of Dll4 induction in tumor vessels. (D) Pericyte coverage around newly forming vessels was examined by α-SMA localization. In wild-type mice, the vessels showed colocalization of PECAM and α-SMA (left panel). In Dll4+/− mice tumor vessels, however, the number of α-SMA–positive cells lining the endothelial cells was profoundly reduced (right panel). Images in panel A were viewed under an Olympus SZX12 stereomicroscope (Tokyo, Japan) with Leica PL Fluotar 0, 5, 20×/0.5 NA dry objective (Wetzlar, Germany), captured with an Olympus C4040 camera, and processed with Olympus DP-Soft 3.2. Images in panels B-D were viewed under a Leica DMRA2 fluorescence microscope with Leica HC PL Fluotar 0, 5, 20×/0.5 NA dry objective, captured using Photometrics CoolSNAP HQ, (Photometrics, Friedland, Denmark), and processed with Metamorph 4.6-5 (Molecular Devices, Sunnyvale, CA).
Dll4+/− mutant mice show defective increase in vascular proliferation. (A) The vasculature of wild-type and Dll4+/− embryos were examined using PECAM whole-mount immunostaining. Dorsal aorta and cardinal vein are labeled as a and v, respectively. Absence of large vessels and an increase in vessel branching and density was seen in Dll4+/− embryos at E10.5 compared to wild type. (B) Vascular response in Dll4+/− adult mice was examined as in panel A after tumor implantation. Wild-type mice showed organized vascular proliferation in the tumor (left half), while mutant mice showed markedly increased vascular response that lacks organization and vascular hierarchy. (C) Expression of Dll4 in tumor and normal regions in Dll4+/− mutant mice was examined by β-gal staining. Dll4 expression was observed (arrows) in a few discrete vessels in the normal tissue, while the tumor region showed many β-gal–positive vessels of similar appearance indicative of Dll4 induction in tumor vessels. (D) Pericyte coverage around newly forming vessels was examined by α-SMA localization. In wild-type mice, the vessels showed colocalization of PECAM and α-SMA (left panel). In Dll4+/− mice tumor vessels, however, the number of α-SMA–positive cells lining the endothelial cells was profoundly reduced (right panel). Images in panel A were viewed under an Olympus SZX12 stereomicroscope (Tokyo, Japan) with Leica PL Fluotar 0, 5, 20×/0.5 NA dry objective (Wetzlar, Germany), captured with an Olympus C4040 camera, and processed with Olympus DP-Soft 3.2. Images in panels B-D were viewed under a Leica DMRA2 fluorescence microscope with Leica HC PL Fluotar 0, 5, 20×/0.5 NA dry objective, captured using Photometrics CoolSNAP HQ, (Photometrics, Friedland, Denmark), and processed with Metamorph 4.6-5 (Molecular Devices, Sunnyvale, CA).
Soluble Dll4 inhibits Dll4-Notch signaling
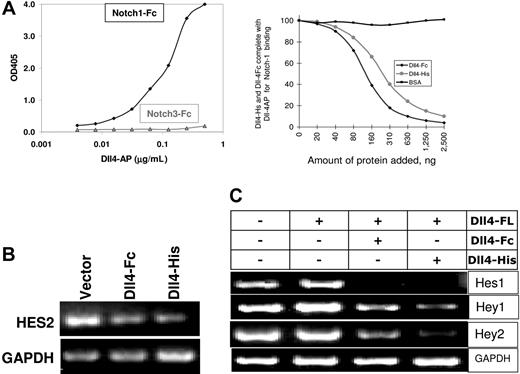
We next wished to determine if soluble Dll4 could antagonize Notch activation. Extracellular domain of human Dll4 fused either to AP, Fc, or His tag were expressed in mammalian cells, purified, and determined to bind Notch1-Fc (Figure 2A) and Notch4-Fc (data not shown) but not Notch3-Fc (Figure 2A) or Fc alone (data not shown). Notch activation is dependent on the expression of Dll4 in the cellular context. To test that the soluble forms of Dll4 do not induce Notch activation, we introduced various Dll4 constructs in endothelial cells and examined the induction of downstream Notch-responsive genes (Hey1, Hey2, Hes1, and Hes2) by RT-PCR. Representative data for the absence of Notch activation are shown by the lack of Hes2 induction by Dll4-Fc or Dll4-His (Figure 2B). Hes2 is downstream of Notch and induced by full-length Dll4 when presented in the cellular context (Figure 2C). We next determined if sDll4 can inhibit the activity of cellular Dll4 in inducing Notch signaling. To this end, full-length Dll4 (Dll4-FL) was introduced into ChoK cells and cocultured with HUVECs expressing target Notch1 and Notch 4. Dll4-FL induces Notch-regulated genes, Hey1, Hey2, Hes1, and Hes2 (Figure 2C), in human endothelial cells using human gene-specific primer pairs. In identical experiments, addition of human sDll4-Fc and sDll4-His blocked Dll4-FL–induced activation of Hey1, Hey2, Hes1, and Hes2 (Figure 2C). Thus soluble Dll4 functions as an antagonist of Dll4-Notch signaling. Quantitation of gene expression showed that Dll4-Fc inhibited Hey1, Hey2, Hes1, and Hes2 to 69%, 26%, 29%, and 46% of control, respectively, while sDll4-His reduced their expression to 48%, 3%, 10%, and 28%, respectively.
Biochemical properties of sDll4. (A) Notch-Fc fusion protein was coated directly on enzyme-linked immunosorbent assay (ELISA) plates. sDll4-AP was allowed to bind Notch-Fc, and the bound Dll4 was quantitated by the addition of AP substrate. sDll4-AP bound efficiently to Notch1 and not Notch3 (left panel). Binding of sDll-4Fc and sDll4-His to Notch1 was examined. (B) HUVECs were transfected with expression vectors for sDll4-Fc, sDll4-His, or vector alone. Notch-responsive Hes-2 gene expression was not induced by sDll4 proteins. (C) Notch activation measured by the induction in Hes-1, Hey-1, and Hes-2 when HUVECs were cocultivated with ChoK expressing Dll4-FL (full length). Addition of recombinant sDll4-Fc and sDll4-His reduced the induction of Notch responsive genes. Two independent experiments produced similar results.
Biochemical properties of sDll4. (A) Notch-Fc fusion protein was coated directly on enzyme-linked immunosorbent assay (ELISA) plates. sDll4-AP was allowed to bind Notch-Fc, and the bound Dll4 was quantitated by the addition of AP substrate. sDll4-AP bound efficiently to Notch1 and not Notch3 (left panel). Binding of sDll-4Fc and sDll4-His to Notch1 was examined. (B) HUVECs were transfected with expression vectors for sDll4-Fc, sDll4-His, or vector alone. Notch-responsive Hes-2 gene expression was not induced by sDll4 proteins. (C) Notch activation measured by the induction in Hes-1, Hey-1, and Hes-2 when HUVECs were cocultivated with ChoK expressing Dll4-FL (full length). Addition of recombinant sDll4-Fc and sDll4-His reduced the induction of Notch responsive genes. Two independent experiments produced similar results.
Soluble Dll4 induces sprouting and tube formation
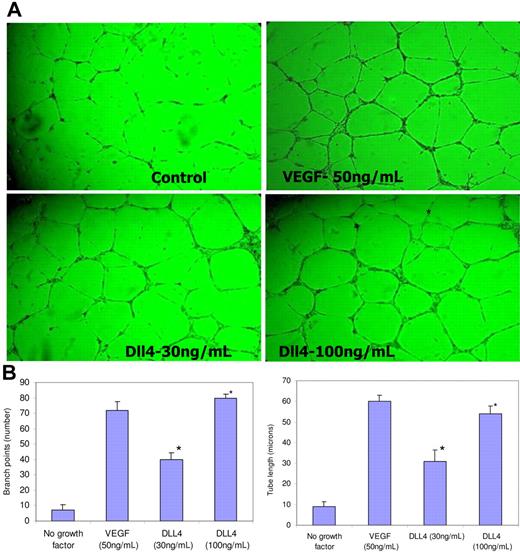
We intended to determine if soluble Dll4 could mimic the dll4 loss-of-function phenotype. sDll4-Fc and sDll4-His were tested in tube formation assays using endothelial cells placed on polymerized Matrigel to promote the formation of tubelike structures. Minimal amounts of tubes were formed in the absence of growth factors, and abundant tube formation was observed with the addition of VEGF. Dll4-His showed a dose-dependent induction of tube formation in the absence of additional growth factors (Figure 3). Quantitative measurement of junction formation and length of tubes increased in a dose-dependent manner (Figure 3). Similar results were seen with Dll4-Fc (data not shown).
sDll4 induces tubule formation in vitro. (A) HUVECs were cultured on standard Matrigel in growth factor–deficient conditions in triplicates in 2 independent experiments with either sDll4 or VEGF for 24 hours. Shown are representative pictures from triplicate wells repeated twice. (B) Quantitative analysis for tube length and the number of junctions in sDll4-treated HUVECs (Bioquant Image Analysis; mean ± SEM from triplicate wells in 2 repetition experiments). Similar results were seen with human arterial endothelial cell assay (data not shown). *P < .05 compared to no growth factor. Photomicrographs in panel A were taken with a Nikon Plan Fluor ∞, 0.17, 4×/0.12 NA objective and 10× eyepiece and processed with Image-Pro Plus 6.0 (Media Cybernetics, Bethesda, MA).
sDll4 induces tubule formation in vitro. (A) HUVECs were cultured on standard Matrigel in growth factor–deficient conditions in triplicates in 2 independent experiments with either sDll4 or VEGF for 24 hours. Shown are representative pictures from triplicate wells repeated twice. (B) Quantitative analysis for tube length and the number of junctions in sDll4-treated HUVECs (Bioquant Image Analysis; mean ± SEM from triplicate wells in 2 repetition experiments). Similar results were seen with human arterial endothelial cell assay (data not shown). *P < .05 compared to no growth factor. Photomicrographs in panel A were taken with a Nikon Plan Fluor ∞, 0.17, 4×/0.12 NA objective and 10× eyepiece and processed with Image-Pro Plus 6.0 (Media Cybernetics, Bethesda, MA).
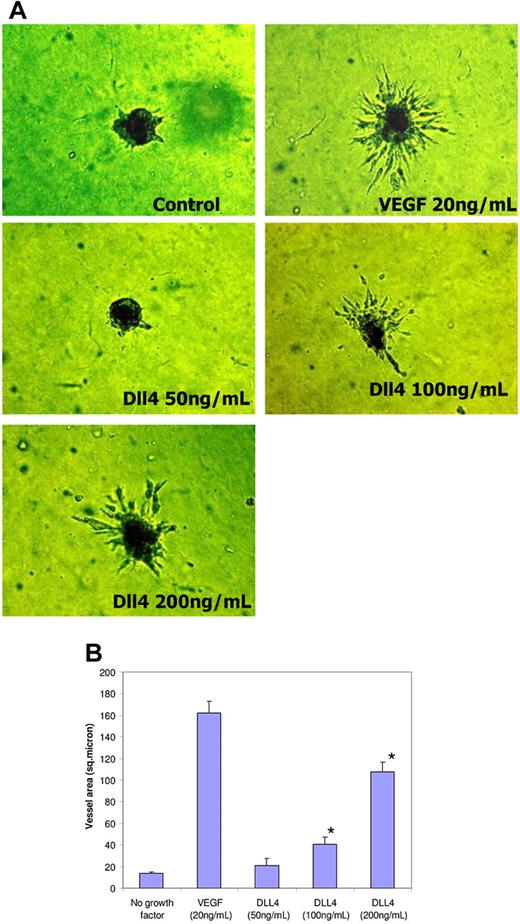
We next extended these studies in an assay in which endothelial cell spheroids placed in collagen show proliferation and outward migration of vessellike structures. VEGF profoundly increases sprouting, while no sprouts were observed in growth factor–deficient conditions. sDll4-Fc and sDll4-His when examined alone induced sprouting in a dose-dependent manner. Representative data with sDll4-His are shown in Figure 4. Similar results were seen with sDll4-Fc (data not shown). These data indicate that inhibition of endogenous Notch signaling leads to increased endothelial cell migration and organization to make tubes and promotes vascular sprouting that is analogous to the observed murine Dll4+/− phenotype.
sDll4 induces sprouting in endothelial cell spheroids in vitro. (A) HUVEC spheroids were cultured on Matrigel in growth factor–deficient conditions in triplicates with either sDll4 or VEGF for 24 to 72 hours. Shown are representative pictures using sDll4-His from triplicate wells repeated twice. (B) Quantitative analysis for vascular area is shown (Bioquant Image Analysis; mean ± SEM from triplicate wells in 2 repetition experiments). Similar results were seen with sDll4-Fc (data not shown). Photomicrographs were taken using a Nikon Coolpix 5000 camera and a Carl Zeiss Invertoskop microscope with a Nikon Plan Fluor ∞, 0.17, 4×/0.12 NA objective and 10× eyepiece and processed with Image-Pro Plus 6.0. Experiments were repeated with similar results. (B) *P < .05 compared to no growth factor.
sDll4 induces sprouting in endothelial cell spheroids in vitro. (A) HUVEC spheroids were cultured on Matrigel in growth factor–deficient conditions in triplicates with either sDll4 or VEGF for 24 to 72 hours. Shown are representative pictures using sDll4-His from triplicate wells repeated twice. (B) Quantitative analysis for vascular area is shown (Bioquant Image Analysis; mean ± SEM from triplicate wells in 2 repetition experiments). Similar results were seen with sDll4-Fc (data not shown). Photomicrographs were taken using a Nikon Coolpix 5000 camera and a Carl Zeiss Invertoskop microscope with a Nikon Plan Fluor ∞, 0.17, 4×/0.12 NA objective and 10× eyepiece and processed with Image-Pro Plus 6.0. Experiments were repeated with similar results. (B) *P < .05 compared to no growth factor.
sDll4 induces vascularization of Matrigel plugs in vivo
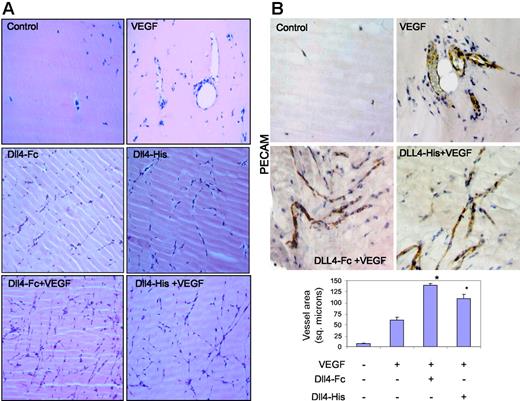
To further demonstrate that sDll4 can directly inhibit angiogenesis in vivo, we performed a murine Matrigel plug experiment. Matrigel was supplemented with VEGF, sDll4-Fc, sDll4-His, or various combinations, and injected into the ventral abdominal subcutaneous tissue of Balb/C nu/nu mice. Matrigel plugs without growth factors had virtually no vascularization after 6 days (Figure 5A), while VEGF recruited endothelial cells and formed various stages of vascular structures including those with open lumen containing red blood cells throughout the plug. sDll4 in the context of VEGF showed a marked increase in vascular structures (Figure 5), which appeared like thin strings, and mostly lacked lumen. Similarly, sDll4 alone, in the absence of VEGF, was also capable of inducing angiogenesis. Immunochemical examination with PECAM further demonstrates the contrast between VEGF-induced large vessel filled with red blood cells and sDll4-induced vessels that express PECAM but lack lumen and lack perfusion defined by the presence of red blood cells (Figure 5B). Hemoglobin was also quantitated specifically by the Drabkin method using a Drabkin reagent kit and fold change was compared with control measurement represented as 1. Median hemoglobin levels thus were 1, 9.7, 2.5, and 3 g/L/g plug in control, VEGF, Dll4-Fc, and Dll4-His groups, respectively. There was a near 4-fold decrease in hemoglobin concentration in sDll4-containing Matrigel plugs, compared to VEGF alone.
sDll4 induces vessel response but lacks perfusion in murine Matrigel assay. (A) Matrigel was injected subcutaneously into Balb/C nu/nu mice. After 6 days, plugs were removed and processed in paraffin. Individual sections were stained with H&E, and representative photographs at × 20 magnification from triplicate plugs in 2 independent experiments are shown. (B) Matrigel plugs were stained for PECAM. Photomicrographs were taken with an Olympus BX51 microscope with an Olympus UPlan FL ∞, 0.17 20×/0.5 NA dry objective mounted with a Retiga 2000R camera (QImaging, Burnaby, BC, Canada) and processed with Image-Pro Plus 6.0. Quantitation of vascularized area averaged (± SEM) from all plugs (Scion Image software; Scion, Frederick, MD) in bar graph. *P < .05 compared to no growth factor.
sDll4 induces vessel response but lacks perfusion in murine Matrigel assay. (A) Matrigel was injected subcutaneously into Balb/C nu/nu mice. After 6 days, plugs were removed and processed in paraffin. Individual sections were stained with H&E, and representative photographs at × 20 magnification from triplicate plugs in 2 independent experiments are shown. (B) Matrigel plugs were stained for PECAM. Photomicrographs were taken with an Olympus BX51 microscope with an Olympus UPlan FL ∞, 0.17 20×/0.5 NA dry objective mounted with a Retiga 2000R camera (QImaging, Burnaby, BC, Canada) and processed with Image-Pro Plus 6.0. Quantitation of vascularized area averaged (± SEM) from all plugs (Scion Image software; Scion, Frederick, MD) in bar graph. *P < .05 compared to no growth factor.
sDll4 inhibits the growth of human tumors in athymic mice
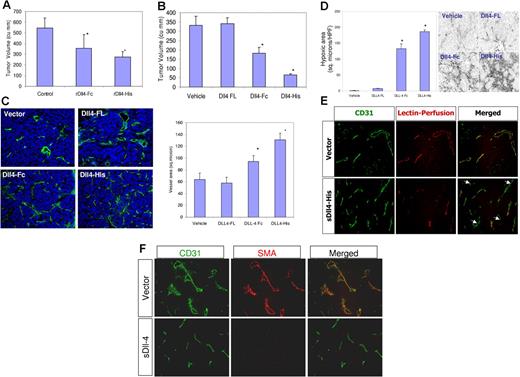
Tumor vessels have distinctive gene expression profile over resting vessels. Dll4 is one of the genes induced in tumor vessels in certain human and murine tumors (Figure 1C). Dll4 induction may be a generalized feature of tumor vessels, one that could be beneficial for tumor growth. Dll4 expression is seen predominantly in the tumor vasculature. To determine the effect of sDll4 on tumor cells, tumor cell viability in vitro with various concentrations of sDll4 was tested (HT29, MCF-7, SCC-15, B16, PC3, and KS-SLK cell lines), and no effect was observed (data not shown). Given the ability of Dll4 to profoundly affect angiogenesis in vivo, and the observed sDll4 alteration of the vascular response, we speculated that sDll4 may modulate tumor growth in vivo. We therefore examined the activity of sDll4 in vivo in tumor xenograft models. HT29 (human colon carcinoma cell line) and KS-SLK (human Kaposi sarcoma cell line) cells were premixed with Matrigel-containing vehicle or sDll4 and implanted subcutaneously. Compared to control tumors, xenografts supplemented with sDll4 exhibited a significantly reduced tumor growth over 2 weeks (Figure 6A). Similar results were obtained in KS-SLK. Median tumor volume of control tumors at 2 weeks was 585 mm3, while that of tumors in Matrigel containing sDll4-His was 267 mm3.
sDll4 inhibits the tumor growth in a murine tumor xenograft model. (A) Mice (n = 6/group) were implanted with 1 × 106 HT29 cells in a Matrigel preparation with PBS or sDll4-Fc or sDll4-His (5 μg/mL) and tumor volumes (mean ± SEM) were measured after 2 weeks; tumors were then harvested and analyzed. Tumor volumes were significantly smaller in the sDll4 arm. *P < .05 compared to control. The experiment was repeated twice. (B) In assessing the effect of endogenous expression of sDll4, HT29 cells were transfected with expression vector with Dll4-FL, sDll4-Fc, sDll4-His, or vector alone. Coexpression of truncated CD4 was done to allow sorting of the transfected cells. Equal numbers of the transfected cells were implanted in mice (n = 6/group). Tumor volumes (mean ± SEM) were assessed. Tumor volumes were significantly smaller in the sDll4 groups. *P < .05 compared to vehicle. (C) Microvasculature was assessed by PECAM immunostaining, and the blood vessel volume was quantitated as described in “Materials and methods.” Mean ± SEM. *P < .05 compared to vehicle. (D) Hypoxy probe was infused prior to tumor harvest; tumor sections were then probed with MAb and fluorescent-labeled secondary antibody as described in “Materials and methods.” Hypoxic areas were quantitated (mean ± SEM) using ImageJ as described in “Materials and methods.” All values are expressed as mean ± SEM. *P < .01. Photomicrographs were taken with a Nikon Eclipse 80 microscope with a Nikon Plan Fluor ∞, 0.17 10×/0.3 NA dry objective mounted with a Photometrics CoolSNAP camera and processed with Metamorph V 6.3r2. (E) Vascular perfusion was determined by injecting fluorescent-labeled lectin 10 to 15 minutes prior to killing mice and harvesting tumors. Lectin was localized to perfused areas, while blood vessels were delineated with PECAM staining. Lectin and PECAM colocalized in control group, while sDll4 group showed marked deficiency of perfusion. (F) Localization of α-SMA in tumor vessel. Control group showed colocalization of α-SMA and PECAM, while sDll4 group had paucity of α-SMA–positive cells in the microvessels.
sDll4 inhibits the tumor growth in a murine tumor xenograft model. (A) Mice (n = 6/group) were implanted with 1 × 106 HT29 cells in a Matrigel preparation with PBS or sDll4-Fc or sDll4-His (5 μg/mL) and tumor volumes (mean ± SEM) were measured after 2 weeks; tumors were then harvested and analyzed. Tumor volumes were significantly smaller in the sDll4 arm. *P < .05 compared to control. The experiment was repeated twice. (B) In assessing the effect of endogenous expression of sDll4, HT29 cells were transfected with expression vector with Dll4-FL, sDll4-Fc, sDll4-His, or vector alone. Coexpression of truncated CD4 was done to allow sorting of the transfected cells. Equal numbers of the transfected cells were implanted in mice (n = 6/group). Tumor volumes (mean ± SEM) were assessed. Tumor volumes were significantly smaller in the sDll4 groups. *P < .05 compared to vehicle. (C) Microvasculature was assessed by PECAM immunostaining, and the blood vessel volume was quantitated as described in “Materials and methods.” Mean ± SEM. *P < .05 compared to vehicle. (D) Hypoxy probe was infused prior to tumor harvest; tumor sections were then probed with MAb and fluorescent-labeled secondary antibody as described in “Materials and methods.” Hypoxic areas were quantitated (mean ± SEM) using ImageJ as described in “Materials and methods.” All values are expressed as mean ± SEM. *P < .01. Photomicrographs were taken with a Nikon Eclipse 80 microscope with a Nikon Plan Fluor ∞, 0.17 10×/0.3 NA dry objective mounted with a Photometrics CoolSNAP camera and processed with Metamorph V 6.3r2. (E) Vascular perfusion was determined by injecting fluorescent-labeled lectin 10 to 15 minutes prior to killing mice and harvesting tumors. Lectin was localized to perfused areas, while blood vessels were delineated with PECAM staining. Lectin and PECAM colocalized in control group, while sDll4 group showed marked deficiency of perfusion. (F) Localization of α-SMA in tumor vessel. Control group showed colocalization of α-SMA and PECAM, while sDll4 group had paucity of α-SMA–positive cells in the microvessels.
We next studied the effect of sDll4 when produced by tumor cells. HT29 and KS-SLK cells were transfected with expression vectors to produce Dll4-FL, sDll4-Fc, and sDll4-His, and expression of each protein was confirmed in Western blot assays (data not shown). Coexpression of truncated CD4 allowed sorting of transfected cells to more than 90% purity. Equal numbers of cells (1 × 106 per injection site) were implanted in athymic mice (6-8 tumors per group), and tumor volume was measured for 2 weeks. Tumor volume was similar in vector alone and Dll4-FL, while sDll4-Fc and sDll4-His had markedly reduced tumor volume (more than 70% reduction with sDll4-His) (Figure 6B). Tumors harvested at the time were examined for vascular density using PECAM immunostaining. Tumors expressing Dll4-FL or vector alone showed highly structured vessels (Figure 6C). In contrast, sDll4-expressing tumors showed marked changes in the vessel architecture. There were many more branching points in sDll4-expressing tumor vasculature compared to vector alone or Dll4-FL (Figure 6). Similar results were obtained in KS-SLK tumor xenografts. Remarkably, the vessels appeared thin and often lacking apparent lumen. These characteristics were reminiscent of blood vessel branching in Dll4+/− mice, and in Matrigel plugs impregnated with sDll4. Consistent with poorly forming thin vessel lacking lumen, we examined the areas of hypoxia. Analysis of hypoxia focused on viable tumor regions only. There were large and wide areas of hypoxia in sDll4-Fc and sDll4-His. Quantitation of these areas showed marked increase in hypoxic regions of sDll4-expressing tumors compared to both Dll4-FL– and vector-expressing tumors (Figure 6D). Tumor perfusion was also measured using fluorescent-labeled lectin, which binds to the luminal surface of the blood vessels. There was very limited perfusion in the sDll4-expressing tumors compared to Dll4-FL– and vector-transfected cells (Figure 6E). In addition, we determined the presence of pericytes on newly forming tumor vessels by localizing α-SMA expression. Tumor vessels in wild-type mice showed normal pericyte coverage, whereas tumor vessels in Dll4+/− mice present a dramatic reduction of pericyte coverage as determined by the number of α-SMA–positive cells lining the endothelial cells. sDll4 similarly reduced the number of pericytes in tumor vessels in athymic mice bearing human tumors. Taken together, these data provide strong evidence for the role of Dll4 in pericyte recruitment to newly forming vessels.
Discussion
Dll4, Notch1, and Notch4 are expressed in endothelial cells. Notch1/Notch4 double-mutant embryos show severe vascular remodeling defects,9,14 and putative downstream targets of Notch pathway including Hey1/Hey2 double mutants develop vascular defects with lesser severity.14,16 Dll4 thus profoundly effects vascular development and vessel maturation. Notably, Dll4−/− and most (∼ 70% on CD1, 100% on C57BL6 and 129Sv/J genetic backgrounds) Dll4+/− mutant embryos die at midgestation due to defective vascular development. Lethal haploinsufficiency of Dll4 is analogous to that of VEGF,5,21 which lies upstream of Notch signaling in arterial development.15
We thus focused on Dll4 in vascular development. Analysis of vasculature in the heterozygote mice has been revealing in that the vasculature develops normally until E8.5 when the defects become apparent and manifest by increased vascular network combined with less mature large vessels that eventually branch and fuse with adjacent vessels and result in short circuit and failing circulatory system. We wished to determine if Dll4 deficiency was manifest in a small fraction of mice that survives to adult life. Use of tumor cell implants in Dll4+/− mice was compared to age-matched wild-type mice for localized vascular response. Vascular response in adult Dll4+/− mice was similar to the embryonic defects including enhanced vascular density, nearly 2-fold higher than in wild-type mice. Secondly, unlike the wild-type mice, the vessels in the mutants have narrow caliber and lack hierarchic branching pattern (Figure 1A-B). Dll4 may thus be required for functions in branching and maturation of newly forming vessels. Furthermore, recruitment of pericytes to the newly forming vessels was markedly reduced in Dll4+/− mice tumor vessels. Similar defect was observed in tumor vessels in response to sDll4. It is thus possible that some of the observed defects in Dll4-deficient signaling are due to the paucity of pericytes and smooth muscle.
VEGF and Dll4 have also been shown to be up-regulated by hypoxia,22,23 which is one of the environmental factors that regulates vascular patterning and growth. We thus wished to determine if Dll4 expression was changed in tumor vessels compared to those in the adjacent normal tissue. Dll4+/− mice tumor implants were examined for Dll4 expression. LacZ insertion in the Dll4 targeting vector allows its expression under the Dll4 promoter. Thus β-gal staining highlighted a few well-organized vessels in the normal subcutaneous tissue. In the tumor tissue, however, we observed a marked increase in vascular β-gal staining and in the density of vascular structures. This role of Dll4 in modifying vascular response and its overexpression in tumor vessels may provide a therapeutic opportunity to alter tumor response.
Dll4 induces Notch signaling when presented in the cellular context, by activating Notch processing and release of the intracellular domain, which is subsequently translocated to the nucleus to modify expression of target genes including Hey and Hes.24 Various Dll4 variants containing only the extracellular domain indeed block Dll4-induced Hey and Hes expression. sDll4 thus allowed us to assess the consequences of blocking Dll4-Notch signaling in various distinct functions. sDll4 treatment recapitulates several features seen in Dll4+/− mutant mice including increased endothelial cell tube formation and vascular sprouting. Using Matrigel plug assays, we noticed that sDll4 markedly increased endothelial cell migration. Endothelial cells created stringlike vascular structures, which do not reveal a prominent lumen and display a notable paucity of red blood cells, unlike those observed upon VEGF treatment, which produces large vascular structures with open lumen filled with red blood cells. sDll4 thus modifies newly forming vessels that are highly deficient in perfusion. Overexpression of Dll4 in tumor vessels and profound alteration of newly forming vessels by disruption of Dll4-Notch signaling provides an opportunity to influence tumor growth. Use of sDll4 indeed showed a marked reduction in tumor growth. Inhibition of tumor growth was also reproduced by engineering tumor cells to produce soluble Dll4. Both sDll4-Fc and sDll4-His markedly reduced the tumor growth combined with changes in tumor vessels similar to those seen in Dll4+/− mutant mice and to the effect of sDll4 in the Matrigel assays. Perfusion deficiency was confirmed by examining the areas of hypoxia in the tumor, which showed a marked increase in sDll4-expressing tumor cells. In summary, Dll4 has profound effect in vascular biology. It appears to be essential for regulating migration of vessels in response to VEGF, and necessary for maturation of newly forming vessels (including regulation of lumen formation) and vascular perfusion. Inhibition of Dll4 function thus has a paradoxical effect in inducing an excessive density of newly forming vessels, but defective vessel maturation, lumen formation, recruitment of pericytes, and perfusion. It raises many questions as to how Dll4 regulates endothelial cell migration, vessel branching, lumen size, and endothelial interaction with pericytes and matrix proteins. Inhibition of Dll4 with soluble protein or neutralizing antibodies or knock down of gene expression with short interference RNA may provide opportunities to treat cancer and vascular proliferative diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by NCI-RO1 CA 079218-07 (P.S.G.), Women's Cancer Research Fund, Mesothelioma Foundation of America, VasGene Therapeutics, and Fundação para a Ciência e Tecnologia (FCT, Portugal) grant POCTI/CVT/48766/2002 (A.D.). A.T., R.B., and D.D. are recipients of FCT PhD studentships. C.B. is an FCT postdoctoral fellow. The authors wish to thank Dr Adrian Harris for the gift of S180 cells and Loubna Hassanieh, PhD, for excellent technical assistance.
National Institutes of Health
Authorship
Contribution: P.S.G., S.R.K., W.J., and A.D. designed the studies and wrote the paper; S.R.K., W.J., V.K., J.S.S., P.S.G., A.T., R.B., D.D., C.B., E.J.L., and A.D. performed research; and A.D., W.J., and P.S.G. analyzed data.
Conflict-of-interest disclosure: W.J. and V.K. are employees of VasGene Therapeutics.
Correspondence: Parkash S. Gill, Norris Cancer Center, Rm 6332, 1441 Eastlake Ave, Los Angeles, CA; e-mail: parkashg@usc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal