Abstract
We investigated whether Toll-like receptor ligands (TLR-Ls) can bypass the requirement for CD4+ T-cell help in the induction of fully efficient memory CD8+ T cells (cytotoxic T lymphocytes [CTLs]). “Helpless” CTLs were induced by a synthetic CD8+ T-cell epitope administered with TLR3-L and TLR9-L, but not with TLR2/6-L, TLR4-L, or TLR7-L. The up-regulation of MHC-I and costimulatory molecules by dendritic cells following TLR stimulation was not sufficient for the priming of “helpless” CTLs, which depended essentially on the induction of a strong IFN-α/β response. The “helpless” CTLs induced by TLR-Ls differentiated into fully functional memory CTLs able to proliferate as well as their “helped” counterparts upon challenge, in the absence of CD4+ T-cell help.
Introduction
The mechanisms underlying effector and memory cytotoxic T lymphocyte (CTL) activation remain unclear and a source of considerable interest. CD4+ T cells have been shown to be required for the induction of optimal primary CTL responses to minor histocompatibility and tumor antigens (Ags), soluble proteins, synthetic peptides, peptide-pulsed dendritic cells (DCs), and herpes simplex virus and influenza virus infection.1–5 However, CTLs can also be primed in the absence of CD4+ T cells (“helpless” CTLs), after infection with lymphocytic choriomeningitis virus (LCMV) or vesicular stomatitis virus6,7 or immunization with the MHC-I–delivery vaccine vector consisting on the adenylate cyclase of Bordetella pertussis.8
The factors determining the requirement for CD4+ T-cell help in CTL priming remain a matter of debate. CD4+ T cells help CTLs by licensing Ag-bearing, CD40-expressing DCs through interaction with the CD40L expressed by CD4+ T cells.5,9,10 In “helpless” CTL responses, the requirement for DC licensing by CD4+ T cells is thought to be overcome by inflammatory mediators induced by infection.11,12 In several systems, the absence of CD4+ T-cell help during CTL induction has been shown to result in defective memory CTLs that mediate effector functions, such as cytotoxicity and cytokine secretion, upon secondary challenge but do not undergo a second round of clonal expansion in the absence of CD4+ T-cell help.4,6,7,13–15 However, other reports have shown that “helpless” memory CTLs can proliferate despite the absence of CD4+ T cells during the recall response.7 The reasons for these discrepancies are unclear, but the requirement for CD4+ T-cell help for memory CTL expansion, like that for CTL induction, may be linked to the infectious agent.
Pathogens stimulate innate immunity by signaling through TLRs, and there is strong evidence to suggest that TLR signaling in DCs predominates over other microbial stimuli. Initiation of the program leading to effector/memory CTL production following TCR activation requires a second signal, provided by costimulation, and a third signal that can be provided by IL-12,16 IFN-α,17 and IL-21.18 It has become increasingly clear that the recognition of TLR-Ls by DCs can shape the adaptive immune response by up-regulating costimulatory molecules and cytokine production. Based on these observations, we suggest that a subset of TLR-Ls can substitute for signals 2 and 3 in the absence of activated CD4+ T cells, and that the signals generated by TLR engagement may be sufficient to cross-prime CD4-independent effector/memory CTLs.
In the present study, we thus investigated whether various TLR-Ls could stimulate effector and memory CTLs in the absence of CD4+ T-cell help, by immunizing mice with synthetic microspheres covalently linked to the OVA257-264 CTL peptide (BOVAp) together with different TLR-Ls. We previously showed that BOVAp can transfer the CTL epitope to the MHC-I pathway of DCs in vivo, without inducing DC maturation and with no stimulatory/anergizing capacity.19 BOVAp therefore fails to induce CTL responses unless further “licensing” signals are delivered to DCs, by an agonist anti-CD40 monoclonal antibody (mAb), for example.19
In this study, we clearly demonstrate that TLR3-L and TLR9-L, but not TLR2/6-L, TLR4-L, and TLR7-L, can bypass the requirement for CD4+ T cells for the induction of fully efficient CTL responses. The ability of TLR3-L and TLR9-L to prime “helpless” CTLs depended on IFN-α/β signaling, although IFN-α/β-derived signals alone are insufficient for the induction of fully efficient CTL priming in the absence of CD4+ T-cell help. Finally, we show that the “helpless” CTL responses induced by TLR3 signaling differentiate into fully functional memory CTLs able to mediate effector functions and to undergo a second round of clonal expansion upon secondary immunization in the absence of CD4+ T-cell help.
Materials and methods
Mice
C57BL/6 and 129Sv mice were obtained from Charles River (L'Arbresle, France). Mice lacking MHC-II molecules were obtained from Taconic Europe A/S (Copenhagen, Denmark). 129Sv mice lacking the type I IFN receptor (IFNARko)20 and C57BL/6 mice lacking TLR3 (TLR3ko)21 were obtained from the Pasteur Institute (Paris, France). Trif−/− mice were obtained from Dr Akira and backcrossed onto a C57BL/6 background (11 backcrosses). Animals were kept under specific pathogen-free conditions. Experiments involving animals were conducted according to institutional guidelines.
Reagents and immunizations
Mice were immunized by intravenous or subcutaneous injection with 109 synthetic latex beads (1-μm diameter; Polysciences, Warrington, PA) covalently linked to the OVA257-264 synthetic peptide (BOVAp), as described,19 either alone or in combination with 25 μg zymosan, poly(I:C), ultrapure LPS, poly(U) (complexed to LyoVec), R837, R848, or with 5 μg CpG-B (1668), CpG-A (2216), or control oligodeoxynucleotide (ODNs) (1982, named ODN-B, and 5′-GGGGGAGCATGCTGCGGGGG-3′, named ODN-A), or with 100 μg anti-CD40 antibodies (3/23) (BD Biosciences, Le Pont de Claix, France). CpGs and control ODNs were administered either free or mixed with DOTAP (30 μL) (Roche Diagnostics, Meylan, France). TLR-Ls were obtained from Invivogen (Toulouse, France), with the exception of CpGs and control ODNs, which were obtained from Genset (Paris, France). In some experiments, mice received beads coated with the following H-2b-epitope–containing peptides: E749-57 from human papilloma virus E7 protein, HY738-746 from the minor histocompatibility (H) male-specific (Y) antigen (H-Y), and LCMV33-41 from LCMV glycoprotein. All peptides were obtained from Neosystem (Strasbourg, France). Some mice were injected subcutaneously with synthetic OVA257-264 peptide (300 μg), either alone or together with poly(I:C). For recall studies, mice primed with BOVAp together with poly(I:C) or anti-CD40 mAb were boosted (day 25) with a single intravenous injection of genetically detoxified adenylate cyclase (CyaA, 50 μg) from B pertussis bearing the OVA257-264 epitope at position 224.8
In vivo killing assay
Naive syngenic splenocytes were pulsed with OVA257-264 peptide (10 μg/mL; 30 minutes, 37°C), washed extensively, and labeled with high concentration (1.25 μM) of CFSE (Molecular Probes, Eugene, OR). The nonpulsed control population was labeled with low concentration (0.125 μM) of CFSE. Both CFSEhigh and CFSElow-labeled cells were mixed at a 1:1 ratio (5 × 106 cells of each population) and then injected intravenously into mice. The number of CFSE-positive cells remaining in the spleen after 20 hours was determined by fluorescence-activated cell sorting (FACS).
ELISPOT
IFN-γ enzyme-linked immunosorbent spot (ELISPOT) was carried out as previously described.19 The number of spot-forming cells (SFCs) was determined using a computer-assisted ELISPOT image analyzer (Bioreader-3000; Bioreader, Karben, Germany). Results are expressed as SFCs/million splenocytes. The number of OVA257-264–specific IFN-γ SFCs was determined by calculating the difference between the number of spots generated in the presence and absence of the OVA257-264 peptide (1 μg/mL).
DC purification
Mice were injected intravenously with PBS or 25 μg zymosan, poly(I:C), LPS, poly(U), R848, or 5 μg CpG-B. CpG was administered either free or mixed with 30 μL DOTAP. At the time indicated, spleens were harvested and treated with 400 U/mL collagenase D and 50 μg/mL DNase I (Roche Diagnostics). Spleen cell suspension was incubated with anti-CD11c antibody-coated magnetic beads (Miltenyi Biotec, Bergisch-Gladbach, Germany) and purified with anti-CD16/32 mAb (BD Biosciences). Cells were selected using an AutoMACS (Miltenyi Biotec).
Flow cytometry analysis
The expression of MHC-I and costimulatory molecules on the surface of DCs was analyzed by staining magnetically sorted splenic DCs with anti-CD11c-APC, anti-CD86-FITC, anti-Kb-FITC, or appropriate isotype control antibodies. For the detection of intracellular IL-12, splenic DCs were magnetically sorted 4 hours after the injection of TLR-Ls. Brefeldin A (10 μg/mL) (Sigma-Aldrich, St Louis, MO) was added during the DC enrichment procedure. Cells were first stained for extracellular markers with anti-CD11c-APC antibodies, then processed with the Fix & Perm kit (BD Biosciences), and finally stained with anti–IL-12p40-APC or an appropriate isotype control mAb. The number of OVA257-264–specific CTLs (OVATetr+) was determined by staining spleen cells first with H-2Kb-OVA257-264-tetramer-PE (Beckman Coulter France, Roissy, France) and then with anti-CD3-APC and anti-CD8-FITC. Results are expressed as the percentage of CD8+ T cells that were OVATetr+. All mAbs were obtained from BD Biosciences. The cells were acquired on a FACScalibur flow cytometer and analyzed with CellQuest Software (BD Biosciences).
Cytokine ELISA assays
IFN-α, IFN-β, and MIG levels in serum were determined with enzyme-linked immunosorbent assay (ELISA) kits (IFN-α and IFN-β were from PBL Biomedical Laboratories, Piscataway, NJ; MIG was from R&D Systems, Minneapolis, MN). Seric IL-12p40 and IL-6 were determined by in-house ELISA as previously described.22
Results
Poly(I:C) and CpG promote the CTL response in the absence of CD4+ T-cell help
Some TLR-Ls, such as LPS, poly(I:C), and ODN containing the CpG motif, have been shown to stimulate CTL responses against coadministered antigens.23–25 However, in all of these studies, the antigens used contained CD4+ T-cell epitopes, and the observed adjuvant effect of these TLR-Ls might have been mediated by CD4+ T-cell help. We aimed to assess the ability of various TLR-Ls to stimulate functional CTLs in the absence of any CD4+ T-cell help.
C57BL/6 mice were injected intravenously with synthetic microspheres covalently linked to the OVA257-264 CTL epitope (BOVAp), either alone or together with 25 μg zymosan, poly(I:C), LPS, poly(U), R837, R848, or with 5 μg CpG-B or CpG-A. CpGs were administered either as naked ODN, or mixed with the cationic lipid formulation DOTAP (CpG(DOTAP)). Control mice were immunized with TLR-L alone or with BOVAp or control ODNs mixed with DOTAP, and CTL responses were analyzed in an in vivo killing assay or by tetramer staining. No OVA-specific CTL response was detected in control mice or in mice injected with BOVAp alone (Figure 1A and data not shown). In contrast, strong in vivo lysis was detected when BOVAp was injected with either poly(I:C), CpG-B(DOTAP), or CpG-A(DOTAP) (Figure 1A). The frequency of OVA-specific CTLs, as determined by tetramer staining (OVATetr+), was similarly high in these 3 groups (Figure 1B). Similar results were obtained when BOVAp was administered subcutaneously with poly(I:C), CpG-B(DOTAP) (Figure 1C), or CpG-A(DOTAP) (data not shown). In contrast, no CTL response was observed when BOVAp was injected with zymosan, LPS, or R837, whereas a weak response was detected in mice immunized with BOVAp and poly(U), R848, or naked CpGs (Figure 1A). The injection of larger amounts of zymosan (500 μg), LPS (100 μg), R837 (100 μg), R848 (200 μg), or naked CpGs (100 μg) did not enhance the CTL response (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article).
Poly(I:C) and CpG promote functional CTL responses in the absence of CD4+ T-cell help. (A) C57BL/6 mice were left untreated or were immunized by a single intravenous injection of BOVAp either alone or in combination with zymosan, poly(I:C), LPS, poly(U), R837, R848, CpG-B, or CpG-A. CpG was administered either naked or mixed with DOTAP (CpG(DOTAP)). The percentage of OVA257-264–specific lysis was determined by in vivo killing assay 7 days after immunization. Control mice were immunized with control ODNs or BOVAp mixed with DOTAP. Specific lysis was calculated as follows: % specific lysis = 100 − [100 × (% CFSEhigh-primed mice/% CFSElow-primed mice)/(% CFSEhigh-naive mice/% CFSElow-naive mice)]. (B) Frequency of OVA257-264–specific CTLs detected in the spleen 7 days after intravenous injection of BOVAp alone or with poly(I:C), CpG-B(DOTAP), or CpG-A(DOTAP). OVA257-264–specific CTLs (OVATetr+) were quantified by tetramer staining. (C) C57BL/6 mice received a single intravenous or subcutaneous injection of BOVAp either alone or with poly(I:C) or CpG-B(DOTAP). Seven days after injection, the CTL response was quantified by in vivo killing assay. (D-E) Poly(I:C) and CpG induce CTL responses against BOVAp in the absence of CD4+ T-cell help. C57BL/6 and MHC-IIko mice were either left untreated or immunized intravenously with BOVAp alone or with poly(I:C) (D-E), CpG-B(DOTAP) (E), or anti-CD40 mAb (D). The specific CTL response was analyzed on day 7 by in vivo killing assay (D) and tetramer staining (E). (F) CTL response induced by poly(I:C) and beads coated with either E749-57 (BE7p), HY738-746 (BHYp), or LCMV33-41 (BLCMVp) peptides. Female C57BL/6 mice were either left untreated or immunized by a single intravenous injection of the corresponding beads either alone or in combination with poly(I:C). (G) CTL response induced by poly(I:C) and soluble OVA257-264 peptide. C57BL/6 mice were left untreated or immunized by a single subcutaneous injection of either BOVAp or soluble OVA257-264 peptide (OVAp) alone or in combination with poly(I:C). (F-G) The CTL response was quantified in the spleen by in vivo killing on day 7 after priming. Dots represent individual mice. All results are representative of at least 2 independent experiments.
Poly(I:C) and CpG promote functional CTL responses in the absence of CD4+ T-cell help. (A) C57BL/6 mice were left untreated or were immunized by a single intravenous injection of BOVAp either alone or in combination with zymosan, poly(I:C), LPS, poly(U), R837, R848, CpG-B, or CpG-A. CpG was administered either naked or mixed with DOTAP (CpG(DOTAP)). The percentage of OVA257-264–specific lysis was determined by in vivo killing assay 7 days after immunization. Control mice were immunized with control ODNs or BOVAp mixed with DOTAP. Specific lysis was calculated as follows: % specific lysis = 100 − [100 × (% CFSEhigh-primed mice/% CFSElow-primed mice)/(% CFSEhigh-naive mice/% CFSElow-naive mice)]. (B) Frequency of OVA257-264–specific CTLs detected in the spleen 7 days after intravenous injection of BOVAp alone or with poly(I:C), CpG-B(DOTAP), or CpG-A(DOTAP). OVA257-264–specific CTLs (OVATetr+) were quantified by tetramer staining. (C) C57BL/6 mice received a single intravenous or subcutaneous injection of BOVAp either alone or with poly(I:C) or CpG-B(DOTAP). Seven days after injection, the CTL response was quantified by in vivo killing assay. (D-E) Poly(I:C) and CpG induce CTL responses against BOVAp in the absence of CD4+ T-cell help. C57BL/6 and MHC-IIko mice were either left untreated or immunized intravenously with BOVAp alone or with poly(I:C) (D-E), CpG-B(DOTAP) (E), or anti-CD40 mAb (D). The specific CTL response was analyzed on day 7 by in vivo killing assay (D) and tetramer staining (E). (F) CTL response induced by poly(I:C) and beads coated with either E749-57 (BE7p), HY738-746 (BHYp), or LCMV33-41 (BLCMVp) peptides. Female C57BL/6 mice were either left untreated or immunized by a single intravenous injection of the corresponding beads either alone or in combination with poly(I:C). (G) CTL response induced by poly(I:C) and soluble OVA257-264 peptide. C57BL/6 mice were left untreated or immunized by a single subcutaneous injection of either BOVAp or soluble OVA257-264 peptide (OVAp) alone or in combination with poly(I:C). (F-G) The CTL response was quantified in the spleen by in vivo killing on day 7 after priming. Dots represent individual mice. All results are representative of at least 2 independent experiments.
We also used adoptive transfer of OT-1 cells to assess the ability of various TLR-Ls to stimulate CTLs in the absence of any CD4+ T-cell help (Figure S2). Forty hours after immunization with BOVAp alone or together with either poly(I:C), zymosan, or LPS, we detected an early proliferation of OT-1 cells (Figure S2A). However, only poly(I:C) significantly enhanced further expansion (70 hours) of primed OT-1 cells. Expression of CD44 by transferred OT-1 cells was not affected by TLR triggering (Figure S2B). However, acquisition of other typical effector markers, such as up-regulation of CD25, down-regulation of CD62L, or production of IFN-γ, was achieved only markedly upon immunization with BOVAp together with poly(I:C) but not with BOVAp alone or with zymosan or LPS (Figure S2B). A similar activation of the transferred OT-1 cells was observed in recipient mice immunized with BOVAp plus CpG-B(DOTAP) (data not shown). Neither proliferation nor activation was detected upon injection of the TLR-Ls alone (data not shown). These results show that only poly(I:C)- and CpG(DOTAP)-derived, but not TLR2/6 or TLR4 ligands–derived, signals fulfill the requirements to prime adoptively transferred OT-1 cells, confirming our previous results with normal mice.
No H-2b-restricted CD4+ T-cell epitope has been identified in the OVA257-264 peptide sequence. Nevertheless, C57BL/6 WT and MHC-IIko mice were immunized (intravenously) with BOVAp and either poly(I:C) or CpG-B(DOTAP) to exclude any possible participation of CD4+ helper T cells in the induction of the CTL response against BOVAp. Both strains of mice mounted similar CTL responses upon immunization with BOVAp and poly(I:C), as shown in the in vivo killing assay (Figure 1D), and this response was similar to that induced by BOVAp in the presence of anti-CD40 mAb. The frequency of OVATetr+ CTLs was similar in WT and MHC-IIko mice after immunization with BOVAp and either poly(I:C) or CpG-B(DOTAP) (Figure 1E). Poly(I:C) also induced a strong CTL response in the absence of CD4+ T cells when injected with beads coated with 3 other peptides containing CTL epitopes (Figure 1F). Finally, we also tested the response induced by poly(I:C) in combination with soluble OVA257-264 peptide, to exclude the possibility that this ability of TLR-Ls to overcome the CD4+ T-cell requirement for CTL activation was limited to particulate antigens. In vivo lytic activity was also detected following the subcutaneous immunization of mice with the soluble OVA257-264 peptide and poly(I:C), although the CTL response was less efficient than that against particulate antigens (Figure 1G). However, no CTL response was detected if mice received the OVA257-264 peptide and poly(I:C) via the intravenous route (data not shown). Thus, TLR3 and TLR9 agonists, but not TLR2/6-L, TLR4-L or TLR7-L, can substitute for CD4+ T-cell help in the induction of an efficient CTL response.
Neither the up-regulation of MHC-I and costimulatory molecules on DCs nor the production of IL-12 induced by TLR stimulation is sufficient for CD4-independent CTL priming
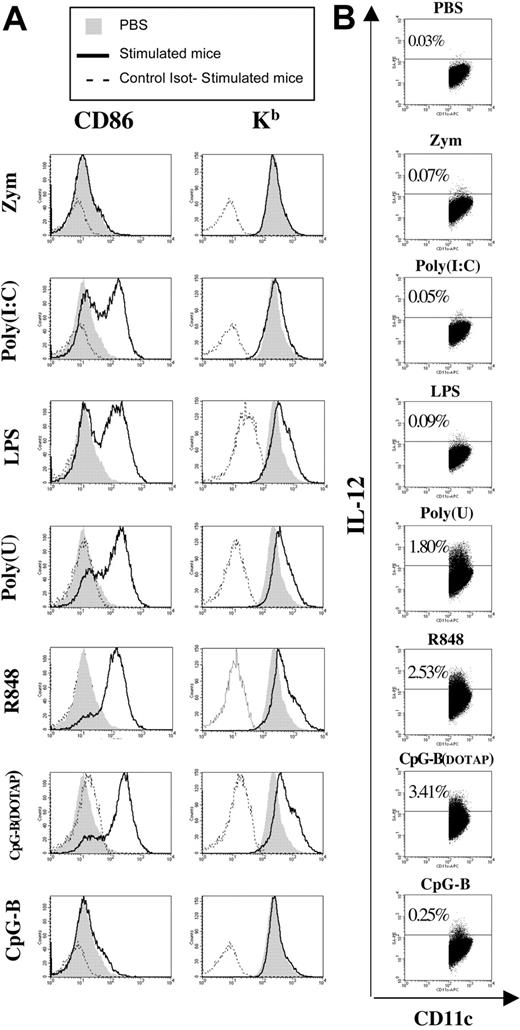
The generation of CTL responses against exogenous cell–associated or soluble antigens requires DC licensing.2 In their resting state, DCs have only low levels of MHC and costimulatory molecules and cannot prime naive CTLs. The maturation of DCs to a functionally mature state is promoted by activated CD4+ T cells, via interactions between CD40 on DCs and CD40L on T cells, in particular.5,9,10 However, resting DCs can also be activated by inflammatory cytokines26 or stimuli from pathogens that up-regulate MHC and costimulatory molecules,27 thereby increasing the ability of these cells to stimulate T cells. We investigated whether the ability of TLR agonists to induce CTL priming in the absence of T-helper activation was dependent on their capacity to activate DCs, by injecting various TLR-Ls intravenously into C57BL/6 mice and analyzing the expression of MHC-I (H-2kb) and costimulatory molecules (CD86) on CD11chigh DCs. DCs displayed similar increases in the levels of CD86 or MHC-I molecules following in vivo stimulation with poly(I:C), LPS, poly(U), R848, and CpG-B(DOTAP) (Figure 2A). In contrast, neither zymosan (Figure 2A) nor R837 (50 μg) (Gillis Dadaglio, unpublished data, June 2005) induced the up-regulation of these molecules. The administration of CpG-B in DOTAP greatly increased the ability of this molecule to up-regulate costimulatory and MHC-I molecules in DCs (Figure 2A). Similar results were obtained when instead of CpG-B we used CpG-A (data not shown). The administration of DOTAP alone did not affect the expression of MHC-I and CD86 molecules (data not shown). These results clearly demonstrate that the expression of MHC-I and CD86 molecules by DCs following TLR-L stimulation is not correlated with the ability of TLR-Ls to promote CTL priming in the absence of CD4+ T-cell help. Similar conclusions were drawn from in vivo analyses of CD40 and CD80 expression upon TLR engagement (data not shown).
In vivo maturation of DCs by different TLR-Ls. C57BL/6 mice received an intravenous injection of PBS or zymosan, poly(I:C), LPS, poly(U), R848, or CpG-B. CpG was administered either naked or mixed with DOTAP. (A) The up-regulation of MHC-I (H-2Kb) and CD86 molecules was analyzed on purified splenic DCs 15 hours after treatment. (B) Intracellular staining of IL-12 was analyzed on sorted splenic DCs 4 hours after TLR injection. (A-B) Cells were gated on the CD11chigh population. Results are representative of 2 independent experiments.
In vivo maturation of DCs by different TLR-Ls. C57BL/6 mice received an intravenous injection of PBS or zymosan, poly(I:C), LPS, poly(U), R848, or CpG-B. CpG was administered either naked or mixed with DOTAP. (A) The up-regulation of MHC-I (H-2Kb) and CD86 molecules was analyzed on purified splenic DCs 15 hours after treatment. (B) Intracellular staining of IL-12 was analyzed on sorted splenic DCs 4 hours after TLR injection. (A-B) Cells were gated on the CD11chigh population. Results are representative of 2 independent experiments.
IL-12 enhances CTL responses in various experimental systems.16 We assessed the correlation between the ability of TLR-Ls to stimulate IL-12 secretion by DCs and their capacity to prime CTLs, by evaluating IL-12 production upon in vivo TLR triggering by intracellular staining in CD11chigh DCs. High levels of IL-12 production were detected in DCs from mice treated with poly(U), R848, CpG-B(DOTAP) (Figure 2B), and CpG-A(DOTAP) (data not shown), but not in DCs from mice treated with zymosan, poly(I:C), and LPS (Figure 2B). A moderate IL-12 response was detected in DCs from mice injected with naked CpGs (Figure 2B and data not shown). Our data indicate that the TLR-mediated up-regulation of MHC-I and costimulatory molecules and IL-12 production in DCs does not fulfill the requirements for CTL priming in the absence of CD4+ T-cell help.
The ability of TLR3-L and TLR9-L to induce CTL responses in the absence of CD4+ T-cell help is dependent on IFN-α/β
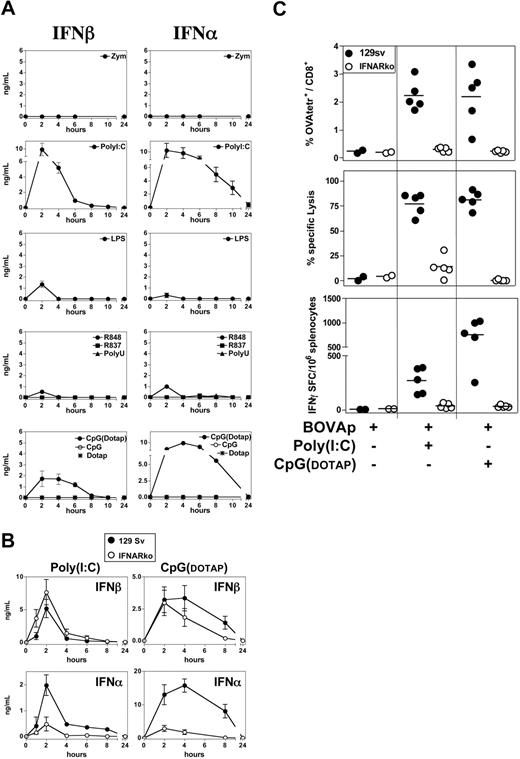
Type I interferons (IFNs) are an essential link between innate and adaptive immunity.28,29 We therefore thought that the ability of some TLR-Ls to promote CTL priming in the absence of CD4+ T-cell help might be mediated by type I IFNs. We investigated the capacity of different TLR-Ls to induce IFN-α/β secretion in vivo. We detected high levels of IFN-α/β in the serum of mice injected with poly(I:C) or CpG-B(DOTAP) and very low, transient levels of these cytokines following the injection of LPS and R848 (Figure 3A). High levels of IFN-α/β were also produced in vivo following the injection of CpG-A(DOTAP) (data not shown). IFN-α/β production was not detected after the administration of zymosan, R837, poly(U), or naked CpGs (Figure 3A and data not shown), even if high doses of CpGs (100 μg) were used (data not shown).
IFN-α/β mediates the adjuvant property of poly(I:C) and CpG. (A) In vivo production of type I IFN in response to various TLR-L. C57BL/6 mice received a single intravenous injection of zymosan, poly(I:C), LPS, R848, R837, poly(U), or CpG-B. CpG was administered either naked or mixed with DOTAP. As a control, one group of mice received DOTAP alone. Serum samples were collected at various time points after injection of the TLR-L, and IFN-α and IFN-β were titrated by ELISA. Results are expressed as means ± SEM for 3 to 4 mice per group tested in 2 independent experiments. (B) 129Sv and IFNARko mice received a single intravenous injection of poly(I:C) or CpG-B(DOTAP), and their sera were titrated for IFN-α and IFN-β by ELISA. Results are expressed as means ± SEM for 3 mice per group. (C) 129Sv and IFNARko mice were left untreated or were injected intravenously with BOVAp either alone or in combination with poly(I:C) or CpG-B(DOTAP). The specific CTL response was analyzed on day 7 by tetramer staining, in vivo killing assay, and ELISPOT (upper, medium, and lower panels, respectively). All data are representative of at least 2 independent experiments.
IFN-α/β mediates the adjuvant property of poly(I:C) and CpG. (A) In vivo production of type I IFN in response to various TLR-L. C57BL/6 mice received a single intravenous injection of zymosan, poly(I:C), LPS, R848, R837, poly(U), or CpG-B. CpG was administered either naked or mixed with DOTAP. As a control, one group of mice received DOTAP alone. Serum samples were collected at various time points after injection of the TLR-L, and IFN-α and IFN-β were titrated by ELISA. Results are expressed as means ± SEM for 3 to 4 mice per group tested in 2 independent experiments. (B) 129Sv and IFNARko mice received a single intravenous injection of poly(I:C) or CpG-B(DOTAP), and their sera were titrated for IFN-α and IFN-β by ELISA. Results are expressed as means ± SEM for 3 mice per group. (C) 129Sv and IFNARko mice were left untreated or were injected intravenously with BOVAp either alone or in combination with poly(I:C) or CpG-B(DOTAP). The specific CTL response was analyzed on day 7 by tetramer staining, in vivo killing assay, and ELISPOT (upper, medium, and lower panels, respectively). All data are representative of at least 2 independent experiments.
We investigated possible regulation by the type I IFN receptor (IFNAR) of the poly(I:C), or CpG-induced production of IFN-α/β in vivo, by injecting WT (129Sv) mice and mice lacking IFNAR (IFNARko) with poly(I:C) or CpG-B(DOTAP). Serum IFN-β concentration was not significantly affected by the absence of IFNAR, and was even slightly higher in IFNARko than in WT mice upon the injection of poly(I:C) (Figure 3B). A lack of IFN-β sequestration by IFNAR may account for the higher levels of IFN-β in IFNARko mice. By contrast, markedly lower levels of IFN-α production were induced by poly(I:C) or CpG(DOTAP) in IFNARko mice than in WT mice. Thus, the production of IFN-α in response to the administration of poly(I:C) or CpG(DOTAP) is strongly regulated by IFNAR in vivo.
We then investigated whether the ability of poly(I:C) and CpG(DOTAP) to promote CTL priming in the absence of CD4+ T-cell help depends on IFN-α/β signaling. We immunized 129Sv and IFNARko mice with BOVAp, together with poly(I:C) or CpG-B(DOTAP), and analyzed the specific CTL response by tetramer staining, ELISPOT, and in vivo killing assays. The induction of CTL priming by BOVAp plus poly(I:C) or CpG-B(DOTAP) was almost completely abolished in IFNARko mice (Figure 3C). These data indicate that the strong IFN-α/β production triggered by TLR3-L and TLR9-L allows the engagement of the IFNAR signaling pathway required to promote CTL priming in the absence of CD4+ T-cell help.
In the absence of T-cell help, a lack of TLR3 triggering decreases the ability of poly(I:C) to promote CTL responses
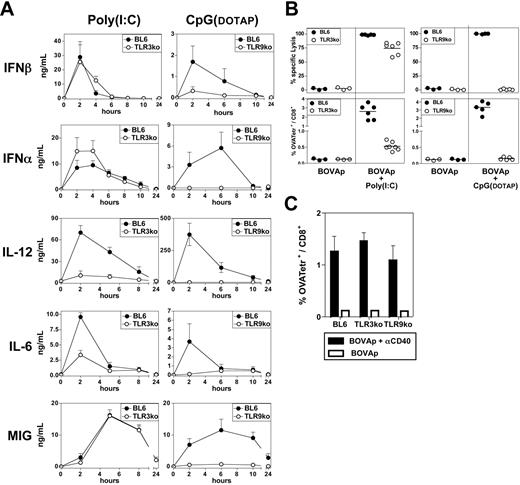
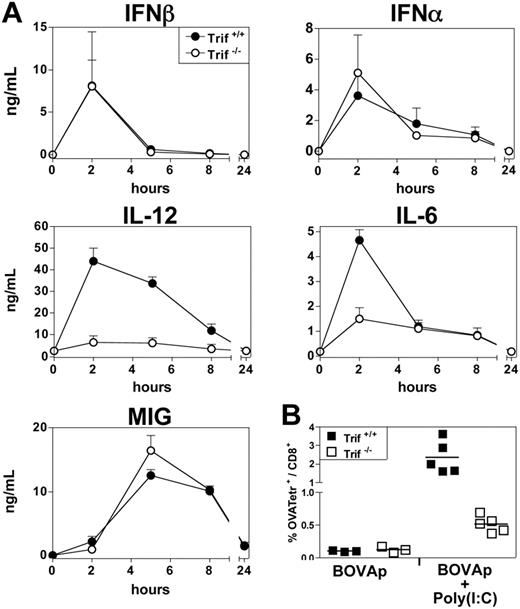
We investigated whether TLR recognition was essential for poly(I:C) and CpG substitution for T-cell help in the induction of CTL priming. We investigated the involvement of TLR triggering in the in vivo production of IFN-α/β and other proinflammatory mediators, such as IL-12, IL-6, and MIG, induced by poly(I:C) and CpG(DOTAP). TLR9ko mice did not produce IFN-α, IL-12, IL-6, or MIG in response to CpG-B(DOTAP) and also displayed severely impaired IFN-β production (Figure 4A). Similar results were obtained with CpG-A(DOTAP) (data not shown). By contrast, TLR3ko mice produced normal amounts of IFN-α/β and MIG, but displayed significant impairment of IL-12 and IL-6 production after poly(I:C) treatment (Figure 4A). A similar cytokine profile was observed following the in vivo treatment of Trif−/− mice with poly(I:C) (Figure 5A). The up-regulation of MHC, CD40 and B7 by DCs from CpG-treated mice was completely abolished in TLR9ko mice, whereas DC phenotypic maturation upon poly(I:C) treatment was similar in WT and TLR3ko mice (data not shown).
The lack of TLR3 triggering decreases the ability of poly(I:C) to promote CTL responses in the absence of T-cell help. (A) C57BL/6, TLR3ko, or TLR9ko mice received a single intravenous injection of either poly(I:C) or CpG-B(DOTAP), and, various times after injection, their sera were titrated for IFN-α, IFN-β, IL-12p40, IL-6, and MIG by ELISA. Results are expressed as means ± SEM for 4 mice per group, tested in 2 independent experiments. (B) C57BL/6, TLR3ko, or TLR9ko mice were left untreated or received intravenous injections of BOVAp either alone or in combination with poly(I:C) or CpG-B(DOTAP). The specific CTL response was analyzed on day 7 by in vivo killing assay (upper panel) or tetramer staining (lower panel). (C) C57BL/6, TLR3ko, or TLR9ko mice were left untreated or were immunized intravenously with BOVAp either alone or in combination with anti-CD40 mAb. The specific CTL response was analyzed on day 7 by in vivo killing assay. Results are expressed as means ± SEM for 3 to 4 mice. (B-C) Data are representative of 3 independent experiments.
The lack of TLR3 triggering decreases the ability of poly(I:C) to promote CTL responses in the absence of T-cell help. (A) C57BL/6, TLR3ko, or TLR9ko mice received a single intravenous injection of either poly(I:C) or CpG-B(DOTAP), and, various times after injection, their sera were titrated for IFN-α, IFN-β, IL-12p40, IL-6, and MIG by ELISA. Results are expressed as means ± SEM for 4 mice per group, tested in 2 independent experiments. (B) C57BL/6, TLR3ko, or TLR9ko mice were left untreated or received intravenous injections of BOVAp either alone or in combination with poly(I:C) or CpG-B(DOTAP). The specific CTL response was analyzed on day 7 by in vivo killing assay (upper panel) or tetramer staining (lower panel). (C) C57BL/6, TLR3ko, or TLR9ko mice were left untreated or were immunized intravenously with BOVAp either alone or in combination with anti-CD40 mAb. The specific CTL response was analyzed on day 7 by in vivo killing assay. Results are expressed as means ± SEM for 3 to 4 mice. (B-C) Data are representative of 3 independent experiments.
Trif dependence of poly(I:C) adjuvant properties. (A) Trif−/− mice and their Trif+/+ littermates received a single intravenous injection of poly(I:C) (25 μg), and, various times after injection, their sera were titrated for IFN-α, IFN-β, IL-12p40, IL-6, and MIG by ELISA. Results are expressed as means ± SEM for 4 mice per group, tested in 2 independent experiments. (B) Trif+/+ and Trif−/− mice were left untreated or were immunized via the intravenous route with BOVAp either alone or in combination with poly(I:C). The specific CTL response was analyzed by tetramer staining on day 7. Data are representative of 2 independent experiments.
Trif dependence of poly(I:C) adjuvant properties. (A) Trif−/− mice and their Trif+/+ littermates received a single intravenous injection of poly(I:C) (25 μg), and, various times after injection, their sera were titrated for IFN-α, IFN-β, IL-12p40, IL-6, and MIG by ELISA. Results are expressed as means ± SEM for 4 mice per group, tested in 2 independent experiments. (B) Trif+/+ and Trif−/− mice were left untreated or were immunized via the intravenous route with BOVAp either alone or in combination with poly(I:C). The specific CTL response was analyzed by tetramer staining on day 7. Data are representative of 2 independent experiments.
We then investigated whether poly(I:C) and CpGs enhanced CTL responses through their recognition by TLR3 and TLR9, respectively. TLR9ko mice were unable to mount a CTL response upon immunization with BOVAp and CpG-B(DOTAP) (Figure 4B) or CpG-A(DOTAP) (data not shown). By contrast, the immunization of TLR3ko mice with BOVAp in the presence of poly(I:C) induced a CTL response, as shown by the in vivo killing of target cells loaded with the OVA257-264 peptide. However, CTLs were much less frequent in these mice than in WT mice (Figure 4B). A similarly impaired CTL response to BOVAp and poly(I:C) immunization was observed in Trif−/− mice (Figure 5B). It should be noted that, following immunization with BOVAp in the presence of anti-CD40 mAb, TLR3ko and TLR9ko mice mounted a CTL response similar to that of control mice (Figure 4C). The impaired immune response observed in TLR3ko and TLR9ko mice injected with BOVAp and poly(I:C) or CpG(DOTAP), respectively, was due to the lack of TLR signaling rather than a general defect in these strains.
Our data show that signaling through TLR9 is strictly required for CTL priming by CpG(DOTAP), whereas an absence of TLR3 triggering significantly decreases, but does not abolish, the ability of poly(I:C) to promote CTL responses. Thus, although the adjuvant effect of poly(I:C) on CTL priming is dependent on type I IFN, IFN-α/β-derived signals alone are not sufficient to induce fully efficient CTL priming in the absence of CD4+ T-cell help.
In the presence of TLR3 agonist, CD4+ T-cell help is not required for the in vivo activation, persistence, and restimulation of memory CTLs
We determined whether CD4+ helper T cells, which are normally required for the activation of fully functional memory CTLs, could be replaced by signals provided through TLR stimulation. We analyzed the effector functions of memory CTLs induced by BOVAp, injected with poly(I:C). Anti-CD40 mAb, which binds CD40, leading to DC activation, was selected as a surrogate for helper T cells. After the injection of BOVAp and poly(I:C), considerable expansion of the OVA-specific CTL population was observed, reaching almost 3% of the total number of CTLs 7 days after injection. This expansion was followed by a prolonged contraction phase and then by a plateau that persisted throughout the memory phase (Figure 6A). By contrast, the expansion phase induced by BOVAp and anti-CD40 mAb was less intense (specific CTLs accounting for 2% of the total number of CTLs) and was followed by a sharp contraction phase, restoring OVATetr+ levels to levels slightly higher than those in naive mice. “Helpless” OVA-specific CTLs generated in the presence of poly(I:C) displayed full effector functions, such as in vivo lytic activity and IFN-γ secretion, even 120 days after priming, similar to those of their “helped” counterparts induced by agonist anti-CD40 mAb (Figure 6B).
TLR3 signaling induces long-lasting effector/memory CD8+ T cells in the absence of CD4+ T-cell help. (A-B) C57BL/6 mice were left untreated or were immunized by a single intravenous injection of BOVAp with poly(I:C) or anti-CD40 mAb. (A) Kinetics of the OVA257-264–specific CD8+ T-cell primary response. At various times after priming, OVA257-264–specific (OVATetr+) CD8+ T cells were quantified in the spleen by tetramer staining. Results are expressed as means ± SEM for 5 to 7 mice, tested in 2 independent experiments. (B) Specific CTL response analyzed in the spleen by in vivo killing assay at 1 week, and 2 and 4 months after priming. Data are representative of 2 independent experiments. (C) Restimulation of the CTL memory response by CyaA-OVA. C57BL/6 mice were left untreated or received an injection (intravenously) of BOVAp with poly(I:C) or anti-CD40 mAb, and, 25 days later, mice remained untreated or received an intravenous injection of CyaA-OVA. The percentage of OVATetr+ CD8+ T cells was quantified in the spleen at various times after the second injection. Results are expressed as means ± SEM for 6 mice, tested in 2 independent experiments. (D) Restimulation by CyaA-OVA of the CTL memory response in MHC-IIko mice. C57BL/6 or MHC-IIko mice were left untreated or received an intravenous injection of BOVAp with poly(I:C), and, 25 days later, mice remained untreated or received a second intravenous injection of CyaA-OVA or of BOVAp with poly(I:C). The percentage of OVATetr+ CD8+ T cells was quantified in the spleen on day 7 after the second injection. Two-tailed unpaired t test with the Welch correction was used to analyze the results presented in panel D. Data are representative of 2 independent experiments.
TLR3 signaling induces long-lasting effector/memory CD8+ T cells in the absence of CD4+ T-cell help. (A-B) C57BL/6 mice were left untreated or were immunized by a single intravenous injection of BOVAp with poly(I:C) or anti-CD40 mAb. (A) Kinetics of the OVA257-264–specific CD8+ T-cell primary response. At various times after priming, OVA257-264–specific (OVATetr+) CD8+ T cells were quantified in the spleen by tetramer staining. Results are expressed as means ± SEM for 5 to 7 mice, tested in 2 independent experiments. (B) Specific CTL response analyzed in the spleen by in vivo killing assay at 1 week, and 2 and 4 months after priming. Data are representative of 2 independent experiments. (C) Restimulation of the CTL memory response by CyaA-OVA. C57BL/6 mice were left untreated or received an injection (intravenously) of BOVAp with poly(I:C) or anti-CD40 mAb, and, 25 days later, mice remained untreated or received an intravenous injection of CyaA-OVA. The percentage of OVATetr+ CD8+ T cells was quantified in the spleen at various times after the second injection. Results are expressed as means ± SEM for 6 mice, tested in 2 independent experiments. (D) Restimulation by CyaA-OVA of the CTL memory response in MHC-IIko mice. C57BL/6 or MHC-IIko mice were left untreated or received an intravenous injection of BOVAp with poly(I:C), and, 25 days later, mice remained untreated or received a second intravenous injection of CyaA-OVA or of BOVAp with poly(I:C). The percentage of OVATetr+ CD8+ T cells was quantified in the spleen on day 7 after the second injection. Two-tailed unpaired t test with the Welch correction was used to analyze the results presented in panel D. Data are representative of 2 independent experiments.
We then investigated whether the memory CTLs generated in the absence of CD4+ T-cell help, in response to BOVAp and poly(I:C), could undergo a second round of clonal expansion after specific restimulation. Mice were primed with BOVAp and either poly(I:C) or anti-CD40 mAb, followed by a boost 25 days later with a single intravenous injection of a MHC-I–vaccine delivery vector consisting on the adenylate cyclase (CyaA) of B pertussis carrying the OVA257-264 epitope (CyaA-OVA).8 The immunization of naive mice with CyaA-OVA induced a primary OVA-specific CTL response accounting for 2% of all CTLs 7 days after injection (Figure 6C). Upon boosting with CyaA-OVA, “helpless” and “helped” memory CTL populations underwent similar, rapid, massive expansions (Figure 6C). The peak of OVA-specific CTLs induced upon secondary immunization was about 5 times higher than the peak of the primary response (Figure 6A,C).
Several studies have demonstrated that the absence of CD4+ T-cell help during CTL priming results in defective memory CTLs that do not undergo a second round of clonal expansion unless CD4+ T-cell help is provided during the recall response.4,6,7,13–15 We investigated whether memory CTLs primed by BOVAp and poly(I:C) could proliferate in response to CyaA-OVA immunization, by priming WT and MHC-IIko mice with BOVAp+poly(I:C), administering a CyaA-OVA boost on day 25 and analyzing the recall response by tetramer staining 7 days later. Poly(I:C)-derived signaling during the primary response generated fully functional memory CTLs able to undergo a second round of marked expansion despite the absence of CD4+ T cells during the recall response (Figure 6D).
Discussion
Several studies have indicated that the requirement for CD4+ T-cell help for the induction of effector and memory CTLs is not absolute and depends on the nature of the stimuli present during the primary response. In particular, some viral infections elicit “helpless” CTL responses. We show here that the requirement for T-cell help to trigger CTLs can be bypassed by TLR3-L and TLR9-L, with administration in liposomes required for TLR9-L, but not by other TLR agonists, such as TLR2/6-L, TLR4-L, or TLR7-L.
TLR4-L and naked TLR9-L have been shown to induce CTL responses when administered with a soluble protein.24 However, the adjuvant effect of these 2 TLR agonists used in combination with soluble antigens containing both CD4+ and CD8+ T-cell epitopes, may be due to a synergic effect between TLR triggering-derived signals and signals provided by antigen-specific CD4+ T cells. By contrast, we used an experimental system allowing the analysis of CTL responses induced by a single CTL epitope, excluding the participation of CD4+ T cells.
Unlike zymosan and R837, all the TLR-Ls tested in this study up-regulated MHC-I and costimulatory molecule levels on DCs in vivo. An increase in the capacity to deliver signal 1 (antigen) and signal 2 (costimulation) is often interpreted as the key feature in the licensing of DCs to prime an effective CTL response. However, several studies,30–32 including this one, have shown that the stimulation of naive CTLs can fail even when the DCs express large amounts of antigen and B7 ligands. It has been suggested that initiation of the program leading to effector/memory CTL activation requires a third signal that can be provided by IL-12,16 IFN-α,17 or IL-21,18 but not by other cytokines, such as IL-1/2/4/6/7/15/18, TNF-α, and IFN-γ.17,33 The third signal may be provided directly (IL-21) or indirectly by CD4+ T cells, but also by alternative pathways. TLR engagement stimulates the production of IL-12 by various cells from the innate system30–32 including DCs. TLR3-L, TLR4-L, TLR7-L, and TLR9-L are strong inducers of IL-12, with TLR9-L effective even if supplied as naked molecules.24,33,34 However, this strong systemic IL-12 response does not account for the different capacities of different TLR-Ls to support a CTL response. Indeed, we show here that TLR7-L and TLR9-L induce high levels of IL-12 production by DCs in vivo, whereas TLR3-L and TLR4-L do not. Thus, the capacity of DCs to produce IL-12 following TLR stimulation is not correlated with their ability to promote CTL priming. IL-12 production by DCs is therefore neither necessary nor sufficient to trigger CTLs in the absence of activated CD4+ T helper cells.
The injection of IFN-α has been shown to enhance CTL responses against soluble proteins.28 The immunostimulatory activity of IFN-α results at least partly from its ability to induce DC maturation directly.35 Several studies have recently demonstrated that type I IFNs also act directly on naive CTLs, inducing clonal expansion and differentiation into effector17,36,37 and memory CTLs.36 We show here that only robust in vivo IFN-α/β inducers, such as poly(I:C) and CpG(DOTAP), were able to support CTL priming in the absence of CD4+ T-cell help. Moreover, the ability of TLR3-L and TLR9-L to prime “helpless” CTLs was strictly dependent on IFN-α/β signaling, because the induction of CTL responses by these TLR-Ls was abolished in IFNARko mice. Thus, in the absence of CD4+ T-cell help, the TLR engagement-mediated up-regulation of MHC-I and costimulatory molecules and IL-12 production by DCs cannot fulfill the requirements for CTL priming, as IFN-α/β signaling is necessary to induce a full CTL response.
In cases of impaired proinflammatory response, such as after poly(I:C) injection in mice deficient for TLR3 signaling, the induction of high levels of IFN-α/β is not sufficient for optimal CTL priming. The poly(I:C)-induced production of cytokines in vivo is differentially regulated by TLR3 and MDA-5 molecules.33,38 TLR3 signaling is critical for IL-12 production and MDA-5 is essential for IFN-α/β production, whereas both sensors regulate IL-6 production in response to poly(I:C). Our results, obtained using TLR3ko and Trifko mice, suggest that IFN-α/β must work in concert with other proinflammatory cytokines for the efficient induction of effector CTLs. This hypothesis is supported by a recent study by Salem et al39 demonstrating that the clonal expansion of OT-1 cells following immunization with OVA257-264 peptide and poly(I:C) requires IFN-α/β and is dependent upon IL-12, TNF-α, IL-15, and IFN-γ.
Microbial DNA can be sensed via TLR9-dependent and TLR9-independent pathways.32 We show here that TLR9 engagement is strictly required for CpG-promoted IFN-α/β production and CTL priming. The injection of naked CpG, at doses ranging from 25 to 100 μg, induces a strong, systemic, TLR-9–dependent proinflammatory response and the up-regulation of MHC-I and costimulatory molecules on DCs (Iwasaki et al32 ) but does not promote IFN-α/β production or CTL responses (data not shown). It has been reported that murine plasmacytoid DCs (pDCs) produce mainly IFN-α and IL-12 upon in vitro stimulation with naked CpG-A and CpG-B, respectively. It remains unknown how different TLR9 agonists lead to such distinct outcomes. In vitro studies have shown that CpG-B is rapidly transferred and degraded in the lysosome, whereas CpG-A DNA is retained for long periods in the endosome of pDCs, together with signal-transducing molecules, such as MyD88 and IFN regulatory factor 7.40,41 If CpG-B is modified by cationic lipids to be retained in the endosome, it can induce type I IFN production. However, as shown here, regardless the type of CpG used, the IFN-α/β in vivo response required to support the CTL priming is produced only when CpG ODNs are administered together with cationic liposomes. These results suggest that, in vivo, cationic liposomes may be strictly necessary to allow the endosomal retention of the ligand-TLR9 complexes and/or the preferential targeting of the ODNs to the endosomes.
LPS and TLR7-Ls do not induce CTL responses, even though they share the common adaptor proteins Trif (poly(I:C)) and MyD88 (CpG) and despite the fact that certain DC subsets have been shown to produce in vitro IFN-β and/or IFN-α in response to LPS and TLR7-Ls.42 Here we show that in vivo engagement of either TLR4 or TLR7 was unable to induce the strong systemic type I IFN response required to support CTL priming. The poor or even undetectable IFN-α/β response elicited upon administration of TLR4 and TLR7 ligands could be due to several reasons: (1) Short half-life of certain TLR agonists. (2) Preferential in vivo targeting by certain TLR agonists of other cell types than DCs and macrophages. There is increasing evidence that cells from nonhematopoietic origin express functional TLR.43,44 The contribution of these cells to the final cytokine pattern induced in vivo by different TLR-Ls remains to be elucidated. (3) Inadequate intracellular location. As we have proposed for naked CpG, an inefficient endosomal translocation and/or retention of TLR7-Ls could limit the in vivo production of type I IFN. (4) Activation of additional pathway(s) by poly(I:C) and CpG, but not by LPS and TLR7 ligands, that could lead to the production of type I IFN in vivo (as the MDA-5 pathway for poly(I:C)) and/or activation of MHC-I–related antigen-processing machinery. Using DC and OT-1 in an in vitro cross-presentation assay, Datta et al45 reported that only TLR3-L and TLR9-L, but not TLR2, TLR4, or TLR5 ligands, induce cross-presentation. It would be interesting to explore if IFN-α/β also play a role in the activation of the MHC-I–related antigen-processing machinery.
No study has yet analyzed the functional properties of the “helpless” memory CTL population promoted by signals derived from TLR triggering. In this study, we show that “helpless” CTLs induced by TLR3-L differentiate into fully functional memory CTLs able to proliferate upon secondary immunization in the absence of any CD4+ T-cell help. This proliferation is equivalent to that of surrogate “helped” memory CTLs induced with anti-CD40 mAb. Our results are consistent with previous reports showing that “helpless” memory CTLs induced by Listeria monocytogenes infection can proliferate upon secondary challenge with the same agent, despite the absence of CD4+ T cells during the recall response.7
Our data clearly indicate that signals derived from TLR triggering can bypass the requirement of CD4+ T-cell help for the in vivo induction of fully efficient effector and memory CTLs. We also show that the TLR engagement-mediated up-regulation of MHC-I and costimulatory molecules and IL-12 production by DCs does not fulfill the requirements for the priming of naive CTLs in the absence of CD4+ T-cell help, as the induction of a strong IFN-α/β response is necessary. However, in the absence of harmonized orchestration between proinflammatory cytokines, IFN-α/β–derived signals alone are insufficient to induce a fully functional CTL response in the absence of CD4+ T-cell help. This study provides information essential for the rational design of vaccines promoting fully efficient CTL responses.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from Association de la Recherche contre le Cancer and Institut National du Cancer and the European Community (Theravac Project) to C.L. S.H-S. was supported by Universidad Pública de Navarra (Spain).
The authors thank Daniel Ladant for providing CyaA-OVA and Richard Lo-Man for fruitful discussions.
Authorship
Contribution: S.H.-S. performed and coordinated most experiments and wrote the first draft of the paper; A.O. performed the experiments suggested by reviewers; F.B. initiated this work and contributed to paper writing; N.T. helped discuss data and contributed with Trifko mice; C.L. provided the original ideas, coordinated the team, obtained funding, and wrote the final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claude LECLERC, Régulation Immunitaire et Vaccinologie, Inserm U883, Institut Pasteur, 25 rue du Docteur Roux, 75724 Paris Cedex 15, France; e-mail: cleclerc@pasteur.fr.

![Figure 1. Poly(I:C) and CpG promote functional CTL responses in the absence of CD4+ T-cell help. (A) C57BL/6 mice were left untreated or were immunized by a single intravenous injection of BOVAp either alone or in combination with zymosan, poly(I:C), LPS, poly(U), R837, R848, CpG-B, or CpG-A. CpG was administered either naked or mixed with DOTAP (CpG(DOTAP)). The percentage of OVA257-264–specific lysis was determined by in vivo killing assay 7 days after immunization. Control mice were immunized with control ODNs or BOVAp mixed with DOTAP. Specific lysis was calculated as follows: % specific lysis = 100 − [100 × (% CFSEhigh-primed mice/% CFSElow-primed mice)/(% CFSEhigh-naive mice/% CFSElow-naive mice)]. (B) Frequency of OVA257-264–specific CTLs detected in the spleen 7 days after intravenous injection of BOVAp alone or with poly(I:C), CpG-B(DOTAP), or CpG-A(DOTAP). OVA257-264–specific CTLs (OVATetr+) were quantified by tetramer staining. (C) C57BL/6 mice received a single intravenous or subcutaneous injection of BOVAp either alone or with poly(I:C) or CpG-B(DOTAP). Seven days after injection, the CTL response was quantified by in vivo killing assay. (D-E) Poly(I:C) and CpG induce CTL responses against BOVAp in the absence of CD4+ T-cell help. C57BL/6 and MHC-IIko mice were either left untreated or immunized intravenously with BOVAp alone or with poly(I:C) (D-E), CpG-B(DOTAP) (E), or anti-CD40 mAb (D). The specific CTL response was analyzed on day 7 by in vivo killing assay (D) and tetramer staining (E). (F) CTL response induced by poly(I:C) and beads coated with either E749-57 (BE7p), HY738-746 (BHYp), or LCMV33-41 (BLCMVp) peptides. Female C57BL/6 mice were either left untreated or immunized by a single intravenous injection of the corresponding beads either alone or in combination with poly(I:C). (G) CTL response induced by poly(I:C) and soluble OVA257-264 peptide. C57BL/6 mice were left untreated or immunized by a single subcutaneous injection of either BOVAp or soluble OVA257-264 peptide (OVAp) alone or in combination with poly(I:C). (F-G) The CTL response was quantified in the spleen by in vivo killing on day 7 after priming. Dots represent individual mice. All results are representative of at least 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-10-053256/4/m_zh80120701850001.jpeg?Expires=1769223862&Signature=YT7imSaxN9vhopPBmrpNjBSka7VscHWgMGWOOvLnQq9q~EFYGPISiGvG48ZZ8rr25IIUr6sIqOWOeMNF2wBdf56h2QlzVJ9QG2uQvLTRRwn8gu0dE-3Qm5jtlA5lEPZi4Km2qr5Ei2K9PXXLmmAApbBnR7mH-1n7l9GwwjkN0vfae9qZopu4bm2sOCBOwwdEySqa3-BBPgXggUMVjGTgas0XE1DvUfTBs6DV1bDD9gXXc9AhFTrCzYtSERjiJ0P6RpIKLeQw7QWsFuBFIobv8YhYP7UQpzFJzag1eFegTTZV1TWqa~-~b1ssQso902MTblzwVVSxpZ4tdvYWYKW8KQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal