Abstract
The development of vertebrate definitive hematopoiesis is featured by temporally and spatially dynamic distribution of hematopoietic stem/progenitor cells (HSPCs). It is proposed that the migration of definitive HSPCs, at least in part, accounts for this unique characteristic; however, compelling in vivo lineage evidence is still lacking. Here we present an in vivo analysis to delineate the migration route of definitive HSPCs in the early zebrafish embryo. Cell-marking analysis was able to first map definitive HSPCs to the ventral wall of dorsal aorta (DA). These cells were subsequently found to migrate to a previously unappreciated organ, posterior blood island (PBI), located between the caudal artery and caudal vein, and finally populate the kidney, the adult hematopoietic organ. These findings demonstrate that the PBI acts as an intermediate hematopoietic organ in a manner analogous to the mammalian fetal liver to sustain definitive hematopoiesis before adult kidney hematopoiesis occurs. Thus our study unambiguously documents the in vivo trafficking of definitive HSPCs among developmentally successive hematopoietic compartments and underscores the ontogenic conservation of definitive hematopoiesis between zebrafish and mammals.
Introduction
The ontogeny of vertebrate hematopoietic system is characterized by 2 phases of development: an early transitory primitive wave generating only primitive erythrocytes and some myeloid cells, and a definitive wave that initiates late and produces all the mature blood lineages (erythroid, myeloid, and lymphoid).1–4 In vertebrates, the first hematopoietic activity can be identified in an extra-embryonic location: yolk sac in mice and chickens, or ventral blood island (VBI) in amphibians, leading to the hypothesis that yolk sac/VBI is the sole source of hematopoietic cells, which will in turn populate downstream hematopoietic compartments.5 However, the singular hematopoietic origin from yolk sac/VBI is challenged by substantial evidence from experiments with various species that reveals another evolutionary conserved intra-embryonic hematopoietic site: the para-aortic splanchnopleurae (pSP) and later referred as aorta-gonad-mesonephros (AGM).6–11 Hence, it is currently believed that yolk sac/VBI mainly supports primitive hematopoiesis, whereas definitive hematopoiesis is autonomously initiated, at least in part, in the pSP/AGM region
The journey of definitive HSPCs is best characterized both temporally and spatially in mice.4 Although multipotential hematopoietic progenitor cells revealed by in vitro colony assays, injection into yolk sac cavity, transplantation into fetal liver of newborn mice or reconstitution of immunodeficient mice, can be detected in the pSP/AGM region and yolk sac at earlier developmental stages,12–15 the first definitive HSPCs, defined as cells fully competent to provide long-term reconstitution of irradiated adult recipients, emerge in the pSP/AGM region at embryonic day (E) 10.5.6,9,11 Subsequently, at around E11.0, definitive HSPC activity can be found in yolk sac.6,9,11 Recently, placenta is recognized as another embryonic reservoir from E10.5 for definitive HSPCs operation.16,17 However, the cellular origin of definitive HSPCs in yolk sac and placenta and their contribution to adult hematopoiesis are not clear. From E11.5, fetal liver begins to be populated by definitive HSPCs and serves as the main site that supports hematopoietic expansion and differentiation during fetal life.4 Finally, from E15.5, definitive hematopoiesis emerges in the bone marrow, which becomes the main hematopoietic organ shortly after birth.4
Despite significant progress in the characterization of anatomical compartments that host definitive HSPCs and determination of the kinetics of definitive HSPCs within these niches during development, several relevant and critical issues, like the in vivo lineage relationship of definitive HSPCs within those hematopoietic compartments, are not fully addressed. Fortunately, in vivo lineage analysis is currently facilitated by the merits of zebrafish, due to their transparent embryos and external development. In addition, zebrafish are shown to possess a hematopoietic program similar to that of higher vertebrates.18,19 As suggested by the expression of c-myb and runx1, 2 critical transcription factors essential for definitive but not primitive hematopoiesis, zebrafish definitive hematopoiesis initiates at approximately 26 hours postfertilization (hpf) from the ventral wall of DA, an equivalent of the mouse AGM.20,21 From 4 days postfertilization (dpf), the kidney begins to be populated by hematopoietic cells and becomes the major hematopoietic organ at a later stage of development.22 In this study, we report the analysis of the migration pathway of definitive HSPCs in zebrafish. Using cell labeling technique such as injection of 4,5-dimethoxy-2-nitrobenzyl (DMNB) caged fluorescein (flu) followed by local uncaging,23,24 we found that flu-labeled cells in the ventral wall of DA at 30 hpf could become rag1-positive T cells, a hematopoietic lineage exclusively generated during definitive hematopoiesis in the thymus, confirming that the cells located in the ventral wall of DA represent the zebrafish counterparts of definitive HSPCs found in the mouse AGM. When the same methodology was applied, we were able to show the migration of definitive HSPCs from the ventral wall of DA to a previously unappreciated hematopoietic organ, the posterior blood island (PBI), located between the caudal artery and caudal vein, prior to populating the kidney.
Materials and methods
Maintenance of fish strains
Laser-activated cell labeling
Lineage-tracking strategy was performed according to previous reports.23,24 Briefly, one cell stage Tg(fli1:eGFP) embryos were injected with 4.6 nl 0.5% DMNB caged, biotinylated, lysine-fixable, fluorescein dextran (10 kDa, Molecular Probes, Eugene, OR) in 0.1 M KCl and allowed to grow at 28°C in the dark until the desired stage. The embryos were subsequently anesthetized with 0.02% tricane and mounted in 0.5% low melting agarose. To achieve uncaging, enhanced green fluorescent protein (EGFP)–positive cells were focused through a 40X objective on an Olympus Fluoview confocal microscope followed by exposure to pulses of 405-nm laser excitation. Successful activation of fluorescein was assessed by examining the local enhancement of green fluorescence through epifluorescence. Finally, uncaged embryos were incubated in the dark until fixation at the required developmental stage for further analysis.
Single-color WISH
Single-color whole-mount in situ hybridization (WISH) was performed as described25 following the high resolution protocol with NBT/BCIP (Sigma, St. Louis, MO) as substrate. The preparation and sequence information of digoxigenin (DIG)–labeled antisense rag1 RNA are available in previous report.27 c-myb (primers: 5′-cggcacagacacagtgtttacagta-3′/5′-gattctcgaaggcaactttggacct-3′)28 was amplified by reverse transcriptase–polymerase chain reaction (RT-PCR) and subcloned into pGEM-T Easy vector (Promega, Madison, WI) for in vitro transcription. Stained embryos were imaged with Olympus DP70 camera on Olympus BX51 microscope.
Two-color fluorescence staining
Two-color fluorescence staining was carried out essentially as described.29 In brief, embryos were first hybridized with DIG-labeled antisense RNA probe at 68°C overnight. After washing and blocking, embryos were incubated at 4°C overnight with peroxidase (POD)-conjugated antiflu antibody (Roche, Mannheim, Germany) at a concentration of 1:500 and detected with Alexa Fluor 488 tyramide substrate (Molecular Probes) according to manufacture's instructions. The color reaction was then stopped by sequentially washing with 25%, 50%, and 75% methanol/phosphate buffered saline Tween-20 (PBST) (10 minutes each); 1% H2O2/methanol (30 minutes); 75%, 50%, and 25% methanol/PBST (10 minutes each); PBST (2 × 5 minutes). Finally, the embryos were subjected to a second color staining with anti-DIG POD (Roche) at a dilution of 1:1000 and Alexa Fluor 555 tyramide as a substrate (Molecular Probes). Fluorescence images were acquired with Olympus Fluoview confocal microscope.
Results
The fli1-positive hematopoietic cells in the ventral wall of DA represent definitive HSPCs
Based on the temporal and spatial expression pattern of transcription factor fli1,30 we reasoned that the Tg(fli1:eGFP) transgenic line, in which the EGFP expression is under the control of the fli1 promoter,26 might provide a unique tool to investigate hematopoietic stem cell development in early zebrafish embryo. First we investigated whether green fluorescence protein could mark putative definitive HSPCs presumably located in the ventral wall of DA during zebrafish embryogenesis. Two-color fluorescence in situ hybridization was performed to examine the co-localization of the EGFP protein and c-myb mRNA, one of the earliest definitive HSPC markers, in the 30 hpf Tg(fli1:eGFP) embryos. As shown in Figure 1, a subset of EGFP-positive cells in the ventral wall of DA were found to express c-myb at 30 hpf (Figures 1A-E′/F′). The co-expression of EGFP and c-myb validates the utilization of Tg(fli1:eGFP) transgenic fish in the further study of definitive HSPCs.
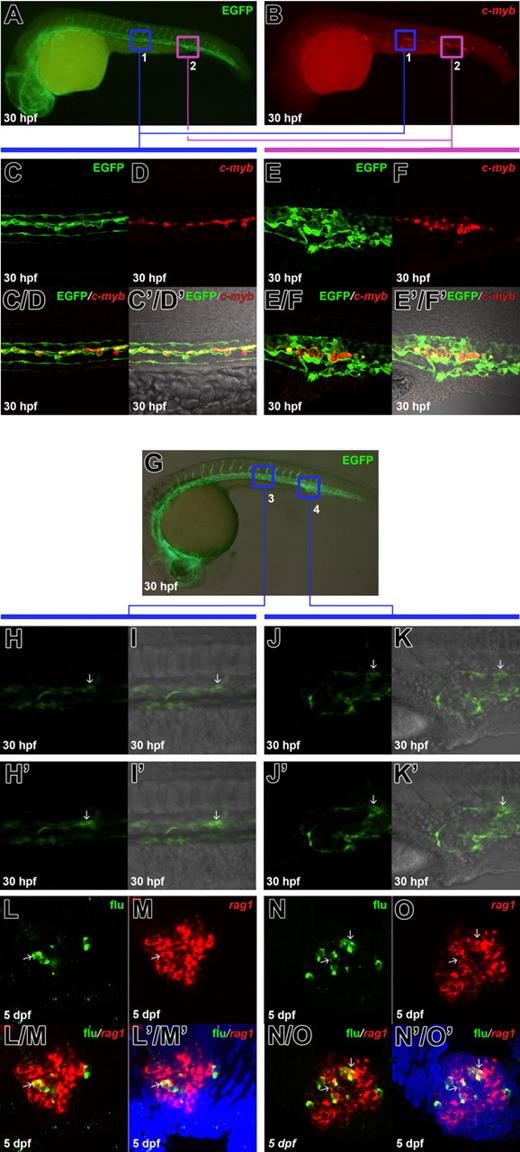
The fli1EGFP-positive hematopoietic cells in the ventral wall of DA co-express c-myb and contribute to T cells. (A) Antibody staining of EGFP in the 30-hpf Tg(fli1:eGFP) transgenic embryo. (B) Fluorescence in situ staining of c-myb mRNA in the same embryo in panel A. (C, D) Confocal images of the boxed region 1 in panels A and B show EGFP and c-myb signals in the anterior part of ventral wall of DA. (C, D) Merged image of panels C and D. (C, D) Superimposed view of panels C, D and differential interference contrast (DIC) image. (E, F) Confocal images of the boxed region 2 in panels A and B show EGFP and c-myb signals in the posterior part of ventral wall of DA. (E, F) Merged image of panels E and F. (E, F) Superimposed view of panels E, F and DIC image. (G) Live image of the 30 hpf Tg(fli1:eGFP) transgenic embryo indicates the uncaging positions in the anterior (boxed region 3) and posterior (boxed region 4) part of ventral wall of DA. (H-K) Confocal images of the boxed area 3 and 4 in G show the elevation of green fluorescence upon uncaging: H-K, images taken before uncaging; H'-K', images taken immediately after uncaging; H, H', J, and J', green fluorescence; I, I', K, and K', superimposed view of H, H', J, and J' with DIC images respectively. Arrows (H-K') indicate the successfully uncaged cells. (L, M) Confocal images of the flu and rag1 signals in the 5-dpf thymus. The embryos were uncaged in boxed region 3 in G, fixed at 5 dpf, and co-stained with flu and rag1 RNA. (L/M) Merged image of L and M. (L'/M') Superimposed view of L/M and 4′6-diamidino-2-phenylindole 2HCl (DAPI) staining (blue). (N, O) Confocal images of the flu and rag1 signals in the 5 dpf thymus. The embryos were uncaged in boxed region 4 in G, fixed at 5 dpf, and co-stained with flu and rag1 RNA. (N/O) Merged image of N and O. (N'/O') Superimposed view of N/O and DAPI staining (blue). Arrows (L-N'/O') indicate the overlapping of flu and rag1 staining.
The fli1EGFP-positive hematopoietic cells in the ventral wall of DA co-express c-myb and contribute to T cells. (A) Antibody staining of EGFP in the 30-hpf Tg(fli1:eGFP) transgenic embryo. (B) Fluorescence in situ staining of c-myb mRNA in the same embryo in panel A. (C, D) Confocal images of the boxed region 1 in panels A and B show EGFP and c-myb signals in the anterior part of ventral wall of DA. (C, D) Merged image of panels C and D. (C, D) Superimposed view of panels C, D and differential interference contrast (DIC) image. (E, F) Confocal images of the boxed region 2 in panels A and B show EGFP and c-myb signals in the posterior part of ventral wall of DA. (E, F) Merged image of panels E and F. (E, F) Superimposed view of panels E, F and DIC image. (G) Live image of the 30 hpf Tg(fli1:eGFP) transgenic embryo indicates the uncaging positions in the anterior (boxed region 3) and posterior (boxed region 4) part of ventral wall of DA. (H-K) Confocal images of the boxed area 3 and 4 in G show the elevation of green fluorescence upon uncaging: H-K, images taken before uncaging; H'-K', images taken immediately after uncaging; H, H', J, and J', green fluorescence; I, I', K, and K', superimposed view of H, H', J, and J' with DIC images respectively. Arrows (H-K') indicate the successfully uncaged cells. (L, M) Confocal images of the flu and rag1 signals in the 5-dpf thymus. The embryos were uncaged in boxed region 3 in G, fixed at 5 dpf, and co-stained with flu and rag1 RNA. (L/M) Merged image of L and M. (L'/M') Superimposed view of L/M and 4′6-diamidino-2-phenylindole 2HCl (DAPI) staining (blue). (N, O) Confocal images of the flu and rag1 signals in the 5 dpf thymus. The embryos were uncaged in boxed region 4 in G, fixed at 5 dpf, and co-stained with flu and rag1 RNA. (N/O) Merged image of N and O. (N'/O') Superimposed view of N/O and DAPI staining (blue). Arrows (L-N'/O') indicate the overlapping of flu and rag1 staining.
One remarkable feature of definitive HSPCs is the ability to give rise to all mature hematopoietic lineages, including rag1-expressing T cells in the thymus. Therefore we directly tested whether in vivo EGFP marked hematopoietic cells in the ventral wall of DA could contribute to T cells in the thymus. To do this, 405-nm laser was used to uncage a population of EGFP-positive cells (2 to 3 cells on the focal plane) in the region of ventral wall of DA of DMNB caged flu dye injected Tg(fli1:eGFP) embryos at 30 hpf from anterior to posterior position (Figures 1G-K′). Uncaged embryos were then survived to 5 dpf, and contribution to T cells was scored by double staining against flu and rag1 RNA. We found that flu-labeled cells from either anterior or posterior position could give rise to rag1-positive T cells (Figures 1L-N′/O′) at comparable frequency. Of 13 anterior uncaged embryos, 3 embryos contained flu/rag1 double-positive cells (Figures 1L-L′/M′), whereas 3 of 12 posterior uncaged embryos were found to have flu/rag1 co-stained cells (Figures 1N-N′/O′). These data suggest that the ventral wall of DA from the anterior to posterior is enriched with definitive HSPCs capable of generating T cells.
Definitive HSPCs are generated de novo in the ventral wall of DA
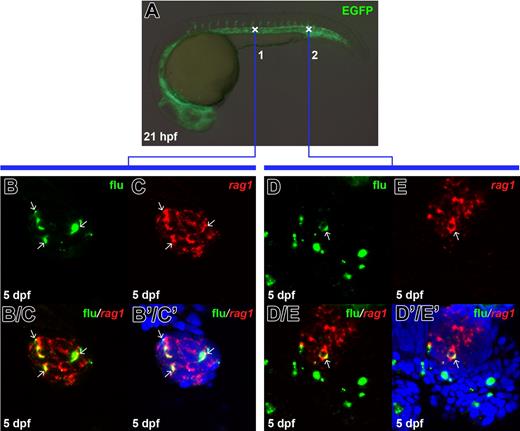
Since circulation already has been established in zebrafish embryos at 30 hpf, it is possible that these early definitive HSPCs in the ventral wall of DA are not generated autonomously but instead come from elsewhere in the embryo through the bloodstream. To rule out this possibility, we performed uncaging analysis in the earlier stage embryo, 21 hpf, before circulation started (Figure 2A). The contribution of uncaged cells to T cells was examined by double staining against flu and rag1 RNA. Results showed that the uncaged cells in the intermediate cell mass (ICM), either at anterior or posterior position, were able to give rise to T cells in the thymus: 2 of 7 anterior uncaged embryos had flu/rag1 double-positive cells in the thymus (Figures 2B-B′/C′), whereas 2 of 8 posterior uncaged embryos contained flu/rag1 double-positive cells (Figures 2D-D′/E′). Together, this in vivo result confirms that definitive HSPCs are generated autonomously and distributed in the ventral wall of DA along the anteroposterior axis. In fact, it appears that definitive HSPCs or perhaps its precursors, presumably the hemangioblast, already have resided in the ICM region by 21 hpf.
The fli1EGFP-positive hematopoietic cells in the ICM labeled before circulation initiation can contribute to T cells. (A) Live image of the 21-hpf Tg(fli1:eGFP) transgenic embryo indicates the uncaging positions in the anterior (cross 1) or posterior (cross 2) part of the ICM. (B, C) Confocal images of the flu and rag1 signals in the 5-dpf thymus. The embryos were uncaged at cross 1 in A, fixed at 5 dpf, and co-stained with flu and rag1 RNA. (B/C) Merged image of panels B and C. (B'/C') Superimposed view of panels B/C and DAPI signal (blue). (D, E) Confocal images of the flu and rag1 signals in the 5-dpf thymus. The embryos were uncaged at cross 2 in panel A, fixed at 5 dpf, and co-stained with flu and rag1 RNA. (D/E) Merged image of panels D and E. (D'/E') Superimposed view of panels D/E and DAPI signal (blue). Arrows indicate the overlapping of flu and rag1 staining.
The fli1EGFP-positive hematopoietic cells in the ICM labeled before circulation initiation can contribute to T cells. (A) Live image of the 21-hpf Tg(fli1:eGFP) transgenic embryo indicates the uncaging positions in the anterior (cross 1) or posterior (cross 2) part of the ICM. (B, C) Confocal images of the flu and rag1 signals in the 5-dpf thymus. The embryos were uncaged at cross 1 in A, fixed at 5 dpf, and co-stained with flu and rag1 RNA. (B/C) Merged image of panels B and C. (B'/C') Superimposed view of panels B/C and DAPI signal (blue). (D, E) Confocal images of the flu and rag1 signals in the 5-dpf thymus. The embryos were uncaged at cross 2 in panel A, fixed at 5 dpf, and co-stained with flu and rag1 RNA. (D/E) Merged image of panels D and E. (D'/E') Superimposed view of panels D/E and DAPI signal (blue). Arrows indicate the overlapping of flu and rag1 staining.
Definitive HSPCs migrate from the ventral wall of DA to the PBI
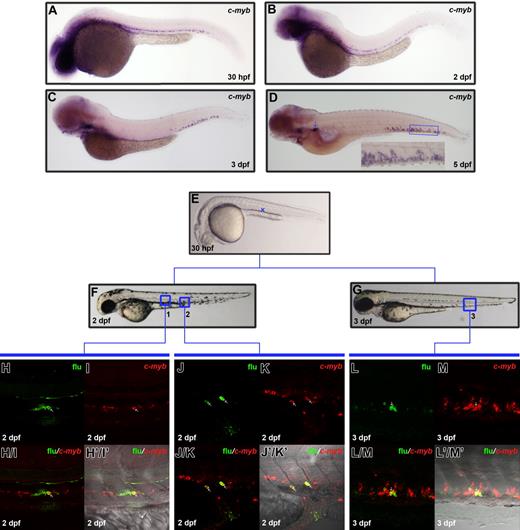
Given that c-myb expression is gradually down-regulated in the ventral wall of DA from 2 dpf onwards (Figures 3A-D) and pronephros is not populated by hematopoietic cells until 4 dpf,22 we hypothesized that there had to be a transitory hematopoietic organ functioning before hematopoiesis in pronephros was launched. To explore the anatomical location of this putative hematopoietic compartment, we closely examined c-myb expression by WISH at various developmental stages. We found that, concomitant with the down-regulation of c-myb expression in the ventral wall of DA, the caudal region between the caudal artery and caudal vein began to express c-myb after 2 dpf (Figures 3A-D). We named this region the posterior blood island (PBI). By 3 dpf, c-myb–positive cells were predominantly located in the PBI, leaving only a few positive cells in the ventral wall of DA, approximately one day earlier than their appearance in the kidney (Figure 3C). And by 5 dpf, c-myb expression was detected in both the PBI and kidney (Figure 3D). Furthermore, these c-myb–positive cells were absent in the runx1 MO (morpholino oligonucleotides)–injected embryos (morphants) (data not shown), in which definitive hematopoiesis is impaired,20,21 suggesting these c-myb–positive cells are of definitive origin. These observations strongly indicate that the PBI is another embryonic site for definitive hematopoiesis before kidney hematopoiesis occurs.
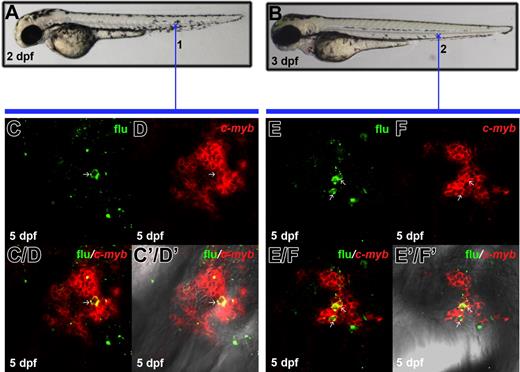
Dynamic c-myb expression and migration of c-myb–positive cells from the ventral wall of DA to PBI. (A-D) WISH shows c-myb expression pattern at 30-hpf, 2-dpf, 3-dpf, and 5-dpf embryos. The inset is a high magnification of the boxed region. The arrow in panel D indicates c-myb expression in the kidney. (E-G) Lateral view of 30-hpf, 2-dpf, and 3-dpf embryo. Embryos were uncaged in the anterior part (blue cross in panel E) of ventral wall of DA, fixed at 2 dpf or 3 dpf, and co-stained with flu and c-myb RNA. (H, I) Confocal images of the boxed region 1 in panel F show flu and c-myb signals in the original uncaged region (corresponding to the region marked by the blue cross in panel E) at 2 dpf. (H/I) Merged view of panels H and I. (H'/I') Superimposed view of panels H/I and DIC image. (J, K) Confocal images of the boxed region 2 in panel F show flu and c-myb signals in the PBI at 2 dpf. (J/K) Merged image of panels J and K. (J'/K') Superimposed view of panels J/K and DIC image. (L, M) Confocal images of the boxed region 3 in panel G show flu and c-myb signals in the PBI at 3 dpf. (L/M) Merged image of panels L and M. (L'/M') Superimposed view of panels L/M and DIC image. Arrows (H-L'/M') indicate the co-staining of flu and c-myb.
Dynamic c-myb expression and migration of c-myb–positive cells from the ventral wall of DA to PBI. (A-D) WISH shows c-myb expression pattern at 30-hpf, 2-dpf, 3-dpf, and 5-dpf embryos. The inset is a high magnification of the boxed region. The arrow in panel D indicates c-myb expression in the kidney. (E-G) Lateral view of 30-hpf, 2-dpf, and 3-dpf embryo. Embryos were uncaged in the anterior part (blue cross in panel E) of ventral wall of DA, fixed at 2 dpf or 3 dpf, and co-stained with flu and c-myb RNA. (H, I) Confocal images of the boxed region 1 in panel F show flu and c-myb signals in the original uncaged region (corresponding to the region marked by the blue cross in panel E) at 2 dpf. (H/I) Merged view of panels H and I. (H'/I') Superimposed view of panels H/I and DIC image. (J, K) Confocal images of the boxed region 2 in panel F show flu and c-myb signals in the PBI at 2 dpf. (J/K) Merged image of panels J and K. (J'/K') Superimposed view of panels J/K and DIC image. (L, M) Confocal images of the boxed region 3 in panel G show flu and c-myb signals in the PBI at 3 dpf. (L/M) Merged image of panels L and M. (L'/M') Superimposed view of panels L/M and DIC image. Arrows (H-L'/M') indicate the co-staining of flu and c-myb.
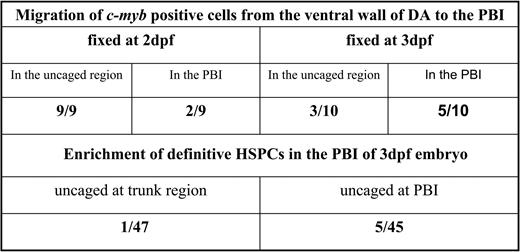
To investigate whether the enrichment of c-myb–positive cells in the PBI reflects the immigration of definitive HSPCs from the ventral wall of DA, we uncaged EGFP-positive cells in the anterior part of the ventral wall of DA in the 30 hpf Tg(fli1:eGFP) embryos and examined the distribution of flu/c-myb double-positive cells in 2 dpf and 3 dpf (Figures 3E-L′/M′). When embryos were examined at 2 dpf, 2 of 9 embryos were found to have flu/c-myb double-positive cells in the caudal region, suggesting homing to the PBI has already begun at 2 dpf (Figures 3J-J′/K′ and Figure 4). In fact, the ratio of embryos containing flu/c-myb double-positive cells in the PBI increased to 5 of 10 when embryos were fixed at 3 dpf (Figures 3L-L′/M′ and Figure 4). On the other hand, 9 of 9 embryos fixed at 2 dpf had flu/c-myb double-positive cells in the original uncaged region (Figures 3H-H′/I′ and Figure 4), and this ratio dropped to 3 of 10 when embryos were examined at 3 dpf (Figure 4). The detection of flu/c-myb double-positive cells in the PBI after embryos were uncaged at the anterior part of the ventral wall of DA strongly indicates the migration of c-myb–positive definitive HSPCs to the PBI, where they presumably undergo further expansion or differentiation.
Migration of definitive HSPCs to the PBI. *EGFP positive cells in the anterior part of ventral wall of DA in the 30hpf Tg(fli1:eGFP) embryos were uncaged. Embryos were then fixed at either 2dpf or 3dpf for double staining against flu and c-myb. The ratio indicates the number of embryos containing flu/c-myb double positive cells found in the corresponding region versus the total number of embryos examined. †Cells in the PBI and trunk region between dorsal aorta and posterior cardinal vein were uncaged at 3dpf. Embryos were survived to 5dpf for double staining against flu and ragI. The ratio indicates the number of embryos containing flu/ragI double positive cells in the thymus versus the total number of embryos examined.
Migration of definitive HSPCs to the PBI. *EGFP positive cells in the anterior part of ventral wall of DA in the 30hpf Tg(fli1:eGFP) embryos were uncaged. Embryos were then fixed at either 2dpf or 3dpf for double staining against flu and c-myb. The ratio indicates the number of embryos containing flu/c-myb double positive cells found in the corresponding region versus the total number of embryos examined. †Cells in the PBI and trunk region between dorsal aorta and posterior cardinal vein were uncaged at 3dpf. Embryos were survived to 5dpf for double staining against flu and ragI. The ratio indicates the number of embryos containing flu/ragI double positive cells in the thymus versus the total number of embryos examined.
An important prediction made by this migration pathway is that, compared to the trunk region, at 3 dpf the PBI should be relatively enriched with definitive HSPCs that can give rise to T cells in the thymus. To prove this, we uncaged cells in the PBI and trunk region between dorsal aorta and posterior cardinal vein at 3 dpf, and contribution to T cells was determined by double staining against flu and rag1 at 5 dpf (Figure 5). Five of 45 PBI uncaged embryos were found to have flu/rag1 double-positive cells in the thymus, whereas only 1 of 47 trunk uncaged embryos had contribution to T cells, showing the relative confinement of definitive HSPCs to the PBI by 3 dpf (Figure 4 and Figures 5B-D′/E′). Taken together, we conclude that the PBI acts as a hematopoietic organ accommodating definitive HSPCs migrated from the ventral wall of DA. Notably, Murayama et al also have recently reported findings showing the migration of definitive HSPCs from the ventral wall of DA to the caudal hematopoietic tissue (CHT), a region identical to the PBI.31
Definitive HSPCs in the PBI can give rise to T cells. (A) Lateral view of 3-dpf fish indicates the uncaging positions (cross 1: the trunk region; cross 2: the PBI region). (B, C) Confocal images of the flu and rag1 signals in the 5-dpf thymus. The embryos were uncaged at cross 1 in panel A, fixed at 5 dpf, and co-stained with flu and rag1 RNA. (B, C) Merged image of panels B and C. (B'/C') Superimposed view of panels B/C and DAPI staining. (D, E) Confocal images of the flu and rag1 signals in the 5-dpf thymus. The embryos were uncaged at cross 2 in panel A, fixed at 5 dpf, and co-stained with flu and rag1 RNA. (D/E) Merged image of panels D and E. (D'/E') Superimposed view of panels D/E and DAPI staining. Arrows indicate the co-staining of flu and rag1.
Definitive HSPCs in the PBI can give rise to T cells. (A) Lateral view of 3-dpf fish indicates the uncaging positions (cross 1: the trunk region; cross 2: the PBI region). (B, C) Confocal images of the flu and rag1 signals in the 5-dpf thymus. The embryos were uncaged at cross 1 in panel A, fixed at 5 dpf, and co-stained with flu and rag1 RNA. (B, C) Merged image of panels B and C. (B'/C') Superimposed view of panels B/C and DAPI staining. (D, E) Confocal images of the flu and rag1 signals in the 5-dpf thymus. The embryos were uncaged at cross 2 in panel A, fixed at 5 dpf, and co-stained with flu and rag1 RNA. (D/E) Merged image of panels D and E. (D'/E') Superimposed view of panels D/E and DAPI staining. Arrows indicate the co-staining of flu and rag1.
Population of the pronephros by definitive HSPCs from the PBI
Next we investigated whether definitive HSPCs within the PBI would further seed the pronephros or kidney, the adult hematopoietic organ. To do this, hematopoietic cells in the PBI were uncaged (in this case, we uncaged ∼50 cells) at 2 dpf or 3 dpf, and the contribution to the c-myb–positive hematopoietic precursors in the pronephros was determined by double staining against flu and c-myb at 5 dpf (Figure 6). While 7 of 15 2-dpf uncaged embryos were found to have flu/c-myb double cells in the pronephros (Figures 6C-C′/D′), 18 of 20 3-dpf uncaged embryos have flu-labeled cells become c-myb–positive hematopoietic cells in the pronephros (Figures 6E-E′/F′). This observation is consistent with the result showing the higher number of the c-myb–positive HSPCs migrated to the PBI at 3 dpf (Figures 3E-L′/M′ and Figure 4). It may also reflect the role of the PBI in supporting definitive HSPCs expansion. Thus our data clearly demonstrate that definitive HSPCs in the PBI migrate to the pronephros during ontogeny.
Definitive HSPCs in the PBI can populate pronephros. (A, B) Lateral view of 2-dpf (A) and 3-dpf (B) fish indicates the uncaging positions (cross 1 and 2). (C, D) Confocal images of the flu and c-myb signals in the 5-dpf kidney. The embryos were uncaged at cross 1 in panel A, fixed at 5 dpf, and co-stained with flu and c-myb RNA. (C/D) Merged image of panles C and D. (C'/D') Superimposed view of panels C/D and DIC image. (E, F) Confocal images of the flu and c-myb signals in the 5-dpf kidney. The embryos were uncaged at cross 2 in panel A, fixed at 5 dpf, and co-stained with flu and c-myb RNA. (E/F) Merged image of panels E and F. (E'/F') Superimposed view of panels E/F and DIC image. Arrows indicate the co-staining of flu and c-myb.
Definitive HSPCs in the PBI can populate pronephros. (A, B) Lateral view of 2-dpf (A) and 3-dpf (B) fish indicates the uncaging positions (cross 1 and 2). (C, D) Confocal images of the flu and c-myb signals in the 5-dpf kidney. The embryos were uncaged at cross 1 in panel A, fixed at 5 dpf, and co-stained with flu and c-myb RNA. (C/D) Merged image of panles C and D. (C'/D') Superimposed view of panels C/D and DIC image. (E, F) Confocal images of the flu and c-myb signals in the 5-dpf kidney. The embryos were uncaged at cross 2 in panel A, fixed at 5 dpf, and co-stained with flu and c-myb RNA. (E/F) Merged image of panels E and F. (E'/F') Superimposed view of panels E/F and DIC image. Arrows indicate the co-staining of flu and c-myb.
Discussion
In this report, we were first able to locate the cells with definitive HSPC activity to the region surrounding the ventral wall of DA, the equivalent of mammalian AGM,20,21 in 30 hpf zebrafish embryos, as those flu-labeled cells could generate rag1-expressing T cells. Subsequently, these definitive HSPCs were found to home to the PBI, a region between caudal artery and caudal vein, as indicated by flu/c-myb double-positive cells in this region at 2 dpf and 3 dpf after uncaging cells in the ventral wall of DA at 30 hpf. Definitive HSPCs in the PBI is further shown to continue their journey to populate the adult hematopoietic organ, pronephros or kidney. Together these findings unravel a previously less-appreciated role of the PBI as an important definitive hematopoietic compartment during early zebrafish embryogenesis and provide convincing in vivo lineage evidence to demonstrate the migratory path of definitive HSPCs during ontogeny. Our data are largely consistent with a recent study by Murayama et al31 except that they reported that HSPCs emigrating from the AGM before day 2 have a distinct fate from that of HSPCs released after day 2. In their report, while cells in the PBI labeled at 3 dpf were able to seed the kidney, cells in the PBI labeled at 2 dpf were not, although both of them were capable of generating T cells. In contrast, we found that either of these 2 populations could populate the kidney, even though the contribution of 2-dpf population was detected at a lower frequency than that of 3 dpf. This discrepancy is possibly due to the low number of 2-dpf labeled cells migrated to the kidney, therefore making it difficult to detect without performing double staining.
Based on the expression of c-myb and runx1, 2 critical transcription factors essential for definitive hematopoiesis, it is believed that zebrafish definitive hematopoiesis initiates at approximately 26 hpf from the ventral wall of DA.20,21 The observation that the fli1 EGFP-positive cells in the ventral wall of DA, when uncaged at 21 hpf, are capable of giving rise to T cells in the thymus strongly indicates that by 21 hpf definitive HSPCs or their precursors, presumably the hemangioblast, that can give rise to only hematopoietic and endothelial lineages, have already localized in the ICM region. As recent in vivo lineage tracing analysis has demonstrated the presence of hemangioblast along the ventral mesoderm of gastrula stage (about 6 hpf) zebrafish embryos,32 it would be of great interest to explore whether these bipotential progenitors exist, presumably in the ICM region, at late stage of zebrafish development using similar labeling technique of single-cell resolution.
One characteristic of the PBI we identified here is that it is a highly vascularized tissue. Parallel to hematopoietic development in the PBI, numerous vessel plexus is reported to spout from the caudal vein.33 Thus these caudal vein plexus may constitute a special hematopoietic niche for the development of definitive hematopoietic cells. The vascularized feature of the PBI is reminiscent of that of placenta and fetal liver in mice. As the PBI is found to be capable of not only sustaining the growth of definitive HSPCs emigrating from the ventral wall of DA but also promoting their myeloid/erythroid differentiation (unpublished data, October 2006), this hematopoietic tissue is more likely to represent an equivalent of mouse fetal liver rather than the placenta, which mainly supports definitive HSPCs without promoting myeloid/erythroid differentiation.16,17 Considering the fact that pronephros is not prepared to support definitive hematopoiesis until 4 dpf,22 we believe that the PBI acts as an obligatory “fetal” hematopoietic organ to sustain definitive hematopoiesis before kidney hematopoiesis is fully launched.
The migration of definitive HSPCs from the ventral wall of DA to the PBI raises an intriguing question as to the route by which definitive HSPCs reach the PBI. It is postulated in mice that definitive HSPCs could populate fetal liver through blood circulation.4 However, an extravascular pathway is not formally excluded. In our experiment, we found the presence of migrating flu/c-myb double-positive definitive HSPCs in the circulation. And among the 9 embryos that were found to have c-myb–positive cells homing to the PBI, we were unable to detect flu/c-myb double-positive cells in the region between the uncaged site and the PBI, suggesting a rapid migration process. Thus our data favors the hypothesis that seeding the PBI by definitive HSPCs is propelled by blood flow rather than via an extravascular path, which is supposed to be slow. It also remains to be shown whether homing to the PBI is compulsory for the definitive HSPCs to give rise to T cells in the thymus. Definitive HSPCs from the ventral wall of DA could either directly populate the thymus via circulation or undergo an essential training step in the PBI before seeding the thymus. To address this question awaits a time course study and the employment of live tracing strategy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Weng Onn Cheong and Wanyu Li (IMCB, Singapore) for their technical assistance.
Z.W. is supported by the Agency for Science, Technology, and Research, Singapore (A*STAR).
Authorship
Contribution: H.J. and J.X. designed the research, performed experiments, and analyzed data. Z.W. designed the research and analyzed data. H.J. and Z.W. wrote this paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests..
H.J. and J.X. contributed equally to this work.
Correspondence: Zilong Wen., Department of Biochemistry, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China; e-mail: zilong@ust.hk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal