Abstract
Pretargeted radioimmunotherapy (PRIT) using streptavidin (SA)–conjugated antibodies (Abs), followed by clearing agent and radiolabeled biotin is a promising method that can increase the effectiveness of RIT, while decreasing the toxicities associated with directly labeled Abs. Although CD20 has been the traditional target antigen for RIT of non-Hodgkin lymphoma (NHL), studies targeting HLA DR and CD22 have yielded promising results. Targeting all 3 antigens at once may further augment the effect of PRIT. This study compares the targeting of Ramos, Raji, and FL-18 lymphoma xenografts with either anti-CD20 Ab/SA (1F5/SA), anti-HLA DR Ab/SA (Lym-1/SA), anti-CD22 Ab/SA (HD39/SA), or all 3 conjugates in combination, followed 24 hours later by a biotin-N-acetyl-galactosamine clearing agent, and 3 hours after that by 111In-DOTA-biotin. The Ab/SA conjugate yielding the best tumor uptake and tumor-to–normal organ ratios of radioactivity varied depending on the target antigen expression on the cell line used, with 1F5/SA and Lym-1/SA yielding the most promising results overall. Also, the best tumor-to–normal organ ratios of absorbed radioactivity were obtained using single conjugates optimized for target tumor antigen expression rather than the combination therapy. This study highlights the importance of screening the antigenic expression on lymphomas to select the optimal reagent for PRIT.
Introduction
Radioimmunotherapy (RIT) using anti-CD20 monoclonal antibodies (Abs) produces response rates of 60% to 95% in patients with relapsed non-Hodgkin lymphoma (NHL).1–5 However, most patients receiving RIT eventually have a relapse because tumor-to–normal organ ratios of absorbed radiation are relatively low, resulting in delivery of insufficient doses of radiation to the tumor sites to eradicate every tumor cell.6 RIT doses are limited by toxicities associated with radiation exposure to normal organs as a result of the long circulating half-lives of conventional directly labeled Abs. Pretargeted RIT (PRIT) is a strategy that has the potential to significantly decrease these toxicities while also increasing efficacy by disassociating the Ab from the delivery of radiation. Although there are several approaches to pretargeting, the method in this study uses a nonradioactive Ab conjugated to streptavidin (Ab/SA), which is injected and localizes to tumor cells. After maximal accumulation of the Ab/SA at tumor sites, a synthetic biotinylated clearing agent (CA) is administered, which removes unbound Ab/SA conjugate from circulation. The CA is followed by a dose of therapeutic radiolabeled DOTA-biotin. The DOTA-biotin is small and penetrates tumors rapidly where it binds to the SA attached to the Ab, delivering a targeted dose of radiation to the tumors. Unbound DOTA-biotin molecules are rapidly cleared from the bloodstream and are excreted through the urine. This method effectively decreases the amount of time that the normal organs are exposed to the radiation, limiting the associated toxicities. Since the levels of radiation in the normal organs are significantly decreased, PRIT consistently yields favorable tumor-to–normal organ ratios of absorbed radiation.7–12
CD20 has been the target traditionally studied for RIT in NHL. However, the optimal antigen-Ab combination remains unknown due to the variable expression of antigens on tumor cells and the paucity of rigorously controlled comparative studies. Several other B-cell antigens have been studied as targets for immunoconjugates, particularly class II HLA DR13–16 and CD2217–19 antigens. In this study, in vitro and in vivo PRIT studies were initiated comparing the anti-CD20 conjugate, 1F5/SA, the anti-HLA DR conjugate, Lym-1/SA, and the anti-CD22 conjugate, HD39/SA. To test the efficacy of targeting these 3 antigens under various conditions, mouse xenograft models of 3 different human B-lymphoma cell lines expressing different levels of the relevant surface antigens were used: Ramos and Raji (both Burkitt lymphomas) and FL-18 (transformed follicular lymphoma). We report here a strong correlation between the in vitro cell-binding results and the in vivo biodistribution data demonstrating that the optimal target varies among the lymphoma cell lines tested.
In addition to comparing the efficacy of targeting CD20, HLA DR, and CD22 individually, we also investigated whether targeting all 3 antigens at once would increase the efficacy of PRIT compared to targeting any single antigen alone. Using all 3 conjugates in concert might be expected to produce additive or synergistic effects, further enhancing the benefit of PRIT. However, in this report, we show that using a single optimal conjugate yields better uptake of radiolabeled DOTA-biotin by tumor nodules and more favorable tumor-to–normal organ ratios of absorbed radiation than using all 3 conjugates in combination.
Materials and methods
Lymphoma cell lines and hybridomas
The Ramos and Raji cell lines (both Burkitt lymphomas) were obtained from American Type Culture Collection (ATCC; Bethesda, MD). The FL-18 cell line (transformed follicular lymphoma) was a gift from Dr David Maloney (Fred Hutchinson Cancer Research Center [FHCRC], Seattle, WA). Hybridoma cell lines for production of 1F5, Lym-1, and a nonspecific IgG2a negative control antibody (HB8181) were obtained from ATCC. The HD39 hybridoma was a kind gift from Dr Edward Clark (University of Washington, Seattle, WA). The B-lymphoma cell lines and hybridoma cells were maintained in log phase growth in RPMI 1640 medium with 10% heat-inactivated bovine calf serum.
Ab/SA chemical conjugates
The 1F5, Lym-1, and HB8181 Abs (all IgG2a Abs) were produced from the respective hybridomas using a hollow fiber bioreactor system in the Biological Production Facility at the FHCRC. The HD39 Ab (IgG1) was produced in ascites generated by pristane-primed BALB/c mice and purified by protein A immunoabsorption column chromatography. All 4 Abs were conjugated to SA to form covalent chemical conjugates using previously described methods.7,20
Radiolabeling
The chemical conjugates were iodinated with Na125I (Perkin Elmer, Boston, MA) by the chloramine T method as previously described.21 DOTA-biotin was synthesized and radiolabeled with 111In (Nordion, Ottawa, ON, Canada) as previously published.7,22 Labeling efficiencies were more than 90% as determined by binding to agarose-avidin beads.
Immunoreactivity/avidity of chemical conjugates
The immunoreactivities and avidities of the chemical conjugates were compared with those of the corresponding unconjugated Abs using a cell-binding assay. Conjugates and native Abs (100 ng/mL) were labeled with 125I and incubated with varying amounts of antigen (0.16-40 × 107 Ramos or Raji cells). Nonspecific binding was determined in the presence of excess unlabeled Ab or Ab/SA (250 μg/mL). After incubation on a rotator for 2 hours at room temperature, bound and free Abs were separated by centrifugation through oil (1:1 dinonyl phthalate-to-dibutyl phthalate) and counted on a γ-counter (Cobra II, Packard Instruments, Downers Grove, IL). Immunoreactivity was calculated from Bmax determined from nonlinear regression analysis of a plot of bound (specific − nonspecific binding)/total (bound + free) versus cell concentration.23,24
Avidity was determined using saturation-binding experiments that measure specific binding of radiolabeled Ab or Ab/SA (0.02-40 ng/mL) at equilibrium in the presence of excess antigen (107 cells). Nonspecific binding was determined in the presence of excess nonlabeled Ab or Ab/SA (250 μg/mL). Cell/Ab mixtures were incubated and centrifuged as described for immunoreactivity. Apparent binding avidity of the naked Abs versus the Ab/SA conjugates was compared by Scatchard analysis using previously published methods.25
Flow cytometry
Cells (1 × 106/tube) were incubated with 10 μg Ab/SA conjugate in 2% FBS-PBS for 30 minutes at 4°C and washed with 2% FBS-PBS. A second incubation for 30 minutes at 4°C was done using 5μg secondary Ab, horse anti–mouse-fluorescein isothiocyanate (FITC). Following a second washing, the cells were fixed in 2% paraformaldehyde in PBS and analyzed in a fluorescence-activated cell sorting (FACS) scan (Becton-Dickinson, San Jose, CA). Mean fluorescence intensity (MFI) was determined using CellQuest Pro 5.2 software (San Jose, CA).
Blood clearance studies
For all mouse studies, female BALB/c nu/nu mice, aged 6 to 8 weeks, were obtained from Harlan Sprague-Dawley (Indianapolis, IN) and Charles River Laboratories (Wilmington, MA) and housed under protocols approved by the FHCRC Institutional Animal Care and Use Committee. To assess whole blood clearance, 1.4 nmol 125I-labeled 1F5/SA, Lym-1/SA, or HD39/SA was injected intravenously via the tail vein into athymic mice. Serial blood samples were drawn from the retro-orbital venus plexus and radioactivity measured by γ-counting. The blood half-life (T1/2) was determined by fitting data for each mouse to a 2-compartmental nonlinear decay model with WinNonLin software (Pharsight, Mountain View, CA) and calculating the mean for each Ab/SA conjugate. To test the effect of the synthetic N-acetyl-galactosamine-biotin (NAGB) CA, groups of 4 mice were first injected intravenously with one of the 3 125I-labeled conjugates followed 24 hours later by an intravenous injection of 5.8 nmol of the CA. Blood samples were drawn before and after CA injection and γ-counted for 125I. The mean values and standard deviations were plotted using GraphPad 4 software (Prism, San Diego, CA).
Biodistribution studies
Female BALB/c athymic mice were injected subcutaneously in each flank with cells from one of the 3 B-lymphoma cell lines, Ramos (1 × 107 cells), Raji (1 × 107 cells), and FL-18 (1.2 × 107 cells). Ramos and Raji cells produced (∼100 mm3) tumors 10 days after tumor cell inoculation; FL-18 tumors reached this size after 14 days. Mice with palpable tumors were placed on a biotin-free diet (Purina Feed, Richmond, IN) for 5 days prior to experiments. Groups of at least 4 mice were injected intravenously with 1.4 nmol of either 1F5/SA, Lym-1/SA, or HD39/SA for single-agent studies. After 24 hours, 5.8 nmol CA was administered intravenously followed 3 hours later by an intravenous injection of 1.2 nmol 111In-DOTA-biotin. Mice receiving either 1F5/SA or Lym-1/SA were coinjected with 2.8 nmol of the nonspecific IgG2a Ab, HB8181, to block nonspecific binding of the conjugates to Fc receptors in the liver, spleen, and bone marrow.8,26 For combination studies, tumor xenograft-bearing mice were coinjected with an equimolar mix of all 3 conjugates. In the low-dose combination groups, mice received 1.4 nmol total conjugate (0.47 nmol of each conjugate), which is equimolar to the amount of total conjugate used in the single-agent studies. After 24 hours, mice received 5.8 nmol CA followed 3 hours later with 1.2 nmol 111In-DOTA-biotin. In the triple-dose combination groups, mice were injected with 4.2 nmol total conjugate (1.4 nmol of each conjugate), 3 times the total dose used in the single-agent studies. To clear the larger dose of conjugate, 3 times the dose of CA (17.4 nmol) was injected 24 hours later followed by 1.2 nmol 111In-DOTA-biotin 3 hours after CA. Mice were bled from the retro-orbital venous plexus, killed, and tumors and normal organs (lung, liver, spleen, stomach, kidneys, small intestine, and colon) were harvested, weighed, and γ-counted for 111In activity 24 and 48 hours after the 111In-DOTA-biotin injection. The percent injected dose of 111In per gram (%ID/g) of blood, tumor, and normal organs was calculated after correcting for radioactive decay using an aliquot of the injectate. Tumor-to–normal organ ratios of absorbed radioactivity were also calculated. Control groups were injected with 1.4 nmol of the nonbinding conjugate HB8181/SA followed by CA and 111In-DOTA-biotin.
Results
In vitro assessment of SA conjugates
After conjugation, each Ab/SA conjugate was evaluated for immunoreactivity to determine whether the conjugation process had altered the binding of the Ab. Quantitation of immunoreactivity and avidity for each conjugate and the corresponding unconjugated Abs were obtained after radioiodination by testing binding at various concentrations using serial dilutions of either Ramos or Raji cells.23,27 The results demonstrate that the conjugation of SA to Abs did not exert deleterious effects on their immunoreactivities or avidities (Table 1).
Comparison of immunoreactivities and avidities of 1F5, Lym-1, and HD39 Abs and Ab/SA conjugates
| . | 1F5 . | 1F5/SA . | Lym-1 . | Lym-1/SA . | HD39 . | HD39/SA . |
|---|---|---|---|---|---|---|
| IR, % | 66.50 | 64.15 | 61.30 | 52.60 | 70.3 | 75.10 |
| Avidity, × 108 M−1* | 0.66 ± 0.08 | 0.72 ± 0.08 | 0.74 ± 0.28 | 0.50 ± 0.12 | 7.75 ± 1.26 | 7.52 ± 1.10 |
| . | 1F5 . | 1F5/SA . | Lym-1 . | Lym-1/SA . | HD39 . | HD39/SA . |
|---|---|---|---|---|---|---|
| IR, % | 66.50 | 64.15 | 61.30 | 52.60 | 70.3 | 75.10 |
| Avidity, × 108 M−1* | 0.66 ± 0.08 | 0.72 ± 0.08 | 0.74 ± 0.28 | 0.50 ± 0.12 | 7.75 ± 1.26 | 7.52 ± 1.10 |
Using standard cell-binding assays, the IR and avidities were determined for 1F5 and 1F5/SA using Ramos cells and Lym-1, Lym-1/SA, HD39, and HD39/SA using Raji cells. No statistically significant differences in avidity were seen between the Ab/SA conjugates and the corresponding unconjugated Abs (paired t test, two-tailed, P < .05).
IR indicates immunoreactivity.
All experiments were done in triplicate. Mean ± SD.
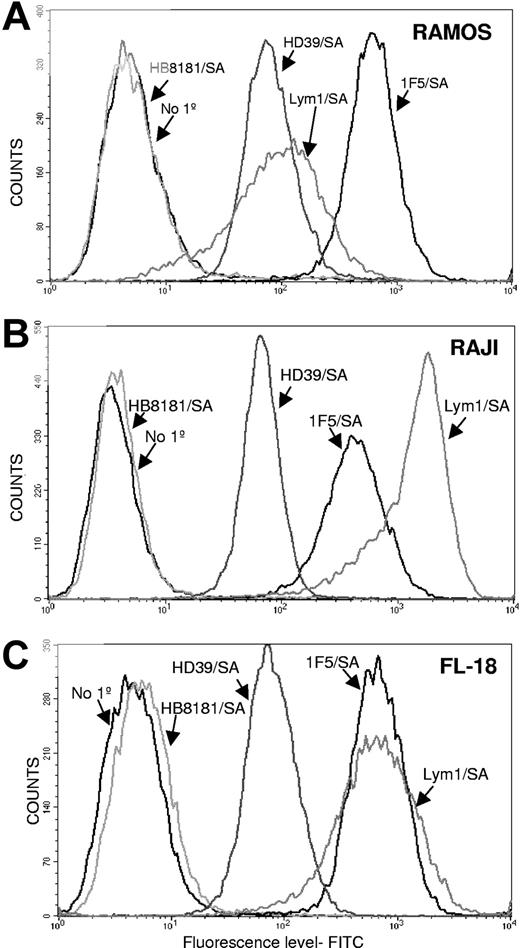
Flow cytometric studies showed that the 3 Ab/SA conjugates differed significantly in binding to the 3 lymphoma cell lines tested (Figure 1). The 1F5/SA conjugate showed the highest level of binding to Ramos cells, whereas Lym-1/SA and HD39/SA showed MFIs that were 83% and 89% lower, respectively (Figure 1A). By contrast, Lym-1/SA demonstrated the highest binding to Raji cells followed by 1F5/SA (MFI 68% of Lym-1/SA), whereas HD39/SA exhibited minimal binding (MFI 96% lower than Lym-1/SA; Figure 1B). Finally, Lym-1/SA and 1F5/SA showed similar high levels of binding to FL18 cells, whereas HD39/SA exhibited a MFI 90% lower than the other 2 Abs (Figure 1C). As can be seen in Figure 1, 1F5/SA exhibited excellent binding to FL-18 cells and Ramos cells, whereas Lym-1/SA bound best to FL-18 and Raji cells. HD39/SA demonstrated the highest level of binding on Ramos cells.
Cell-binding studies. Flow cytometric analysis of the cell-binding capabilities of 1F5/SA, Lym-1/SA, and HD39/SA to 3 different B lymphoma cell lines: Ramos (A), Raji (B), and FL-18 (C). For all studies, 2 controls were included, one omitting the primary conjugate and one using a nonbinding control conjugate HB8181/SA.
Cell-binding studies. Flow cytometric analysis of the cell-binding capabilities of 1F5/SA, Lym-1/SA, and HD39/SA to 3 different B lymphoma cell lines: Ramos (A), Raji (B), and FL-18 (C). For all studies, 2 controls were included, one omitting the primary conjugate and one using a nonbinding control conjugate HB8181/SA.
Blood clearance
Blood clearance studies were conducted in female BALB/c athymic mice to assess the pharmacokinetics of the 3 conjugates (Figure 2A). The half-lives were 39 ± 1 hours for 1F5/SA, 36 ± 2 hours for Lym 1/SA, and 24 ± 8 hours for HD39/SA after injection of 1.4 nmol of each conjugate. The efficacy of the NAGB CA was also tested with each conjugate by injecting 5.8 nmol of the CA 24 hours after 125I-Ab/SA injection. In each case, the %ID/g of the conjugates in the blood fell by 60% to 90% within the first hour after CA injection (Figure 2B-D).
Pharmacokinetics and blood clearance of Ab/SA conjugates. (A) Whole blood clearance of 1.4 nmol of either 125I-labeled 1F5/SA (●), Lym-1/SA (○) or HD39/SA (◇) injected intravenously into athymic BALB/c mice (n = 4/group). (B-D) The effect of the NAGB CA on circulating levels of each of the Ab/SA conjugates: 1F5/SA (B), Lym-1/SA (C), and HD39/SA (D). Athymic BALB/c mice (n = 4/group) were injected intravenously at t = 0 with 1.4 nmol 125I-labeled conjugate followed 24 hours later with an intravenous injection of 5.8 nmol CA. In each study, serial blood samples were obtained from the retro-orbital venous plexus and radiation levels present determined by γ-counting and percent injected dose/gram (%ID/g) calculated.
Pharmacokinetics and blood clearance of Ab/SA conjugates. (A) Whole blood clearance of 1.4 nmol of either 125I-labeled 1F5/SA (●), Lym-1/SA (○) or HD39/SA (◇) injected intravenously into athymic BALB/c mice (n = 4/group). (B-D) The effect of the NAGB CA on circulating levels of each of the Ab/SA conjugates: 1F5/SA (B), Lym-1/SA (C), and HD39/SA (D). Athymic BALB/c mice (n = 4/group) were injected intravenously at t = 0 with 1.4 nmol 125I-labeled conjugate followed 24 hours later with an intravenous injection of 5.8 nmol CA. In each study, serial blood samples were obtained from the retro-orbital venous plexus and radiation levels present determined by γ-counting and percent injected dose/gram (%ID/g) calculated.
Biodistributions
The biodistributions of the 3 Ab/SA conjugates were assessed individually and in combination using 3 types of lymphoma xenografts. Athymic mice bearing Ramos, Raji, or FL-18 tumors were injected intravenously with 1.4 nmol of one of the 3 Ab/SA conjugates at the initiation of the experiments. Mice injected with IgG2a conjugates (1F5/SA and Lym-1/SA) also received 2.8 nmol of the nonspecific IgG2a Ab, HB8181, to prevent nonspecific binding of the Ab/SA conjugates to Fc receptors in the liver, spleen, and marrow.8,26 Twenty-four hours later, mice received 5.8 nmol CA followed 3 hours later by 1.2 nmol 111In-DOTA-biotin. Mice were killed 24 or 48 hours after 111In-DOTA-biotin administration and tissues were harvested and γ-counted to calculate the %ID/g of tissue (Figure 3A-C). To display results for each Ab/SA on a single graph, the normal organ values were averaged across studies using the same conjugate since the levels of radiation present were very similar. A pretargeted biodistribution was also performed for each type of xenograft using a control, nonbinding conjugate, HB8181/SA (Figure 3D). The amounts of radioactivity localized to the xenografts correlated closely with the relative binding of the Ab/SA conjugates for the cell lines in vitro, as illustrated by flow cytometry (Figure 1). FL-18 tumors exhibited the best targeting using 1F5/SA with 14.1% ± 11.9% ID/g 111In-DOTA-biotin present in tumor xenografts after 48 hours compared to 5.4% ± 1.5% with Ramos and 3.3% ± 0.7% ID/g with Raji xenografts, using this anti-CD20 conjugate. Mice pretargeted with Lym-1/SA exhibited high levels of tumor-associated 111In-DOTA-biotin in FL-18 and Raji tumors (24.0% ± 16.3% and 22.2% ± 15.5% ID/g at 48 hours, respectively), whereas targeting to Ramos tumors was significantly worse (3.0% ± 2.4% ID/g). Finally, pretargeting with HD39/SA resulted in similarly low levels of 111In-DOTA-biotin uptake in all 3 types of tumor after 48 hours (Ramos, 4.7% ± 1.6% ID/g; Raji, 2.4% ± 0.8% ID/g; and FL-18, 2.4% ± 1.2% ID/g). Biodistribution studies using pretargeting with the nonspecific HB8181/SA conjugate exhibited negligible tumor uptake of radioactivity in xenografts of all 3 cell lines after 48 hours (Ramos, 0.5% ± 0.2% ID/g; Raji, 1.6% ± 1.5% ID/g; and FL-18, 0.6% ± 0.2% ID/g) confirming the specificity of tumor targeting.
Biodistributions of 111In-DOTA-biotin in tumor xenografts and normal organs after pretargeting with each Ab/SA conjugate. Athymic BALB/c mice bearing Ramos, Raji, or FL-18 tumor xenografts were injected intravenously with 1.4 nmol of a single conjugate. After 24 hours, mice were injected with 5.8 nmol CA followed 3 hours later with 1.2 nmol 111In-labeled DOTA-biotin. Mice were killed 24 and 48 hours later and blood, tumors, and normal organs were harvested, weighed, and analyzed for levels of radioactivity by γ-counting to determine %ID/g. Results are shown for PRIT with 1F5/SA (A), Lym-1 (B), HD39/SA (C), and the nonbinding control conjugate HB8181/SA (D). For each organ, the first bar represents results 24 hours after 111In-DOTA-biotin injection and the second bar represents results after 48 hours.
Biodistributions of 111In-DOTA-biotin in tumor xenografts and normal organs after pretargeting with each Ab/SA conjugate. Athymic BALB/c mice bearing Ramos, Raji, or FL-18 tumor xenografts were injected intravenously with 1.4 nmol of a single conjugate. After 24 hours, mice were injected with 5.8 nmol CA followed 3 hours later with 1.2 nmol 111In-labeled DOTA-biotin. Mice were killed 24 and 48 hours later and blood, tumors, and normal organs were harvested, weighed, and analyzed for levels of radioactivity by γ-counting to determine %ID/g. Results are shown for PRIT with 1F5/SA (A), Lym-1 (B), HD39/SA (C), and the nonbinding control conjugate HB8181/SA (D). For each organ, the first bar represents results 24 hours after 111In-DOTA-biotin injection and the second bar represents results after 48 hours.
The biodistributions of 111In-DOTA-biotin in the normal organs were similar for all 3 Abs with Lym-1/SA showing the highest relative background with an average of 2.0 ± 0.9% ID/g in the blood and 2.% ± 1.0% ID/g in the kidneys at 24 hours. In contrast, 1F5/SA and HD39/SA displayed lower levels of radiation in the blood (0.6% ± 0.3% and 1.3% ± 0.3% ID/g, respectively) and the kidneys (0.8% ± 0.2% and 1.3 ± 0.2% ID/g, respectively) after 24 hours.
Pretargeted biodistribution studies were also conducted using all 3 conjugates in combination at 2 different doses to assess whether targeting multiple antigens yielded additive or synergistic effects (Figures 4–5). For the lower dose study, 0.47 nmol of each Ab/SA was injected intravenously into athymic mice bearing tumor xenografts. In these studies, the total amount of conjugate was equivalent to the amounts used in the previous single-agent biodistribution experiments. Conjugate injections were followed 24 hours later with 5.8 nmol NAGB CA and then, 3 hours later, with 1.2 nmol 111In-DOTA-biotin. For studies testing the higher conjugate dose, 1.4 nmol of each Ab/SA was injected, yielding a cumulative dose 3 times the total amount of protein used in all other studies. Conjugate injections were followed 24 hours later by 17.4 nmol NAGB CA and then 3 hours later with 1.2 nmol 111In-DOTA-biotin. For both combination schemas, the control Ab, HB8181, was coinjected to block nonspecific binding of the IgG2a conjugates to Fc receptors. The higher dose of the Ab/SA combination resulted in significantly higher tumor uptake of radioactivity for all 3 types of xenografts than the lower dose of conjugate. For example, in mice bearing Ramos tumors, animals receiving of the higher dose combination accumulated 11.2% ± 1.2% ID/g 111In-DOTA-biotin in the tumor after 48 hours compared to 3.1% ± 0.4% ID/g with the lower dose combination. Similarly, FL-18 tumors in mice receiving the higher dose combination accumulated 20.5% ± 21.9% ID/g 111In-DOTA-biotin after 48 hours compared to 6.1% ± 3.7% ID/g in tumors of mice receiving the lower dose combination. As illustrated in Figures 4 and 5, Ramos tumor xenografts demonstrated the most significant increase in tumor targeting with the higher dose combination, with tumor levels after 24 and 48 hours greater than observed using any of the 3 Ab/SA conjugates singly. However, targeting FL-18 tumor xenografts with the combination of Ab/SA conjugates resulted in levels of tumor-associated radioactivity similar to those obtained using either 1F5/SA or Lym-1/SA alone. Surprisingly, the amount of 111In-DOTA-biotin concentrated in Raji tumors after pretargeting with all 3 Ab/SA conjugates was less than the amount pretargeted with Lym-1/SA alone (Figures 4B and 5B).
Comparative biodistributions using all 3 conjugates in combination or with each conjugate singly at 24 hours. For the combination studies, mice bearing Ramos (A), Raji (B), or FL-18 (C) tumor xenografts were injected intravenously with a cocktail of either 0.47 or 1.4 nmol each of 1F5/SA, Lym-1/SA, and HD39/SA followed 24 hours later with 5.8 or 17.4 nmol, respectively, of CA and 3 hours after that with 1.2 nmol 111In-DOTA-biotin. Mice were killed 24 and 48 hours after injection of 111In-DOTA-biotin and tumor and organs were harvested and analyzed as for the studies in Figure 3. Each graph shows %ID/g 24 hours after injecting 1.4 nmol 1F5/SA alone (□), Lym-1/SA alone (▩), HD39 alone (■), and all 3 used in combination at 0.47 nmol/conjugate (▤) and at 1.4 nmol/conjugate (▧).
Comparative biodistributions using all 3 conjugates in combination or with each conjugate singly at 24 hours. For the combination studies, mice bearing Ramos (A), Raji (B), or FL-18 (C) tumor xenografts were injected intravenously with a cocktail of either 0.47 or 1.4 nmol each of 1F5/SA, Lym-1/SA, and HD39/SA followed 24 hours later with 5.8 or 17.4 nmol, respectively, of CA and 3 hours after that with 1.2 nmol 111In-DOTA-biotin. Mice were killed 24 and 48 hours after injection of 111In-DOTA-biotin and tumor and organs were harvested and analyzed as for the studies in Figure 3. Each graph shows %ID/g 24 hours after injecting 1.4 nmol 1F5/SA alone (□), Lym-1/SA alone (▩), HD39 alone (■), and all 3 used in combination at 0.47 nmol/conjugate (▤) and at 1.4 nmol/conjugate (▧).
Comparative biodistributions using all 3 conjugates in combination or with each conjugate singly at 48 hours. Combination studies in Ramos (A), Raji (B), and FL-18 (C) as described for Figure 4. Each graph shows results 48 hours after injecting 111In-DOTA-biotin pretargeted with either 1.4 nmol 1F5/SA alone (□), Lym-1/SA alone (▩), HD39 alone (■), and all 3 used in combination at 0.47 nmol/conjugate (▤) and at 1.4 nmol/conjugate (▧).
Comparative biodistributions using all 3 conjugates in combination or with each conjugate singly at 48 hours. Combination studies in Ramos (A), Raji (B), and FL-18 (C) as described for Figure 4. Each graph shows results 48 hours after injecting 111In-DOTA-biotin pretargeted with either 1.4 nmol 1F5/SA alone (□), Lym-1/SA alone (▩), HD39 alone (■), and all 3 used in combination at 0.47 nmol/conjugate (▤) and at 1.4 nmol/conjugate (▧).
Most notable in these combination studies, however, was the distinct increase in the amount of radioactivity seen in normal organs when the conjugate dose was escalated. For example, in mice bearing Ramos tumors the amount of radioactivity seen in the liver was 8.4% ± 2.6% ID/g after 24 hours, significantly higher than the levels seen using each Ab/SA individually (0.5% ± 1.4% ID/g) or in combination at 0.47 nmol each (1.8% ± 0.4% ID/g). Similar high levels of liver uptake were seen in mice bearing Raji and FL18 tumors after 24 hours (6.7% ± 1.1% and 8.8% ± 1.6% ID/g, respectively). These combination studies were repeated using escalating doses of CA to test whether tripling the dose of CA would improve clearance of the conjugates from the bloodstream and from nonspecific organs sufficiently to mitigate the high levels of normal organ radioactivity. However, even administration of 5 times the normal dose of CA did not significantly diminish the radioactivity in the blood, liver, and other normal organs (data not shown) suggesting that our dosing schema has exceeded the capacity of the liver to efficiently clear and metabolize conjugate-CA complexes prior to 111In-DOTA-biotin administration.
Tumor-to–normal organ ratios of radioactivity were calculated 48 hours after 111In-DOTA-biotin administration for studies using the conjugates individually or in combination (Figure 6). These graphs demonstrate the effectiveness of the pretargeting approach with all 3 Ab/SA conjugates demonstrating positive ratios for all normal organs tested. In general, ratios were most favorable when 1F5/SA or Lym-1/SA was used to target either Raji or FL-18 tumors. For example, in the FL-18 tumor model, pretargeting with 1F5/SA yielded tumor-to–normal organ ratios of 12.6:1 in blood and 7.5:1 in kidney. In the same model, pretargeting with Lym-1/SA resulted in ratios of 23:1 in blood and 8.7:1 in kidneys. On the other hand, the tumor-to–normal organ ratios were generally lower in experiments using combinations of conjugates due to either decreased tumor uptake of 111In-DOTA-biotin (lower dose of each conjugate), or higher background (higher dose of conjugate). Most significant is the decrease in the tumor-to–normal organ ratio for the liver in animals receiving the higher dose combination with a tumor-to-liver ratio as high as 29:1 after 48 hours for Lym-1/SA alone in FL-18 tumors, but as low as 1.4:1 for the triple dose combination in Ramos and Raji models and 3.8:1 in the FL-18 model.
Tumor-to–normal organ ratios of absorbed radioactivity using each Ab/SA conjugate individually or all 3 in combination. Mice were treated as described in Figure 3. Tumor-to–normal organ ratios of radioactivity are shown 48 hours after injection of 111In-DOTA-biotin. Animals were pretargeted with 1.4 nmol of either 1F5/SA (□), Lym-1/SA (▩), or HD39/SA (■) or a combination of either 0.47 nmol (▤) or 1.4 nmol of each conjugate (▧). Results are shown for mice bearing Ramos (A), Raji (B), and FL-18 (C) xenografts.
Tumor-to–normal organ ratios of absorbed radioactivity using each Ab/SA conjugate individually or all 3 in combination. Mice were treated as described in Figure 3. Tumor-to–normal organ ratios of radioactivity are shown 48 hours after injection of 111In-DOTA-biotin. Animals were pretargeted with 1.4 nmol of either 1F5/SA (□), Lym-1/SA (▩), or HD39/SA (■) or a combination of either 0.47 nmol (▤) or 1.4 nmol of each conjugate (▧). Results are shown for mice bearing Ramos (A), Raji (B), and FL-18 (C) xenografts.
Discussion
This report demonstrates the advantages of a pretargeted approach to RIT for NHL and illustrates the high levels of radiation that can be specifically localized to lymphoma xenografts while minimizing the radiation exposure of normal organs. This is the first report to investigate the potential utility of Ab/SA conjugates targeting CD22 and HLA DR for PRIT, and it provides confirmatory results concerning the effectiveness of anti-CD20 PRIT.7,8,10–12 Furthermore, this study rigorously compares parallel conjugates directed against the 3 antigenic targets head-to-head for the first time using 3 different B-lymphoma cell lines with differing surface antigen expression levels. These results demonstrate that HLA DR, CD20, and CD22 are all effective targets for PRIT. In fact, in some settings (eg, for Raji xenografts), targeting HLA DR with Lym-1/SA proved superior to targeting CD20. Finally, this study is the first to investigate whether targeting multiple antigens simultaneously can augment the efficacy of PRIT. Surprisingly, the results suggest that targeting CD20, CD22, and HLA DR in combination does not increase the amount of radiation that is specifically localized at the tumor site or improve tumor-to–normal organ ratios compared to targeting the optimal antigen alone.
Preclinical and clinical studies have shown that the pretargeting strategy has significant pharmacokinetic advantages over directly labeled RIT especially in treating radiosensitive hematopoietic malignancies. Conventional RIT is dose limiting due to the long circulating half-life of Ab, which results in nonspecific delivery of radiation to normal organs. By dissociating the Ab from the delivery of radiation, these pharmacokinetic limitations can be markedly ameliorated. Several methods of pretargeting have been described including bispecific Abs, which recognize both a tumor antigen as well as a radiolabeled hapten,29,30 biotinylated antibodies capable of binding avidin or SA,31 and either SA-conjugated Abs7–9 or genetically engineered tetravalent fusion proteins containing SA11,27 used with DOTA-biotin. We have investigated the SA/biotin approach, based on promising experience both in our laboratory and at other institutions.9,28,31,32 Murine studies have demonstrated substantially improved tumor-to–normal organ ratios of absorbed radioactivity while exposure of normal organs was minimal.7–12 Because of the favorable ratios, PRIT allows a significant dose escalation with minimal toxicity permitting much higher amounts of absorbed radiation to be delivered directly to tumor sites resulting in enhanced cure rates in animal models.7–11 Preliminary clinical studies using 90Y-DOTA-biotin pretargeted with either an anti-CD20 Ab/SA (rituximab/SA)33 or anti-CD20 fusion protein (B9E9FP)34 have yielded encouraging results with several objective clinical remissions.
Although the SA/biotin system for PRIT is quite promising, this approach has some potential clinically relevant limitations. For example, it requires multiple injections of reagents at specified time intervals. In addition, SA is immunogenic, potentially limiting the ability of patients to undergo multiple treatments. Finally, endogenous biotin may compete with radiolabeled biotin for binding to SA, thereby limiting therapeutic efficacy. Although we acknowledge these theoretical concerns, we do not believe they undermine the promise of this approach for lymphoma patients. Hematologists and oncologists routinely administer complex multiagent chemotherapy regimens that are much more complicated than the PRIT regimen used in this paper. Two clinical trials have already demonstrated the feasibility of administering PRIT to patients with advanced lymphoma, the favorable biodistributions that can be obtained with anti-CD20 PRIT and the fact that endogenous biotin does not pose a formidable obstacle.34,35 Furthermore, only a minority of lymphoma patients mounted a strong immune response to SA in these trials, with most patients showing either low titer or no response.34 Finally, currently approved RIT regimens such as 131I-tositumomab and 90Y-ibritumomab are generally only administered a single time, rendering the concern about repetitive therapy moot.
CD20 has been the primary target for most studies investigating Ab-mediated immunotherapy of B-cell lymphomas.1–12,36–40 Although targeting this antigen has shown great promise, RIT studies targeting other B-cell surface antigens, particularly class II HLA DR13–16 and CD22,17–19 have also yielded promising results and merit further consideration.41–45 Work by the DeNardo group has convincingly shown that RIT targeting HLA DR achieves clinical remissions in a high proportion of patients with NHL treated with either directly labeled 131I-Lym-114 or 90Y-Lym-143 and may increase survival in patients with NHL.42 In addition, NHL patients with splenomegaly who were treated with directly labeled Lym-1 exhibited significant shrinkage of splenic volume, resulting in improved targeting of lymph nodes and partial and complete remissions.16 The anti-CD22 Ab, epratuzumab, has also yielded encouraging data for treatment of B-cell malignancies. Dose-fractionated RIT with 90Y-labeled epratuzumab resulted in a 62% response rate with 25% of patients achieving a complete remission.45 In addition, clinical studies using unconjugated epratuzumab have also shown low toxicity and encouraging response rates in indolent follicular lymphoma.44
We complement this published work in a companion manuscript comparing the targeting and biodistribution of directly radiolabeled anti-CD20, anti-CD22, and anti–HLA-DR Abs and demonstrate that all 3 Abs produce favorable results as directly labeled immunoconjugates.46 In this present manuscript, we extend this experience to the field of PRIT, which we believe offers significant advantages over conventional RIT. Indeed, we have recently published studies demonstrating the superior efficacy and diminished toxicity of PRIT using anti-CD20 Ab/SA conjugates compared with conventional RIT with directly radiolabeled anti-CD20 Ab.10 We now amplify and extend these findings by confirming that PRIT also produces impressive advantages for anti-CD22 and anti-HLA DR Abs. Cell-binding studies using flow cytometry demonstrated that the Ab/SA conjugates differed in their binding to the 3 lymphoma cell lines, based on varying surface antigen expression levels (Figure 1). These antigen expression levels were highly predictive of the amounts of the radiation accumulating in lymphoma xenografts in the in vivo biodistribution studies. This observation suggests that cell-binding information obtained on tumor biopsies in vitro can assist the selection of optimal in vivo targeting reagents for patients.
Few prior studies have investigated combination RIT regimens targeting 2 or more antigens. Tuscano et al treated mice bearing Raji xenografts with unlabeled anti-CD22 Ab in addition to radiolabeled Lym-1 and demonstrated increased survival rates with the combination compared with anti-DR RIT alone.47 Similarly, Stein et al reported administering an anti-CD20 Ab in combination with epratuzumab to Raji-bearing SCID mice suggests that these agents in combination are more active than either agent alone.48 Clinically, combined immunotherapy using rituximab with either epratuzumab49 or the anti-HLA DR antibody Hu1D1050 in patients with B-cell lymphomas have shown an increase in response. The present study explored whether this approach has the potential to increase the efficacy of PRIT. The 2 doses tested in these combination studies were chosen to best compare with the results obtained in the single-agent biodistributions. For all 3 cell lines, studies in mice receiving the lower dose (1.4 nmol) of the conjugates yielded levels of absorbed radiation in tumor sites that were uniformly lower than those observed in studies using the single best Ab/SA for that cell line.
Combination studies using the higher dose (4.2 nmol) of the conjugates yielded enhanced tumor uptake in comparison to the lower dose. However, only in the Ramos model were increased amounts of radiation localized to xenografts compared to the single agent biodistributions. The average %ID/g in the Ramos tumors after 24 hours was 41% greater for the combination than the total %ID/g for all 3 of the conjugate used as single agents. After 48 hours, the amount of radiobiotin measured in the tumor in the combination study was nearly additive when compared to the total of the single agents. In combination experiments done in mice bearing either Raji or FL-18 tumors, the average %ID/g observed in the tumors was either equal to or less than the %ID/g observed in studies using the most favorable single Ab/SA. Additionally, in all 3 cell lines higher levels of radiation were seen in the normal organs. Most notably, the %ID/g of the liver increased at least 5-fold compared to PRIT using any of the conjugates singly, suggesting that the total amount of conjugate used in these studies was too high for the liver to effectively process before 111In-DOTA-biotin administration. As a result of the higher background, the tumor-to–normal organ ratios calculated for these biodistributions are suboptimal compared with those achieved with single-agent PRIT. Although it is conceivable that cross-blocking, steric hindrance, or coordinate down-modulation of antigenic expression may also be partially responsible for the results observed with antibody combinations, we have seen no evidence of these phenomena in preliminary studies (Daming Shan and O.W.P., unpublished results, August 1998). These unexpected findings suggest that combination therapy for PRIT is less promising than targeting the most highly expressed antigen alone.
Based on our findings, we conclude that the most favorable biodistribution is obtained when a single antigen is targeted using the Ab/SA conjugate that binds most effectively to the tumor cells. These conclusions suggest the importance of immunophenotyping NHL patients prior to therapy to determine which antigen may be the most highly expressed on the malignant cells and testing the binding of each conjugate to discern the optimal antigen-antibody combination to use in PRIT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by R01 grant CA76287, P01 grant 44991, and K08 grant CA95448 from the National Cancer Institute, a Damon Runyan Award (J.M.P.), the Lymphoma Research Foundation Career Development Award (J.M.P.), and an endowed Chair from James and Shirley Raisbeck.
National Institutes of Health
Authorship
Contribution: A.P. designed and performed research, analyzed data, and drafted the manuscript; J.M.P. contributed to the conception, design, analysis, and interpretation of the research and revised the manuscript; N.H. contributed vital reagents; L.S. performed research and collected data; S.W. performed research and collected data; D.K.H. contributed vital reagents; D.S.W. contributed vital reagents and contributed to the interpretation of data; Y.L. contributed to the conception and interpretation of research; D.S. designed and performed research and collected data; D.A. contributed to the conception and design of the research; A.K.G. contributed to the interpretation of data; and O.W.P. contributed to the conception, design, analysis, and interpretation of the research and revised the manuscript.
Conflict-of-interest disclosure: D.A. is employed by Aletheon Pharmaceuticals, whose potential products were studied in this paper. The authors declare no competing financial interests.
A.P. and J.M.P. contributed equally to this research and manuscript.
Correspondence: John M. Pagel, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, M/S D5-385, Seattle, WA 98109; e-mail: jpagel@fhcrc.org.