To the editor:
In the June 1, 2003, issue of Blood, Krijanovski et al presented a single–base pair deletion in cDNA position 1492 of exon 10 in a family with isolated high-molecular-weight kininogen (HK) deficiency.1 This deletion affected amino acid 498 of the mature protein and resulted in a frameshift and a premature stop codon at position 1597 (amino acid 532). In this letter, we show that a novel frameshift mutation in exon 10 is responsible for isolated HK deficiency inherited in a Japanese family. Previously, Hayashi et al detected a partial deletion in intron 7 of the kininogen gene in the proband of this family.2 They assumed that this deletion was related to an abnormality of the alternative RNA splicing of high-molecular-weight prekininogen mRNA.
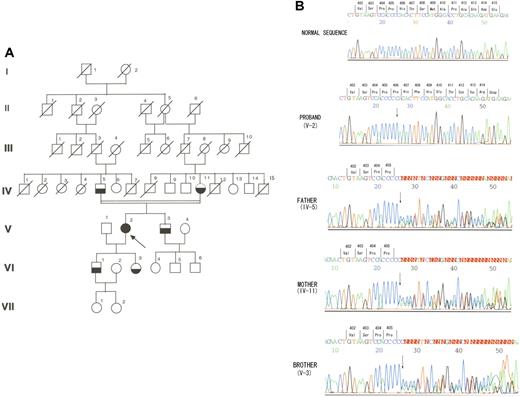
Clinical and laboratory data relevant to the isolated HK deficiency in this report have been presented.3 In brief, the proband (Figure 1A; V-2), whose parents were second cousins, was born in 1948. She had no bleeding or thrombotic tendency. When she entered our hospital because of pneumonia in 1983, her kaolin-activated partial thromboplastin time was found to be prolonged at 242.0 seconds (control, 38.0 seconds). Her prothrombin time was normal. Her plasma HK level assessed by the functional clotting method was less than 1%, and the other clotting factors were present at normal levels except for prekallikrein, which was present at 39% of the normal level. Her plasma low-molecular-weight kininogen (LK) level assessed by the method of Uchida and Katori was 2.82 μg bradykinin (BK) equivalent/mL (control, 2.70 μg BK equivalent/mL).4 Plasma HK levels of her father (IV-5), mother (IV-11), brother (V-3), son (VI-1), and daughter (VI-3) were 62%, 44%, 50%, 52%, and 47%, respectively. The proband was diagnosed as having homozygous isolated HK deficiency. Her parents, brother, and children were diagnosed as heterozygous for this deficiency.
A novel frameshift mutation in exon 10 of the kininogen gene found in a Japanese family with isolated HK deficiency. (A) Pedigree of the family with isolated HK deficiency. Arrow indicates the proband; filled symbol, homozygous isolated HK deficiency; half-filled symbols, heterozygous isolated HK deficiency; symbols with diagonal line, deceased family members; and open symbols, unexplored subjects. (B) Identification of a base-pair insertion within exon 10 of the kininogen gene. The proband exhibited a homozygous base pair insertion of a cytidine at nucleotide position 1217 in exon 10. The proband's father, mother, and brother were heterozygous for the same defect.
A novel frameshift mutation in exon 10 of the kininogen gene found in a Japanese family with isolated HK deficiency. (A) Pedigree of the family with isolated HK deficiency. Arrow indicates the proband; filled symbol, homozygous isolated HK deficiency; half-filled symbols, heterozygous isolated HK deficiency; symbols with diagonal line, deceased family members; and open symbols, unexplored subjects. (B) Identification of a base-pair insertion within exon 10 of the kininogen gene. The proband exhibited a homozygous base pair insertion of a cytidine at nucleotide position 1217 in exon 10. The proband's father, mother, and brother were heterozygous for the same defect.
To elucidate the genetic basis of isolated HK deficiency in this family, we first examined the nucleotide sequences of exon 10 of the proband's kininogen gene. Since she had a normal LK plasma level, we thought that the defect causing the absence of HK in her plasma should reside in exon 10.1,5 This study was approved by the ethical committee of Tokushima Prefecture Central Hospital. Informed consent was provided according to the Declaration of Helsinki. Genomic DNA was isolated from whole blood, and exon 10 was amplified in standard polymerase chain reaction (PCR) conditions. The primer sequences used for PCR were the same as those used by Krijanovski et al.1 DNA sequences were determined on an ABI 3130 × 1 Genetic Analyzer (Applied Biosystems, Foster City, CA). A single cytidine nucleotide insertion was found at nucleotide position 1217 (Figure 1B).6 There were 4 consecutive cytidine nucleotides before the insertion of an extra cytidine. This insertion resulted in a frameshift in codon 406, and a premature stop signal (TGA) was generated at codon 415. We next examined the proband's family members and found that her parents and brother were heterozygous for this mutation (Figure 1B). These results indicate that a cytidine nucleotide insertion found in the proband is responsible for the isolated HK deficiency inherited in this family.
In conclusion, we identified a novel frameshift mutation in exon 10 of the kininogen gene in a Japanese family with isolated HK deficiency.
Authorship
Correspondence: Toshio Shigekiyo, Department of Internal Medicine, Tokushima Prefectural Central Hospital, 1-10-3 Kuramoto-cho, Tokushima 770-0042, Japan: e-mail: shigekiyo@tph.gr.jp.
The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal