Abstract
The carboxylation of glutamic acid residues to γ-carboxyglutamic acid (Gla) by the vitamin K–dependent γ-glutamyl carboxylase (γ-carboxylase) is an essential posttranslational modification required for the biological activity of a number of proteins, including proteins involved in blood coagulation and its regulation. Heterozygous mice carrying a null mutation at the γ-carboxylase (Ggcx) gene exhibit normal development and survival with no evidence of hemorrhage and normal functional activity of the vitamin K–dependent clotting factors IX, X, and prothrombin. Analysis of a Ggcx+/− intercross revealed a partial developmental block with only 50% of expected Ggcx−/− offspring surviving to term, with the latter animals dying uniformly at birth of massive intra-abdominal hemorrhage. This phenotype closely resembles the partial midembryonic loss and postnatal hemorrhage previously reported for both prothrombin- and factor V (F5)–deficient mice. These data exclude the existence of a redundant carboxylase pathway and suggest that functionally critical substrates for γ-carboxylation, at least in the developing embryo and neonate, are primarily restricted to components of the blood coagulation cascade.
Introduction
The vitamin K–dependent γ-glutamyl caboxylase (γ-carboxylase) is localized to the endoplasmic reticulum (ER) and is predicted to be a transmembrane protein. The enzyme functions posttranslationally to convert selected glutamic acid (Glu) residues within a specific subset of proteins to Gla.1,2 γ-carboxylase functional activity can be detected in most or all mammalian tissues,3 and the carboxylase gene has been demonstrated to be conserved not only in other vertebrates such as zebrafish,4 but also in chordates such as Ciona intestinalis5 and diverse invertebrates including Drosophila6 and Conus, the cone snail.7,8 The most thoroughly characterized γ-carboxylase substrates are the vitamin K–dependent coagulation factors prothrombin, factor VII, factor IX, factor X, protein C, protein S, and protein Z.1,2 Prothrombin and factors VII, IX, and X are involved in the coagulant response leading to the generation of thrombin at sites of injury, whereas protein C and protein S play roles in an antithrombotic pathway that limits coagulation by inactivating the important coagulation cofactors Va and VIIIa. Deficiencies for a number of the vitamin K–dependent coagulation proteins have been well characterized in both mice and humans. Of note, knockout mice with complete deficiency of prothrombinase or prothrombin exhibit an embryonic lethal phenotype in approximately one-half of animals, possibly due to loss of a required early developmental signal through the thrombin receptor, with the remaining mice dying at term of massive hemorrhage.9–12
Other mammalian proteins known to undergo γ-carboxylation include osteocalcin, matrix Gla protein (MGP),13 Gas6,14 nephrocalcin A-D,15 the proline-rich γ-carboxylated proteins (PRGP-1 and PRGP-2),16 and the transmembrane proteins TMG3 and TMG4.17 γ-Carboxylation is absolutely required for function of the vitamin K–dependent blood coagulation factors.1,2,18 Although the functional importance of γ-carboxyglutamic acid in other proteins is less well characterized, this processing appears to be necessary for full function of osteocalcin19 and MGP.19 High levels of undercarboxylated osteocalcin are associated with low bone mineral density and increased fractures,20 and osteocalcin knockout mice exhibit increased bone mass evident by 4 to 6 months of age.21 In contrast, deletion of the MGP gene results in widespread vascular calcification and uniform lethal aortic rupture by 2 months of age.22,23 Given the generalized expression of the γ-carboxylase in most or all tissues, it is likely that a number of other Gla-containing proteins with specialized functions remain to be identified, including additional members of a recently characterized class of proteins lacking a conventional γ-carboxylase recognition site.24
The γ-carboxylase requires reduced vitamin K as an obligate cofactor. The oral anticoagulant warfarin functions by inhibiting vitamin K epoxide reductase (VKOR) and thereby blocking the regeneration of active reduced vitamin K.2 The VKOR gene has recently been cloned and shown to encode a small transmembrane protein localized to the ER.25,26 Coumarin-like compounds, including warfarin, are currently the only active oral anticoagulant drugs approved in the United States and are widely used in the treatment of thromboembolic diseases. Administration of warfarin during pregnancy results in the fetal warfarin syndrome, characterized by a spectrum of birth defects including abnormal midfacial development, stippling of the epiphyses, and mental retardation.27 Although there are data to suggest an effect of warfarin on bone health, bone abnormalities in warfarin-treated adult patients have not been consistently demonstrated. However, aberrant vascular calcification and other abnormalities have been reported with warfarin treatment in animal systems,28 and accelerated vascular calcification has also been observed in patients with renal failure treated with warfarin.29
Mutations in the γ-carboxylase cause a rare autosomal recessive bleeding disorder referred to as combined deficiency of the vitamin K–dependent coagulation factors (VKCFD).30,31 A few cases of VKCFD are also now known to result from missense mutations in VKOR.26 Although patients with VKCFD exhibit variable levels of bleeding, abnormalities related to other γ-carboxylase substrates are not generally observed. However, in a recent report,32 2 affected patients were noted to have skeletal and cardiac developmental abnormalities possibly related to γ-carboxylase deficiency. Of note, all the reported human mutations appear to be hypomorphic, with no individuals yet identified with complete γ-carboxylase deficiency. To investigate the importance of γ-glutamyl carboxylation for the function of the Gla-containing proteins, we generated mice completely lacking γ-carboxylase activity by gene targeting.
Materials and methods
γ-Carboxylase gene targeting
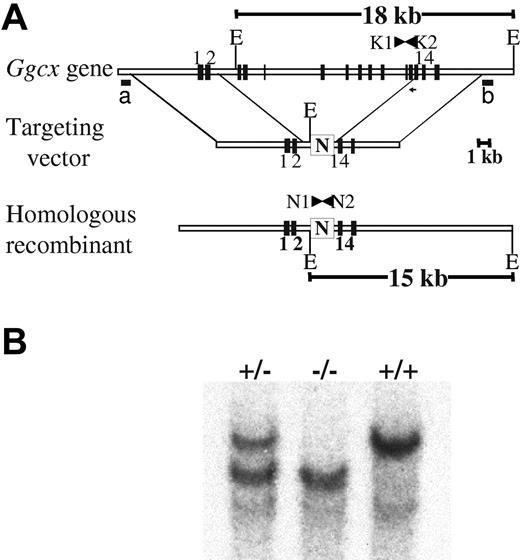
CJ7 and R1 embryonic stem (ES) cells were cultured as described previously.9,33 The genomic structure of the Ggcx allele was determined by comparing the sequence of the Ggcx cDNA with the sequence of identified genomic clones. Genomic fragments were generated from a 129Sv genomic library (Stratagene, La Jolla, CA) or by polymerase chain reaction (PCR) from 129Sv DNA, as previously reported.9,33 Further characterization of the intron/exon boundaries of the Ggcx locus was determined by direct sequencing of genomic clones and comparison of genomic PCR data with RNA-derived reverse transcriptase (RT)–PCR products. A 5.8-kb SpeI/SpeI fragment from the genomic clone mGgcx-A, encompassing Ggcx exons 1 to 2 (Figure 1A) was cloned into the NheI site in the pFlox vector and served as the 5′ arm of the homologous recombination cassette. The 3′ homology arm was constructed by cloning a 7-kb BamHI/BamHI fragment from the genomic clone mGgcx-B, which contains exons 14 to 15 of the Ggcx genomic sequence, into the BamHI site of the pFlox vector. The final targeting construct (25 μg) was linearized with SalI and electroporated into both CJ7 and R1 ES cells at a density of 107/0.6 mL at 320 V and 250 μF (GenePulser; BioRad, Hercules, CA). Cells were plated on mouse embryonic fibroblast (MEF) feeder cells to a final density of 2 × 106 ES cells per 100-mm plate. At 24 hours after plating, G418 was added to the growth media to a final concentration of 400 μg/mL, and the cells were allowed to grow in this media for 24 hours. The media were then changed, and fresh growth media were added containing G418 at a final concentration of 300 μg/mL. A 1-kb BamHI/HindIII fragment (Figure 1Al probe b) was used as probe for screening Southern blots of EcoRV-digested genomic DNA to identify individual ES cell clones that had undergone homologous recombination at the Ggcx locus. Correct genomic structure of the targeted Ggcx allele at the 5′ end of positive ES clones was confirmed by Southern blotting of XbaI-digested genomic DNA with hybridization probe a (Figure 1A). Targeting efficiency was approximately 1%, with 5 successfully targeted clones identified among 474 screened R1 colonies and 4 among 500 CJ7 colonies. Two independent R1 and 2 CJ7 ES clones each were injected into C57Bl/6J blastocysts. High-level chimeric animals were then bred to C57Bl/6J females to obtain mice heterozygous for γ-carboxylase. Germline transmission was obtained from 2 R1-derived clones, but neither of the CJ7 clones.
Targeting of the murine γ-carboxylase gene by homologous recombination. (A) structure of the endogenous γ-carboxylase gene. The targeting vector contains a neomycin resistance cassette (N), driven by the phosphoglycerokinase promoter, that replaces murine exons 3 to 13 flanked by 5.8 kb of 5′ and 7 kb of 3′ homologous DNA arms. The predicted product of successful homologous recombination is shown at the bottom. The location of hybridization probes a and b, used to detect successful targeting, are indicated. (B) Southern blot analyses demonstrating the expected genomic fragments from the 3′ end of the locus. Genomic DNA was prepared from tail biopsies of Ggcx+/+, Ggcx+/−, and Ggcx−/− mice and was analyzed by restriction digestion with EcoRV and hybridization with probe b. Restriction of Ggcx+/+ genomic DNA yields a fragment of 18 kb after hybridization with probe b. The targeted Ggcx+/− DNA yields 2 distinct bands of 15 kb and 18 kb, indicating that correct targeting of the Ggcx gene has occurred. Animals harboring copies of the targeted allele at both chromosomal loci show the expected hybridization band only at 15 kb, indicating that both copies of the Ggcx gene have been successfully deleted. The presence of the PCR primers (N1, N2, K1, and K2) used for subsequent genotyping are indicated in panel A.
Targeting of the murine γ-carboxylase gene by homologous recombination. (A) structure of the endogenous γ-carboxylase gene. The targeting vector contains a neomycin resistance cassette (N), driven by the phosphoglycerokinase promoter, that replaces murine exons 3 to 13 flanked by 5.8 kb of 5′ and 7 kb of 3′ homologous DNA arms. The predicted product of successful homologous recombination is shown at the bottom. The location of hybridization probes a and b, used to detect successful targeting, are indicated. (B) Southern blot analyses demonstrating the expected genomic fragments from the 3′ end of the locus. Genomic DNA was prepared from tail biopsies of Ggcx+/+, Ggcx+/−, and Ggcx−/− mice and was analyzed by restriction digestion with EcoRV and hybridization with probe b. Restriction of Ggcx+/+ genomic DNA yields a fragment of 18 kb after hybridization with probe b. The targeted Ggcx+/− DNA yields 2 distinct bands of 15 kb and 18 kb, indicating that correct targeting of the Ggcx gene has occurred. Animals harboring copies of the targeted allele at both chromosomal loci show the expected hybridization band only at 15 kb, indicating that both copies of the Ggcx gene have been successfully deleted. The presence of the PCR primers (N1, N2, K1, and K2) used for subsequent genotyping are indicated in panel A.
PCR genotyping
Two pairs of primers were used for genotyping (Figure 1A). N1 (5′ GGACTGGCTGCTATTGGGCGAAGTG) and N2 (5′ GAAGAACTCGTCAAGAAGGCGATAGAAGG) are specific for amplification of the neomycin sequence unique to the targeted allele and give rise to a 525-bp product. Primers K1 (5′ GGCTGTCTGGTCCCCCTTCC) (NM_019 802; Genbank nt 1544-1563) and K2 (5′ GTCTGATTTTTCTGTTCTGCCACCAA) (NM_019 802; Genbank nt 1764-1789) are located within the region that was deleted in the homologously recombined γ-carboxylase allele and thus give rise to a 416-bp amplification product derived exclusively from the wild-type Ggcx allele. Both sets of primers were cycled as follows: 94°C for 1 minute, 60°C for 1 minute and 20 seconds, and 72°C for 1 minute for 35 cycles.
Timed mating
Timed matings were performed by intercrossing Ggcx+/− mice that had been backcrossed into C57BL/6J mice for 4 or more generations (N4 or greater). The embryos from each intercross were harvested at either 9.5 or 18 dpc (days after coitus). DNA was prepared from 9.5-dpc yolk sacs or tail biopsies of 18-dpc embryos for subsequent PCR genotyping.
Preparation of solubilized γ-carboxlase
Livers were quick frozen in liquid nitrogen, cut into several pieces, suspended in 1 mL PBS (2.7 mM KCl/1.5 mM KH2PO4/137 mM NaCl/6.5 mM Na2HPO4)/20% glycerol/PIC (2 mM dithiothreitol/2 mM EDTA/leupeptin [0.5 μg/mL]/pepstatin A [1 μg/mL]/aprotinin [1 μg/mL]) and homogenized with 10 strokes of a Potter tissue grinder with a Contorque Power Unit (Eberback, Ann Arbor, MI) 4 times, cooling the sample on ice after 10 strokes. The sample was separated by centrifugation at 10 000g for 10 minutes at 4°C, and the supernatant was collected. The pellet was resuspended in 1 mL PBS/20% glycerol/PIC, and the homogenization procedure was repeated. The supernatants from 4 cycles of homogenization and centrifugation were pooled, and the microsomes were isolated by centrifugation at 38 000g for 1 hour at 4°C. The microsomal pellet was resuspended in 1 mL PBS/20% glycerol/PIC/0.5% CHAPS/0.1% phospatidylcholine and solubilized with sonication using a Ultrasonics Processor W22 with a Ultrasonics Converter C2 with a microprobe (Heat Systems-Ultronics Inc, Farmingdale, NY) at power level 4 for 5 seconds. The sample was cooled on ice and the sonication was repeated. Insoluble material was removed by centrifugation at 38 000g for 1 hour at 4°C, and the supernatant was removed. The solubilization was repeated on the pellet. The supernatants containing the γ-carboxylase were combined and stored at −80°C.
Assay for vitamin K–dependent carboxylase activity
Vitamin K1 (10 mg/mL), purchased from Abbott (Chicago, IL), was chemically reduced with 8 mg NaBH4 for 30 minutes at ambient temperature under a N2 atmosphere protected from light. It was diluted 1:4 with PBS/1% CHAPS prior to use. Carboxylase activity was measured as the incorporation of 14CO2 into a synthetic peptide substrate (FLEEL) in the presence of propeptide as described previously.34 Incorporation of 14CO2 into FLEEL by solubilized γ-carboxylase from the livers of Ggcx+/+ mice was considered to be 100% γ-carboxylase activity, and the incorporation of 14CO2 by solubilized carboxylase from Ggcx+/− and Ggcx−/− mice were expressed as a percentage of wild-type.
Western blot analyses
Monoclonal antibovine carboxylase antibody EB3 was used to detect mouse γ-carboxylase. The epitope for this antibody is in the C-terminal 14 amino acids of the enzyme (B. Bouchard, B.F., and B.C.F., unpublished results, December 2006). Western blot analyses were performed as previously described.35
Coagulation assays
Assays for factor IX, factor X, and prothrombin coagulant activity were performed on plasma samples from Ggcx+/+ and Ggcx+/− mice. Blood was anticoagulated with 3.8% sodium citrate (vol/vol 9:1) and reduced to platelet-poor plasma. Pooled citrated plasma from normal C57BL/6J mice was used as the standard for normal levels of prothrombin, factor IX, and factor X coagulant activity. Factor-deficient human plasmas and Russell viper venom in cephalin were purchased from Sigma Diagnostics (St Louis, MO).
Results
Construction of the γ-carboxylase knockout allele
The structure of the murine γ-carboxylase gene (Figure 1A) closely resembles that previously reported for the human gene.36 The murine γ-carboxylase gene was targeted by homologous recombination to replace murine exons 3 to 13 with a neomycin resistance cassette (N), removing the coding sequence corresponding to 74% of the γ-carboxylase protein (Figure 1). Germline transmission was obtained from 2 independent targeted ES cell clones. Southern blot analyses confirmed the expected structure of the knockout allele (Figure 1B).
Heterozygous γ-carboxylase–deficient mice are phenotypically normal
Mice carrying the targeted allele produced a stable mRNA containing only exons 1 and 2 of the γ-carboxylase gene, as determined by ribonuclease protection assay (data not shown). Mice heterozygous for the disrupted γ-carboxylase gene (Ggcx+/−) develop normally, exhibit normal survival and fertility, and are indistinguishable from their wild-type littermates. γ-Carboxylase expression was abolished by the targeting, as demonstrated by the analysis of liver homogenates prepared from 18-dpc fetuses that were wild-type, heterozygous, or homozygous null for γ-carboxylase (Figure 2). Western blot analysis (Figure 2A) and γ-carboxylase activity assays (Figure 2B) demonstrate parallel reductions of γ-carboxylase antigen and activity in heterozygous animals to approximately half of the wild-type level, whereas both antigen and activity were undetectable in the livers of null embryos.
Analysis of γ-carboxylase expression. (A) Western blot analysis of mouse fetal liver microsomes probed with an antibody raised against recombinant bovine γ-carboxylase. All lanes were loaded with 5 ng of microsomal protein except for the 2x −/− lane, which contains 10 ng of microsomal protein. (B) Incorporation of 14CO2 into FLEEL by solubilized γ-carboxylase from the livers of Ggcx+/+ mice was considered 100% γ-carboxylase activity, and the incorporation of 14CO2 by solubilized carboxylase from Ggcx+/− and Ggcx−/− mice were expressed as a percentage of the wild-type. Mean values; error bars indicate ± SD.
Analysis of γ-carboxylase expression. (A) Western blot analysis of mouse fetal liver microsomes probed with an antibody raised against recombinant bovine γ-carboxylase. All lanes were loaded with 5 ng of microsomal protein except for the 2x −/− lane, which contains 10 ng of microsomal protein. (B) Incorporation of 14CO2 into FLEEL by solubilized γ-carboxylase from the livers of Ggcx+/+ mice was considered 100% γ-carboxylase activity, and the incorporation of 14CO2 by solubilized carboxylase from Ggcx+/− and Ggcx−/− mice were expressed as a percentage of the wild-type. Mean values; error bars indicate ± SD.
The functional activity for the vitamin K–dependent coagulation factors IX and X and prothrombin in plasma from adult Ggcx+/− mice are indistinguishable from wild-type (Table 1), indicating that heterozygous levels of the γ-carboxylase are sufficient to maintain full γ-carboxylation of these factors.
Plasma activities of factor IX, factor X, and prothrombin from both Ggcx+/+ and Ggcx+/−mice
| . | +/+ (n = 7), % . | +/− (n = 6), % . |
|---|---|---|
| Factor IX activity | 100 ± 13 | 89 ± 13 |
| Factor X activity | 100 ± 4 | 100 ± 6 |
| Prothrombin activity | 100 ± 6 | 98 ± 7 |
| . | +/+ (n = 7), % . | +/− (n = 6), % . |
|---|---|---|
| Factor IX activity | 100 ± 13 | 89 ± 13 |
| Factor X activity | 100 ± 4 | 100 ± 6 |
| Prothrombin activity | 100 ± 6 | 98 ± 7 |
The level of functional activity for these 3 vitamin K-dependent coagulation factors as measured in plasma is expressed as a percentage of the activity found in a plasma pool generated from independently bled C57BI/6J mice. Data are expressed as mean ± SD. n indicates number of mice.
Homozygous γ-carboxylase deficiency
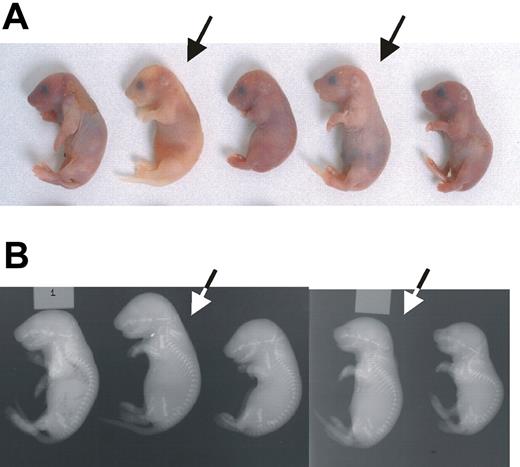
Although Ggcx+/− mice appear entirely normal, intercrosses between these mice yielded only rare homozygous null (Ggcx−/−) animals, all of which succumbed to massive intra-abdominal hemorrhage shortly after birth (Figure 3A). To determine the stage of embryogenesis at which Ggcx−/− mice are lost, a Ggcx+/− intercross was performed, and the embryos were harvested for genotyping at either 9.5 or 18 dpc (Table 2). Analysis of 146 embryos at 9.5 dpc yielded genotype results indistinguishable from the expected Mendelian distribution. However, at 18 dpc the percentage of homozygous null embryos observed was approximately half of the expected number (P < .001). The observation that approximately half of the Ggcx−/− embryos are lost between 9.5 and 18 dpc suggests a critical role for 1 or more γ-carboxyglutamic acid–containing proteins in mammalian development. No ectopic calcification was observed in the null embryos, either histologically or by x-ray (Figure 3B).
Phenotype of Ggcx−/− mice. (A) Newborn progeny from a Ggcx+/− intercross. The homozygous null animals (arrows) exhibit intra-abdominal hemorrhage. (B) X-rays from the same animals shown in panel A. Arrows again indicate the homozygous null mice.
Phenotype of Ggcx−/− mice. (A) Newborn progeny from a Ggcx+/− intercross. The homozygous null animals (arrows) exhibit intra-abdominal hemorrhage. (B) X-rays from the same animals shown in panel A. Arrows again indicate the homozygous null mice.
Genotype distribution of progeny obtained from intercross of Ggcx+/− mice
| . | Total no. . | +/+, n (%) . | +/−, n (%) . | −/−, n (%) . | P . |
|---|---|---|---|---|---|
| Term pups | 217 | 85 (39.2) | 128 (59) | 5 (2.3) | < .001 |
| 18 dpc | 164 | 56 (34.2) | 88 (53.7) | 20 (12.2) | < .001 |
| 9.5 dpc | 146 | 37 (25.3) | 72 (49.3) | 37 (25.3) | > .9 |
| . | Total no. . | +/+, n (%) . | +/−, n (%) . | −/−, n (%) . | P . |
|---|---|---|---|---|---|
| Term pups | 217 | 85 (39.2) | 128 (59) | 5 (2.3) | < .001 |
| 18 dpc | 164 | 56 (34.2) | 88 (53.7) | 20 (12.2) | < .001 |
| 9.5 dpc | 146 | 37 (25.3) | 72 (49.3) | 37 (25.3) | > .9 |
The data were derived from an intercross of Ggcx+/− animals derived from a backcross to C57BL/6 mice of 4 generations or greater. The expected percentages for each genotype from this cross are 25% (Ggcx+/+), 50% (Ggcx+/−), and 25% (Ggcx−/−). The number of Ggcx−/− embryos observed at 18 dpc is approximately one-half of the expected 25%. P values obtained by a χ2 test comparing the expected and observed numbers of animals for each indicated genotype are given in the last column. n indicates number of mice.
Discussion
The vitamin K–dependent coagulation factors have long been known to be critical substrates for the γ-carboxylase in vivo, as evident from the anticoagulant action of warfarin. However, the identification of additional substrates with diverse functions such as the bone proteins osteocalcin and MGP, the kidney proteins nephrocalcin A-D, and the signaling molecule GAS6,13–16 together with the expression of γ-carboxylase in multiple tissues,3 all suggest a broader function for this posttranslational modification, in addition to its role in forming the membrane-binding domains of the vitamin K–dependent blood coagulation and regulatory proteins.37 Further support for this notion comes from the identification of the conservation of the γ-carboxylase gene in Ciona intestinalis and other chordates,5 as well as in Drosophila and Conus snail species,6,7 organisms which arose well before the evolution of the known vitamin K–dependent coagulation factors. γ-Carboxylation is important in the snail for the biosynthesis of a range of specific peptide toxins.1,24,38 Four Gla-domain containing proteins have been identified in C. intestinalis, including a receptor tyrosine kinase and a possible homolog of a Notch receptor family ligand.5 The function and specific substrates of γ-carboxylase in Drosophila are unknown,6 although some studies suggest a possible role for insect carboxylase in the regulation of circadian rhythm. However, a recent analysis of flies deficient in γ-carboxylase demonstrated no obvious phenotype.6 Taken together with evidence for the widespread expression of γ-carboxylase in the mammalian embryo,3 these observations suggest broad functional significance of γ-carboxylation, potentially affecting multiple biologic processes both in the developing embryo and the adult animal. We were thus surprised to observe grossly normal development of the completely γ-carboxylase–deficient mouse embryo through embryonic day 9.5, with approximately half of the embryos developing normally to term.
This phenotype is strikingly similar to that observed in prothrombin null mice, as well as other mouse knockouts completely deficient in thrombin generation. Specifically, nearly identical to the γ-carboxylase null phenotype reported here, factor V–deficient9 and factor X–deficient39 mice null for prothrombinase activity and prothrombin null mice10,11 all exhibit similar approximately 50% midembryonic lethality, with the remaining animals dying of hemorrhage at birth. Tissue factor null mice exhibit a similar but more severe embryonic phenotype,40 though maternal transfer appears to rescue factor VII deficient mice from embryonic lethality.41
Fetal death in these animals may be due to a defect in the development of the yolk sac vasculature,9,10,42 or may result directly from hemorrhage secondary to the coagulation deficiency.43,44 The midembryonic lethal phenotype may reflect a requirement for thrombin signaling through the thrombin receptor (Par-1), since about half of Par-1–deficient (Par1−/−) mouse embryos are also lost at approximately 9.5 dpc,45 though the involvement of Par1 in other signaling pathways may also contribute.12 Of note, adult Par-1−/− mice exhibit normal hemostasis,45 suggesting that the mechanisms responsible for the midembryonic loss are distinct from those leading to perinatal death, the latter appearing to be purely hemorrhagic.9,45 The identical phenotypes observed for deficiency of the γ-carboxylase or for its substrates prothrombin or factor X suggest that the Ggcx−/− embryonic lethality is due to the dependence of prothrombin on γ-carboxylation for activity and thus the resulting complete functional deficiency of prothrombin in Ggcx−/− mice. However, we cannot exclude the possibility that trace residual function of 1 or more uncarboxylated vitamin K–dependent proteins, and/or maternal transfer, might be contributing to the survival of a subset of Ggcx−/− mice to term. Of note, knockouts of other γ-carboxylase substrates, including factor IX,46 protein C,47 protein Z,48 factor VII,41 MGP,22 osteocalcin,21 and Gas6,49 do not result in embryonic lethality.
Partial deficiency of γ-carboxylase has been reported in several human patients with VKCFD,30–32 with the decrease in γ-carboxylase activity resulting in combined deficiency of all of the vitamin K–dependent coagulation factors, though without any apparent fetal or embryonic loss. Two compound heterozygous individuals were recently reported to exhibit skeletal and cardiac developmental abnormalities potentially related to γ-carboxylase deficiency.32 These observations suggest that partial activity of γ-carboxylase is sufficient for embryonic developmental signaling. Of note, low-level expression of a prothrombin or factor V transgene rescues the prenatal as well as the midembryonic lethal phenotypes of prothrombin- and factor V–deficient mice, respectively,10,50,51 consistent with the notion that a low level of thrombin generation is sufficient for normal development.
Warfarin embryopathy results from fetal exposure during gestation to warfarin ingested by the mother. This serious disorder is the primary contraindiction to the use of warfarin during pregnancy. Affected children exhibit nasal hypoplasia and stippled epiphyses.27 Warfarin embryopathy shows remarkable phenotypic similarities to X-linked recessive chrondrodysplasia punctata (CDPX).52 CDPX is caused by an inherited deficiency of an arylsulfatase whose activity can be inhibited by warfarin, suggesting a role for inhibition of this enzyme in the pathophysiology of warfarin embryopathy. However, Keutel syndrome, a human disorder characterized by abnormal cartilage calcification, peripheral pulmonary stenosis, and midfacial hypoplasia, is due to mutations in the gene encoding the γ-carboxylated protein MGP.53 In parallel, a rare case of VKOR deficiency resulted in a similar phenotype of nasal hypoplasia, distal digital hypoplasia, and epiphyseal stippling.54 These latter observations suggest a critical role for 1 or more γ-carboxylase–dependent proteins in the pathogenesis of warfarin embryopathy. However, the early mortality of Ggcx−/− mice precludes a direct assessment in these animals of the importance of γ-carboxylation for the biologic functions of MGP, osteocalcin, or other γ-carboxylated proteins and their contributions to warfarin embryopathy. Although initial analysis of Ggcx−/− embryos at 18 dpc suggested a subtle developmental abnormality of the forebrain and midface, these changes were not consistently observed.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) nos. P01HL057346 (D.G. and R.J.K.), R01R37-HL39693 (D.G.), and RO1-HL52172 (R.J.K.); a National Hemophilia Foundation Judith Graham Pool fellowship (H.S.); and additional NIH grants to B.C.F. and B.F. D.G. and R.J.K. are Howard Hughes Medical Institute investigators.
Authorship
Contribution: A.Z. performed research, analyzed data, and wrote the paper; H.S. performed research, analyzed data, and wrote the paper; R.R. performed research, analyzed data, and wrote the paper; B.C.F. wrote the paper; B.F. wrote the paper; M.B. performed research and analyzed data; R.J.K. analyzed data and wrote the paper; R.W. performed research; and D.G. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Ginsburg, Howard Hughes Medical Institute, University of Michigan, Life Sciences Institute, Rm 5028, 210 Washtenaw Ave, Ann Arbor, MI 48109; e-mail: ginsburg@umich.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal