Abstract
Germinal center (GC) and non-GC phenotypes are predictors of outcome in diffuse large B-cell lymphoma (DLBCL) and can be used to stratify chemotherapy-treated patients into low- and high-risk groups. To determine how combination of rituximab with chemotherapy influences GC-associated clinical outcome, GC and non-GC phenotypes were identified immunohistochemically from samples of 90 de novo DLBCL patients treated with rituximab in combination with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)–like regimen (immunochemotherapy). One hundred and four patients previously treated with chemotherapy served as a control group. Consistent with previous studies, chemotherapy-treated patients with immunohistochemically defined GC phenotype displayed a significantly better overall (OS) and failure-free survival (FFS) than the non-GC group (OS, 70% vs 47%, P = .012; FFS, 59% vs 30%, P = .001). In contrast, immunohistochemically defined GC phenotype did not predict outcome in immunochemotherapy-treated patients (OS, 77% vs 76%, P = ns; FFS, 68% vs 63%, P = ns). In comparison, International Prognostic Index (IPI) could separate the high-risk patients from low- and intermediate-risk groups (OS, 84% vs 63%, P = .030; FFS, 79% vs 52%, P = .028). We conclude that rituximab in combination with chemotherapy seems to eliminate the prognostic value of immunohistochemically defined GC- and non-GC phenotypes in DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of all non-Hodgkin lymphomas (NHLs). It is an aggressive disease, of which less than half can be cured with anthracyclin-based combination chemotherapy. Recently, however, a significant improvement of the outcome of both young and elderly patients has been obtained by combining a monoclonal anti-CD20 antibody, rituximab, with chemotherapy (immunochemotherapy).1–4 Despite the advances, response to treatment is heterogeneous and outcome is often unpredictable. Furthermore, treatment is costly. These facts raise the need to identify more accurately the patients who benefit from immunochemotherapy
In DLBCL, International Prognostic Index (IPI)5 is considered to be the most important prognostic factor for survival, and therefore the strongest indicator for identification of high-risk patients, who are unlikely to be cured with standard chemotherapy. However, the 5 clinical characteristics of IPI (age, WHO performance status, stage, extranodal involvement, and LDH level) do not provide any information of the biologic features of DLBCL, nor predict the responsiveness to therapies. Therefore, there is a need for biomarkers that accurately predict outcome of these patients.
Individual biomarkers may provide prognostic information for patients with DLBCL. For example, expression of Bcl-2 and Bcl-6 has been associated with adverse and favorable outcome of chemotherapy-treated patients, respectively.6–12 Different studies, however, yield conflicting and inconclusive results, reflecting the heterogeneity of the patient populations, as well as technical factors related to staining, interpretation, and scoring of the data. In addition, single genes or molecules may simply be unable to reflect the heterogeneity of DLBCL accurately. A development of DNA microarray techniques has supplied a more comprehensive tool to explore the relation of prognosis and the molecular features of NHLs. For example, as initially identified by gene expression profiling, DLBCL appears to include at least 3 distinct subtypes, germinal center–type (GC), activated B-cell–type, and additional type 3, which differ in cell of origin and survival parameters.13,14 Recently, these results have been translated into a clinically applicable approach using immunohistochemistry. Based on the expression of Bcl-6, CD10, and MUM-1, DLBCL can be subdivided into GC and non-GC subtypes, which have been shown to be important outcome predictors for chemotherapy-treated patients.15–17 However, the immunohistochemically defined cell-of-origin distinction appears not to predict the outcome of patients with relapsed or refractory DLBCL.18 To date, it is not known whether the cell of origin is a relevant prognostic factor for immunochemotherapy-treated DLBCL. In the present study, we addressed how the addition of rituximab into CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)–like regimen has improved the outcome in our institutes and whether immunohistochemically defined GC versus non-GC distinction of DLBCL could be used to predict a patient's outcome in response to a combination of rituximab and chemotherapy.
Patients, materials, and methods
Patients and treatments
The study population consisted of 194 de novo DLBCL patients treated at the University Hospitals of Helsinki and Uppsala between 1994 and 2004. The characteristics of the patients with respect to age, sex, stage, IPI, and treatment are listed in Table 1, and in Tables S1-S2 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Ninety patients received immunochemotherapy (postrituximab era), whereas 104 patients treated with chemotherapy before rituximab was adapted into clinical routine served as a control group (prerituximab era). All patients received anthracyclin-based regimens. Of these, the majority was treated with CHOP (n = 113) or CHOEP (CHOP plus etoposide; n = 49). Other regimens (n = 32) included VACOP (etoposide, doxorubucin, cyclophosphamide, vincristine, and prednisone), MACOP-B (methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin), EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin), and CNOP (cyclophosphamide, mitoxantrone, vincristine, and prednisone). The median follow-up for chemotherapy- and immunochemotherapy-treated patients was 52 and 27 months, respectively. The study protocol and sampling were approved by institutional review boards of the departments of oncology in Helsinki and Uppsala University Hospitals and Finnish National Authority for Medicolegal Affairs. Due to the retrospective nature of the study, the patients were not contacted directly.
Patient characteristics according to immunohistochemically defined GC and non-GC phenotypes for rituximab and control group
| Characteristic . | Rituximab group . | Control group . | P, all . | ||||
|---|---|---|---|---|---|---|---|
| All . | GC . | Non-GC . | All . | GC . | Non-GC . | ||
| No. of patients (%) | 90 (100) | 50 (55.6) | 40 (44.4) | 104 (100) | 47 (45.2) | 57 (54.8) | .20 |
| Median age, y (range) | 60.9 (23-82) | 59.9 (27-77) | 62.3 (23-82) | 62.0 (25-83) | 60.0 (25-83) | 67.0 (29-80) | .64 |
| Age, no. (%) | |||||||
| Younger than 60 y | 40 (55.6) | 25 (50.0) | 15 (37.5) | 44 (42.3) | 22 (46.8) | 22 (38.6) | .77 |
| 60 y or older | 50 (44.4) | 25 (50.0) | 25 (62.5) | 60 (57.7) | 25 (53.2) | 35 (61.4) | |
| Sex, no. (%) | |||||||
| Male | 48 (53.3) | 21 (42.0) | 27 (67.5) | 59 (56.7) | 24 (51.1) | 35 (61.4) | .67 |
| Female | 42 (46.7) | 29 (58.0) | 13 (32.5) | 45 (43.3) | 23 (48.9) | 22 (38.6) | |
| Stage, no. (%) | |||||||
| I to II | 24 (26.7) | 15 (30.0) | 9 (22.5) | 33 (31.7) | 14 (29.8) | 19 (33.3) | .53 |
| III to IV | 66 (73.3) | 35 (70.0) | 31 (77.5) | 71 (68.3) | 33 (70.2) | 38 (66.7) | |
| IPI, no. (%) | |||||||
| 0 to 2 | 54 (60.0) | 32 (64.0) | 22 (55.0) | 65 (63.1) | 31 (66.0) | 34 (60.7) | .77 |
| 3 to 5 | 36 (40.0) | 18 (36.0) | 18 (45.0) | 38 (36.9) | 16 (34.0) | 22 (39.3) | |
| Missing | 0 | 0 | 0 | 1 | 0 | 1 | |
| Treatment, no. (%) | |||||||
| CHOP | 53 (58.9) | 29 (58.0) | 24 (60.0) | 60 (57.7) | 24 (51.1) | 36 (63.1) | .89 |
| Other | 37 (41.1) | 21 (42.0) | 16 (40.0) | 44 (42.3) | 23 (48.9) | 21 (36.9) | |
| Characteristic . | Rituximab group . | Control group . | P, all . | ||||
|---|---|---|---|---|---|---|---|
| All . | GC . | Non-GC . | All . | GC . | Non-GC . | ||
| No. of patients (%) | 90 (100) | 50 (55.6) | 40 (44.4) | 104 (100) | 47 (45.2) | 57 (54.8) | .20 |
| Median age, y (range) | 60.9 (23-82) | 59.9 (27-77) | 62.3 (23-82) | 62.0 (25-83) | 60.0 (25-83) | 67.0 (29-80) | .64 |
| Age, no. (%) | |||||||
| Younger than 60 y | 40 (55.6) | 25 (50.0) | 15 (37.5) | 44 (42.3) | 22 (46.8) | 22 (38.6) | .77 |
| 60 y or older | 50 (44.4) | 25 (50.0) | 25 (62.5) | 60 (57.7) | 25 (53.2) | 35 (61.4) | |
| Sex, no. (%) | |||||||
| Male | 48 (53.3) | 21 (42.0) | 27 (67.5) | 59 (56.7) | 24 (51.1) | 35 (61.4) | .67 |
| Female | 42 (46.7) | 29 (58.0) | 13 (32.5) | 45 (43.3) | 23 (48.9) | 22 (38.6) | |
| Stage, no. (%) | |||||||
| I to II | 24 (26.7) | 15 (30.0) | 9 (22.5) | 33 (31.7) | 14 (29.8) | 19 (33.3) | .53 |
| III to IV | 66 (73.3) | 35 (70.0) | 31 (77.5) | 71 (68.3) | 33 (70.2) | 38 (66.7) | |
| IPI, no. (%) | |||||||
| 0 to 2 | 54 (60.0) | 32 (64.0) | 22 (55.0) | 65 (63.1) | 31 (66.0) | 34 (60.7) | .77 |
| 3 to 5 | 36 (40.0) | 18 (36.0) | 18 (45.0) | 38 (36.9) | 16 (34.0) | 22 (39.3) | |
| Missing | 0 | 0 | 0 | 1 | 0 | 1 | |
| Treatment, no. (%) | |||||||
| CHOP | 53 (58.9) | 29 (58.0) | 24 (60.0) | 60 (57.7) | 24 (51.1) | 36 (63.1) | .89 |
| Other | 37 (41.1) | 21 (42.0) | 16 (40.0) | 44 (42.3) | 23 (48.9) | 21 (36.9) | |
Immunohistochemistry
Immunohistochemical stainings were performed on formalin-fixed, paraffin-embedded, 4-μm sections from patient samples collected at the time of diagnosis. The sections were deparaffinized, rehydrated, treated in an autoclave in sodium citrate (pH 6.0), and washed with phosphate-buffered saline. Stainings for CD10 (Novocastra Laboratories, Newcastle upon Tyne, United Kingdom), Bcl-6, and MUM-1 (Dako Cytomation, Glostrup, Denmark) were performed at room temperature using antibody dilutions 1:200, 1:20, and 1:100, respectively. The immunoreactions were visualized with ABC (avidin-biotin-peroxidase) method and sections counterstained with Mayer hematoxylin. Immunoreactivity was determined without any knowledge of the survival or other clinical data. The scoring was based on the algorithm described by Hans et al,16 and validated by others.15,17 Accordingly, the samples were scored positive for CD10, Bcl-6, and MUM-1, if 30% or more of the tumor cells were stained with an antibody. The cases were assigned to the immunohistochemically defined GC group if CD10 alone or together with Bcl-6 was positive. If both CD10 and BCL-6 were negative, the cases were classified to the immunohistochemically defined non-GC group. If CD10 was negative and Bcl-6 positive, the classification was based on MUM-1 expression; if MUM-1 was negative, the cases were assigned to the immunohistochemically defined GC group, whereas MUM-1–positive cases were classified to the immunohistochemically defined non-GC group. The samples were analyzed independently by 2 pathologists (M.-L.K.-L. and R.-M.A.) and 2 students (H.N. and M.B.), and disagreements resolved by a joint review on a multihead microscope.
To test the reproducibility of the immunohistochemical data, a subset of 40 samples was restained and evaluated for CD10, and Bcl-6 positivities. A comparison of immunoreactivities between 2 series demonstrated a 100% and 93.1% reproducibility for CD10 and Bcl-6, with kappa values of 1.0 and 0.601, respectively. In all cases, a measure of agreement was highly significant (P < .001).
Statistical analyses
The chi-square test was used to assess differences in the frequency of individual prognostic factors. Analyses of the reproducibility of immunohistochemical stainings were carried out with a kappa statistic. Survival rates were estimated by the Kaplan-Meier method and the differences were compared by the log-rank test. Overall survival (OS) was measured from the date of diagnosis until the last follow-up or death from any cause. Failure-free survival (FFS) was determined as an interval between the date of diagnosis and relapse, or death. Cox multivariate analysis was used to test the prognostic impact of identified genes on OS and FFS. Statistical data processing was carried out with SPSS software for Macintosh (SPSS, Chicago, IL). Probability values less than .05 were considered statistically significant. All P values were 2-tailed.
Results
Immunohistochemically defined cell of origin in relation to patient and disease characteristics
Of the 194 patients included into study, the rituximab group consisted of 90 patients, whereas 104 patients treated with chemotherapy before rituximab was adapted into clinical practice served as a control group. Patient and disease characteristics were well balanced between rituximab and control groups (Table 1). The distribution of immunohistochemically defined GC and non-GC phenotypes was also equal. However, in the rituximab group, the immunohistochemically defined non-GC subgroup contained more males than females (P = .020).
The baseline characteristics were also analyzed according to immunohistochemically defined GC and non-GC phenotypes (Tables S1-S2). In the immunohistochemically defined GC subgroup, the rituximab group included more women (n = 29) than men (n = 21) with a median age of 59 years, whereas the control group consisted of 24 men and 23 women with a median age of 60 years. In the immunohistochemically defined non-GC subgroup, in turn, both rituximab and control groups included more men (n = 27 and n = 35) than women (n = 13 and n = 22). The median age for the rituximab group was somewhat lower than for the control patients (62 vs 67 years, P = ns). No significant differences were observed in age (< 60 vs ≥ 60 years), stage, and IPI scores between rituximab and control groups (for all comparisons, P > .27).
Survival analyses
To assess how the survival of DLBCL patients has improved during the postrituximab era of antilymphoma therapy, we compared the outcomes of patients treated before and after rituximab was incorporated into DLBCL therapies. A significant difference in outcome was observed between immunochemotherapy (rituximab)– and chemotherapy (control)–treated patients. According to Kaplan-Meier estimates, OS at 27 months was 76% in the rituximab group, and 57% in the control group who received only chemotherapy (P = .004). Conversely, the FFS rates were 67% and 43% for rituximab and control groups, respectively. These differences were seen in all age and IPI subgroups. When regimens other than CHOP were excluded from the analyses, the difference in OS and FFS remained significant (OS, 72% vs 51%, P = .013; FFS, 67% vs 39%, P = .002). The data confirm previous clinical studies showing that addition of rituximab to chemotherapy results in a dramatic improvement in outcome for DLBCL patients of all ages and risk groups.1–4
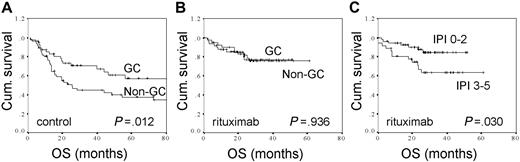
The clinical outcomes according to treatment and immunohistochemically defined GC versus non-GC phenotypes during prerituximab and postrituximab era are shown in Table 2 and Figure 1. As previously demonstrated,15,16 the survival rates in the control group were significantly better for the patients with immunohistochemically defined GC phenotype than for the ones with non-GC phenotype (Table 2; Figure 1A). However, when the outcome of immunochemotherapy-treated patients was compared between immunohistochemically defined GC and non-GC groups, no significant differences were found in survival parameters (Table 2; Figure 1B). In comparison to molecular predictors, a clinically based IPI could separate the high-risk patients from low- and intermediate-risk groups (OS, 84% vs 63%, P = .030; FFS, 79% vs 52%, P = .028) (Figure 1C).
Analysis of clinical outcome according to immunohistochemically defined GC and non-GC phenotypes for rituximab and control groups
| Survival . | Rituximab . | Control . | ||||||
|---|---|---|---|---|---|---|---|---|
| All, % . | GC, % . | Non-GC, % . | P . | All, % . | GC, % . | Non-GC, % . | P . | |
| OS | 76 | 77 | 76 | .936 | 57 | 70 | 47 | .012 |
| FFS | 67 | 68 | 63 | .593 | 43 | 59 | 30 | .001 |
| Survival . | Rituximab . | Control . | ||||||
|---|---|---|---|---|---|---|---|---|
| All, % . | GC, % . | Non-GC, % . | P . | All, % . | GC, % . | Non-GC, % . | P . | |
| OS | 76 | 77 | 76 | .936 | 57 | 70 | 47 | .012 |
| FFS | 67 | 68 | 63 | .593 | 43 | 59 | 30 | .001 |
Cumulative survival was estimated by Kaplan-Meier method. The significance of the differences was analyzed by log-rank test. The values represent the percentage of surviving patients after follow-up of 27 months.
The overall survival rates for control and immunochemotherapy-treated DLBCL patients according to molecular and clinical factors. (A) OS according to immunohistochemically defined GC (n = 47) versus non-GC (n = 57) distinction for patients treated with chemotherapy. (B) OS according to immunohistochemically defined GC (n = 50) versus non-GC (n = 40) distinction for patients treated with immunochemotherapy. (C) OS according to IPI (0-2, n = 54 vs 3-5, n = 36) for patients treated with immunochemotherapy.
The overall survival rates for control and immunochemotherapy-treated DLBCL patients according to molecular and clinical factors. (A) OS according to immunohistochemically defined GC (n = 47) versus non-GC (n = 57) distinction for patients treated with chemotherapy. (B) OS according to immunohistochemically defined GC (n = 50) versus non-GC (n = 40) distinction for patients treated with immunochemotherapy. (C) OS according to IPI (0-2, n = 54 vs 3-5, n = 36) for patients treated with immunochemotherapy.
To further investigate the prognostic impact of immunohistochemically defined cell-of-origin distinction during postrituximab era, Cox analyses were performed for control and immunochemotherapy-treated patients. As shown in Table 3, both the immunohistochemically defined cell of origin and IPI had prognostic value on OS and FFS in chemotherapy-treated control patients, whereas only IPI was a significant prognostic factor for OS and FFS in immunochemotherapy-treated patients.
Cox proportional hazard regression analysis for rituximab and control groups
| Survival . | Rituximab . | Control . | ||||
|---|---|---|---|---|---|---|
| RR . | 95% CI . | P . | RR . | 95% CI . | P . | |
| FFS | ||||||
| IPI | ||||||
| 0 to 2 or 3 to 5 | 2.210 | 1.052-4.645 | .036 | 2.102 | 1.271-3.479 | .004 |
| Phenotype | ||||||
| ihc-defined GC or non-GC | 0.884 | 0.425-1.839 | .742 | 0.457 | 0.272-0.769 | .003 |
| OS | ||||||
| IPI | ||||||
| 0 to 2 or 3 to 5 | 2.736 | 1.076-6.957 | .035 | 3.533 | 2.011-6.208 | < .001 |
| Phenotype | ||||||
| ihc-defined GC or non-GC | 1.075 | 0.436-2.654 | .875 | 0.542 | 0.305-0.964 | .037 |
| Survival . | Rituximab . | Control . | ||||
|---|---|---|---|---|---|---|
| RR . | 95% CI . | P . | RR . | 95% CI . | P . | |
| FFS | ||||||
| IPI | ||||||
| 0 to 2 or 3 to 5 | 2.210 | 1.052-4.645 | .036 | 2.102 | 1.271-3.479 | .004 |
| Phenotype | ||||||
| ihc-defined GC or non-GC | 0.884 | 0.425-1.839 | .742 | 0.457 | 0.272-0.769 | .003 |
| OS | ||||||
| IPI | ||||||
| 0 to 2 or 3 to 5 | 2.736 | 1.076-6.957 | .035 | 3.533 | 2.011-6.208 | < .001 |
| Phenotype | ||||||
| ihc-defined GC or non-GC | 1.075 | 0.436-2.654 | .875 | 0.542 | 0.305-0.964 | .037 |
RR indicates relative risk; CI, confidence interval; IPI, higher IPI worse; ihc, immunohistochemically; and
GC vs non-GC, non-GC worse.
To study the impact of rituximab on the predictive value of immunohistochemically defined GC phenotype, we examined the clinical outcome according to treatment in GC and non-GC groups. After a follow-up of 27 months, immunohistochemically defined non-GC patients who received rituximab in combination with chemotherapy had a significantly better OS than patients treated with chemotherapy alone (76% vs 47%, P = .003). The FFS at 27 months was estimated at 63% for rituximab and 30% for control patients (P = .001). For the patients with immunohistochemically defined GC phenotype, the influence of rituximab on OS or FFS was not significant (OS, 77% vs 70%, P = .400; FFS, 68% vs 59%, P = .286).
The results of Cox multivariate analyses confirmed the prognostic effect of rituximab in the immunohistochemically defined non-GC group (Table 4). Both IPI score and treatment were independent prognostic factors for OS and FFS. In contrast, IPI score was the only statistically significant prognostic factor for survival in the immunohistochemically defined GC group.
Cox proportional hazard regression analysis for immunohistochemically defined non-GC and GC groups
| Survival . | Non-GC . | GC . | ||||
|---|---|---|---|---|---|---|
| RR . | 95% CI . | P . | RR . | 95% CI . | P . | |
| FFS | ||||||
| IPI | ||||||
| 0 to 2 or 3 to 5 | 1.704 | 1.001-2.903 | .050 | 3.274 | 1.645-6.518 | .001 |
| Treatment | ||||||
| Control or rituximab | 0.341 | 0.186-0.627 | .001 | 0.596 | 0.298-1.191 | .143 |
| OS | ||||||
| IPI | ||||||
| 0 to 2 or 3 to 5 | 2.610 | 1.412-4.824 | .002 | 4.383 | 1.964-9.779 | < .001 |
| Treatment | ||||||
| Control or rituximab | 0.296 | 0.139-0.631 | .002 | 0.600 | 0.268-1.343 | .214 |
| Survival . | Non-GC . | GC . | ||||
|---|---|---|---|---|---|---|
| RR . | 95% CI . | P . | RR . | 95% CI . | P . | |
| FFS | ||||||
| IPI | ||||||
| 0 to 2 or 3 to 5 | 1.704 | 1.001-2.903 | .050 | 3.274 | 1.645-6.518 | .001 |
| Treatment | ||||||
| Control or rituximab | 0.341 | 0.186-0.627 | .001 | 0.596 | 0.298-1.191 | .143 |
| OS | ||||||
| IPI | ||||||
| 0 to 2 or 3 to 5 | 2.610 | 1.412-4.824 | .002 | 4.383 | 1.964-9.779 | < .001 |
| Treatment | ||||||
| Control or rituximab | 0.296 | 0.139-0.631 | .002 | 0.600 | 0.268-1.343 | .214 |
RR indicates relative risk; CI, confidence interval; IPI, higher IPI worse; and treatment, control worse than rituximab.
Discussion
The aim of our study was to identify whether cell-of-origin distinction has prognostic impact for DLBCL patients treated with combination of rituximab and chemotherapy. Our findings confirm previous studies showing that chemotherapy-treated patients with immunohistochemically defined GC phenotype have a significantly better outcome compared to patients with the non-GC phenotype.15–17 In line with previous clinical studies,1,3 our data demonstrate that addition of rituximab to chemotherapy in routine clinical practice improves the outcome of DLBCL patients of all ages and risk groups. In contrast, we found no correlation between the immunohistochemically defined cell of origin and outcome in the patients treated with combination of rituximab and chemotherapy. Together, our data suggest that rituximab eliminates the adverse impact of immunohistochemically defined non-GC phenotype for survival. Considering that the positive predictive value of the immunohistochemical approach in comparison to gene expression–based classification is 87% for the GC and 73% for the non-GC phenotypes with a misclassification rate of 20%,16 it is important to note that our conclusions are restricted to immunohistochemically defined GC and non-GC subtypes, and cannot be extrapolated to phenotypes identified by gene expression–based microarrays. Such a misclassification rate may also partially explain why the immunohistochemically defined cell-of-origin distinction does predict the outcome of chemotherapy-treated patients in some but not all studies.15–17,19,20
Since this study is not a concurrent comparison of treatment options, and any conclusions between nonrandomized groups may be subject to differences in observed and unobserved prognostic factors, it is also possible that the findings seen in control and rituximab groups are related to factors other than treatment. Nevertheless, the distribution of baseline characteristics, including IPI scores, was similar between the 2 cohorts. The similarities with previously published data on Bcl-6 showing that the adverse impact of Bcl-6 expression on the outcome of DLBCL patients was overcome by adding rituximab to CHOP- chemotherapy9 provide further support that the findings are real. In addition, identical data on the loss of prognostic value for immunohistochemically defined cell-of-origin distinction in immunochemotherapy-treated patients have been recently presented in brief abstract form.21–23 Although it is premature to make final conclusions, the data suggest that a significant benefit of rituximab is seen only for patients having immunohistochemically defined non-GC phenotype. If confirmed in prospective clinical trials, these findings would have immediate clinical and economic value, because they could lead to the use of rituximab with chemotherapy only in the subgroups of DLBCL patients.
The mechanism by which the addition of rituximab to chemotherapy improves outcomes significantly in only the immunohistochemically defined non-GC group is unknown but may represent a chemosensitizing effect of the antibody. As suggested by studies in cell culture conditions, rituximab may suppress the constitutively active NF-κB pathway in the non-GC–type DLBCL24 or perturb Bcl-2–related antiapoptotic proteins thereby leading to increased sensitivity of lymphoma cells to chemotherapy.25–27 The latter hypothesis is supported by a recent study showing that the adverse impact of BCL-2 expression is associated with non-GC phenotype in chemotherapy-treated patients.6
The intent of this study was not to identify novel prognostic factors, but to assess the utility of previously identified and validated predictors of chemotherapy-treated DLBCL patients in the setting of rituximab-containing treatment practice. Based on the marked improvement in the outcome of DLBCL patients, it was recognized that the addition of rituximab to chemotherapy may alter the significance of previously identified prognostic factors. Consistent with a recent study,28 the IPI score had a prognostic impact on FFS and OS in our cohort of immunochemotherapy-treated patients. In contrast, the prognostic significance of immunohistochemically defined cell-of-origin distinction was lost. Potential explanations for the failure of cell of origin to predict the outcome after immunochemotherapy remain to be shown.
To date, only few prognostic factors have been re-evaluated for immunochemotherapy-treated patients. Consistent with our findings, clinical IPI score has remained predictive,28 whereas previously identified molecular prognostic factors no longer retain their prognostic significance. For example, Bcl-2 expression, which has been associated with adverse outcome in chemotherapy-treated DLBCL patients,8,10–12 had no prognostic value in elderly patients treated with rituximab in combination with chemotherapy.8,29 Likewise, the adverse impact of Bcl-6 expression on the outcome of DLBCL patients was overcome by adding rituximab to CHOP chemotherapy. As no prognostic molecular factors are yet available in postrituximab era, IPI remains the only tool to predict the outcome of immunochemotherapy-treated patients.
In conclusion, we have confirmed the prognostic value of immunohistochemically defined GC phenotype in chemotherapy-treated patients and observed that addition of rituximab eliminates the prognostic influence of immunohistochemically defined GC phenotype. Clearly, the results should be confirmed prospectively in an independent cohort of immunochemotherapy-treated DLBCL patients. Nevertheless, our study illustrates that the molecular prognostic factors in the postrituximab era have to be re-evaluated to obtain additional tools for risk assessment in the current treatment practice of DLBCL.
Presented in part in abstract form at the 48th annual meeting of the American Society of Hematology, Orlando, FL, December 10, 2006.30
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Anitra Ahonen and Anneli Grönqvist for excellent technical assistance. The study was supported by grants from Finnish Medical Foundation, Finnish Cancer Societies, University of Helsinki, Helsinki University Central Hospital, the Swedish Cancer Society, the Lions Research Foundation, and the Research Foundation of the Department of Oncology, Uppsala University Hospital.
Authorship
Contribution: H.N. scored immunohistochemical stainings, collected clinical data, analyzed the data, and assisted in writing the paper; M.A. treated the patients, collected clinical data, and analyzed the data; M.-L.K.-L. was responsible for verifying histology and scoring immunohistochemical stainings; M.T. assisted in stainings and data analyses; M.B. and R.-M.A. assisted in stainings and scoring immunohistochemical stainings; C.B. assisted in data analyses and writing the paper; G.E. supervised and participated in data collection and assisted in writing the paper; and S.L. designed the study, supervised all aspects of the research and analyses, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sirpa Leppä, Department of Oncology, Helsinki University Central Hospital, PO Box 180, FIN-00029 Helsinki, Finland; e-mail: sirpa.leppa@helsinki.fi.