Abstract
Defects in erythrocyte ankyrin are the most common cause of typical, dominant hereditary spherocytosis (HS). Detection of ankyrin gene mutations has been complicated by allelic heterogeneity, large gene size, frequent de novo mutations, and associated mRNA instability. Using denaturing high-performance liquid chromatography (DHPLC)–based mutation detection, a mutation in the splice acceptor of exon 17 was discovered in a Turkish family. Reticulocyte RNA and functional minigene splicing assays in heterologous cells revealed that this mutation was associated with a complex pattern of aberrant splicing, suggesting that removal of intron 16 is important for ordered ankyrin mRNA splicing. As predicted by clinical, laboratory, and biochemical studies, the parents were heterozygous and the proband was homozygous for this mutation. These data indicate that DHPLC offers a highly sensitive, economic, and rapid method for mutation detection and, unlike previously suggested, homozygosity for a mutation associated with dominant ankyrin-linked HS may be compatible with life.
Introduction
Mutations of erythrocyte ankyrin are the most common cause of typical, dominant hereditary spherocytosis (HS).1–3 We developed a denaturing high-performance liquid chromatography (DHPLC)–based mutation screening method and used it to study a Turkish HS kindred. The proband suffered from severe, ankyrin-deficient HS. His parents, first cousins, both suffered from typical, ankyrin-deficient HS.
Patients, materials, and methods
Patients
The clinical, laboratory, and biochemical characteristics of this kindred have been previously reported.4 Approval was obtained from the Ondokuz May's Üniversitesi institutional review board for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
Mutation detection
Genomic DNA was amplified using primers flanking the 42 coding exons and promoter of the ankyrin-1 gene. DHPLC was performed on a WAVE analysis system (Transgenomic, Omaha, NE) using conditions described (Table S1, available on the Blood website; see the Supplemental Tables link at the top of the online article). Primers were designed using Primer 3 and WAVEmaker 4.1.40 software to optimize polymerase chain reaction (PCR) and DHPLC conditions. Nucleotide sequence was determined on amplification products demonstrating an abnormal chromatograph.
Splicing assays
Reticulocyte RNA was reverse transcribed using oligo d(T). cDNA was amplified using primers I+D or I+G (Table S2). [32P]-dCTP was added to some reactions. Amplification products were separated by gel electrophoresis, transferred to a filter, and exposed to a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Wild-type and ankyrinAnkara minigenes were created with a cytomegalovirus (CMV) promoter, the ankyrin ATG/Kozak consensus, ankyrin exons 15-20, a termination codon, and a bGH polyadenylation signal. COS or K562 cells (107) were transiently transfected via lipofection with 10 μg minigene plasmid. Cells were harvested after 48 hours, RNA was prepared, and reverse transcriptase–PCR (RT-PCR) was performed using primers I+G. Amplification products were subcloned and sequenced.
Results and discussion
Mutation detection
Initially, the ankyrin gene of the proband was screened by single-stranded conformational polymorphism and heteroduplex analyses.2 Because no mutations were identified, a DHPLC mutation detection technique was developed. DHPLC identified several variants (Table S3) including one, −17G>A in intron 16, changing the sequence upstream of exon 17 from ctctgggcGGcttctt to ctctgggcAGcttctt, creating a new splice acceptor site.
Splicing assays: reticulocyte RNA
To determine if this mutation was associated with altered mRNA splicing, RT-PCR of reticulocyte RNA was performed with primers in exons 15 and 17 (I+D). Control RT-PCR products yielded the expected cDNA fragment of 248 bp. In the father, the expected band of 248 bp and additional bands of higher molecular weight were visualized (Figure 1A). In the proband, the normal 248-bp cDNA product was not seen and only the additional higher molecular weight bands visualized in the father's cDNA were found.
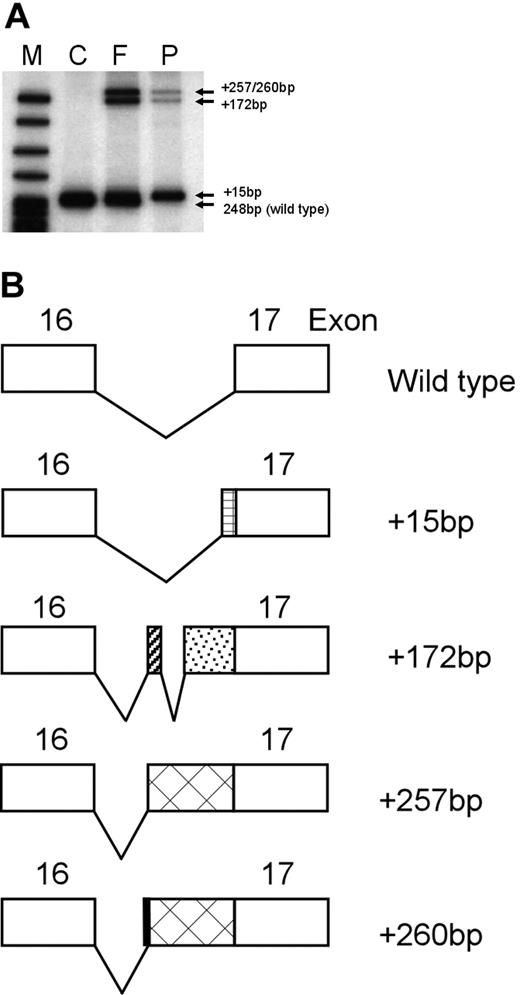
Splicing analyses of the ankyrinAnkara mutation in reticulocyte RNA. (A) To determine if the ankyrinAnkara mutation was associated with altered mRNA splicing, reticulocyte RNA was reverse transcribed with oligo d(T). RT products were PCR amplified with primers in exons 15 and 17. M indicates markers. Control (C) reticulocyte RT-PCR product yielded the expected cDNA fragment of 248 bp. In the father (F), the expected band of 248 bp and 3 additional bands of higher molecular weight were visualized. In the proband (P), the normal 248-bp cDNA product was not seen and only the 3 additional higher molecular weight bands visualized in the father's cDNA were found. (B) Determination of the nucleotide sequence of these higher molecular weight bands yielded novel ankyrin cDNA isoforms containing additional sequence of +15 bp, +172 bp, +257 bp and +260 bp, respectively, from the 3′ end of intron 16. The corresponding predicted splicing patterns of these isoforms are shown.
Splicing analyses of the ankyrinAnkara mutation in reticulocyte RNA. (A) To determine if the ankyrinAnkara mutation was associated with altered mRNA splicing, reticulocyte RNA was reverse transcribed with oligo d(T). RT products were PCR amplified with primers in exons 15 and 17. M indicates markers. Control (C) reticulocyte RT-PCR product yielded the expected cDNA fragment of 248 bp. In the father (F), the expected band of 248 bp and 3 additional bands of higher molecular weight were visualized. In the proband (P), the normal 248-bp cDNA product was not seen and only the 3 additional higher molecular weight bands visualized in the father's cDNA were found. (B) Determination of the nucleotide sequence of these higher molecular weight bands yielded novel ankyrin cDNA isoforms containing additional sequence of +15 bp, +172 bp, +257 bp and +260 bp, respectively, from the 3′ end of intron 16. The corresponding predicted splicing patterns of these isoforms are shown.
The sequence of these higher molecular weight bands corresponded to 4 novel ankyrin cDNA isoforms containing additional sequence of 15 bp, 172 bp, 257 bp, and 260 bp, respectively, from the 3′ end of intron 16. The corresponding splicing patterns of these isoforms are shown in Figure 1B. In all but the 15-bp insert, a premature termination codon is present, predicting, if stable, a protein product truncated after ankyrin repeat 18.5–7 The 15-bp insert is predicted to produce an extended loop in ankyrin repeat 18, and, if assembled onto the membrane, predicted to disrupt ankyrin–band 3 interactions.5–7 No wild-type ankyrin isoforms were found in the proband's cDNA.
Mutations in splice acceptor regions have been associated with skipping of the downstream exon and/or other splicing defects.8,9 To search for additional aberrant spliceoforms, RT-PCR was performed using primers from exons 15 and 19 (I+G). Nine abnormal spliceoforms were identified, including the 4 variants previously found and spliceoforms that skipped exon 17, exons 16 + 17, 17 + 18, 16 + 17 + 18, and skipped exon 16 and added 18 bp from the 3′ end of intron 16 (Table S4). At the protein level, these isoforms would be predicted to delete repeats 19 + 20, 18 + 19 + 20, 19 + 20 + 21, 18 + 19 + 20 + 21, and repeat 18 with 6 novel amino acids inserted, respectively. These repeats are critical for ankyrin–band 3 interactions.5–7 In cDNA prepared from the proband's reticulocyte RNA, no spliceoforms were wild type.
Splicing assays: ankyrin minigenes
Ankyrin mRNA processing was analyzed in an in vitro functional splicing assay using ankyrin minigenes, a valuable technique for study of normal and aberrant splicing.10,11 Wild-type and mutant ankyrinAnkara minigenes from exon 15 to 20 were prepared and transfected into COS or K562 cells (Figure S1A). COS cells offer the advantage of not expressing endogenous ankyrin-1.
RT-PCR products from COS cells transfected with the wild-type minigene contained the expected normally sized product with correct mRNA splicing from exons 15 to 19. RT-PCR products from COS cells transfected with the ankyrinAnkara minigene yielded the same 9 mutant spliceoforms identified in reticulocyte RNA, 2 additional spliceoforms, +26 bp and +144 bp (Figure 2; Table S4), but no wild-type product. No ankyrin spliceoforms were visualized in mock-transfected COS cells.
Minigene analyses of ankyrin mRNA splicing. Wild-type and mutant ankyrinAnkara minigenes from exon 15 to exon 20 were prepared and transfected into COS and K562 cells. The sequence of the RT-PCR products derived from RNA of transfected cells was analyzed. RNA from cells transfected with the wild-type minigene contained the expected normally sized and spliced product (wild-type [WT], top line). RT-PCR products derived from RNA of cells transfected with the mutant ankyrinAnkara minigene yielded 11 mutant spliceoforms, shown schematically, but no wild-type product. Similar studies with reticulocyte RNA from the proband yielded the same results, except that the +26-bp and +144-bp spliceoforms were not found.
Minigene analyses of ankyrin mRNA splicing. Wild-type and mutant ankyrinAnkara minigenes from exon 15 to exon 20 were prepared and transfected into COS and K562 cells. The sequence of the RT-PCR products derived from RNA of transfected cells was analyzed. RNA from cells transfected with the wild-type minigene contained the expected normally sized and spliced product (wild-type [WT], top line). RT-PCR products derived from RNA of cells transfected with the mutant ankyrinAnkara minigene yielded 11 mutant spliceoforms, shown schematically, but no wild-type product. Similar studies with reticulocyte RNA from the proband yielded the same results, except that the +26-bp and +144-bp spliceoforms were not found.
Mock-transfected K562 cells yielded the endogenous, correctly spliced wild-type ankyrin product (Figure S1B). K562 cell RT-PCR products with the wild-type minigene contained the expected normally sized product with correct mRNA splicing from exons 15-19, impossible to distinguish from endogenous ankyrin (Figure S1B). K562 cell RT-PCR products with the ankyrinAnkara minigene yielded the same mutant spliceoforms identified in reticulocyte RNA. PhosphorImager quantitation of the K562 cell RT-PCR products was performed (Figure S1C). The most abundant spliceoforms were the +260 bp, +172 bp, and the spliceoform skipping exons 16 + 17 + 18. It was impossible to distinguish the amounts represented by the +15 and +26 bp or the skipped exon 16 + 17 and 17 + 18 spliceoforms, but these were of relatively low abundance. Slightly more than a third of the spliceoforms were created by the use of alternate AG splice acceptors in intron 16 (+260, +172, +144, +26, and +15 bp).
Molecular genetic analyses
The mutation creates a novel site for the enzyme AluI, allowing for PCR-based detection in family members (Figure S2). These studies revealed that the parents were heterozygous and the proband was homozygous for this mutation. The majority of defects in typical, dominant HS are heterozygous mutations in ankyrin.1 Rare patients homozygous for ankyrin defects would be expected, but such patients have not been described. Because of the importance of ankyrin in linking the spectrin-based membrane skeleton and the lipid bilayer, Bennett12 postulated that homozygosity for an ankyrin mutation would result in fetal death. Murine nb/nb erythrocyte membranes, which lack normal full-length ankyrin, are approximately 50% deficient in spectrin and assemble a highly ordered hexagonal membrane skeletal lattice, indicating that ankyrin is not required for formation of a stable, spectrin-based skeleton.13 These results suggest that homozygosity for a mutation associated with dominant ankyrin-linked HS may be compatible with life. Of note, erythrocyte membranes from the proband and his parents did not demonstrate qualitative or quantitative abnormalities of band 3 on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analyses.4
Splicing assays revealed that the ankyrinAnkara mutation is associated with a complex pattern of aberrant splicing, suggesting that removal of intron 16 is important for ordered splicing of ankyrin mRNA. Two well-characterized splicing mutations, in the COL1A1 gene (osteogenesis imperfecta) and the COL5A1 gene (Ehlers-Danlos), are due to single mutations leading to multiple aberrant mRNA species due to perturbed order of intron removal.14,15 The process of ordered splicing is fundamental to production of appropriate protein products, yet the mechanisms controlling correct order of exon joining are poorly understood.16
Detection of ankyrin gene mutations has been complicated by allelic heterogeneity, large gene size, frequent de novo mutations, and associated mRNA instability.1 DHPLC has emerged as a highly sensitive, high-capacity, semiautomated, low-cost method for mutation detection, DNA methylation analysis, and detection of somatic mosaicism.17–21 DHPLC identifies mutations in DNA with high G+C content, a significant advantage over Taq-based direct sequencing.22,23
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grant DK062039 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIDDK, NIH; P.G.G.).
National Institutes of Health
Authorship
Contribution: E.J.E. designed and performed experiments. Y.M. designed and performed experiments and prepared figures. F.D. and C.A. performed phenotyping, prepared patient samples, and reviewed manuscript. P.G.G. designed and interpreted experiments and prepared manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Patrick G. Gallagher, Department of Pediatrics, Yale University School of Medicine, 333 Cedar Street, PO Box 208064, New Haven, CT 06520-8064; e-mail: patrick.gallagher@yale.edu.

![Figure 2. Minigene analyses of ankyrin mRNA splicing. Wild-type and mutant ankyrinAnkara minigenes from exon 15 to exon 20 were prepared and transfected into COS and K562 cells. The sequence of the RT-PCR products derived from RNA of transfected cells was analyzed. RNA from cells transfected with the wild-type minigene contained the expected normally sized and spliced product (wild-type [WT], top line). RT-PCR products derived from RNA of cells transfected with the mutant ankyrinAnkara minigene yielded 11 mutant spliceoforms, shown schematically, but no wild-type product. Similar studies with reticulocyte RNA from the proband yielded the same results, except that the +26-bp and +144-bp spliceoforms were not found.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-09-046573/4/m_zh80120701240002.jpeg?Expires=1765900573&Signature=RbCgYZWVkLyYXJc4wM0v1L2gLiNwNQTnjiRyUOOfeL~TAyi2am9bKISlA3QdG4o-rjkTy7vXeIBcbHpRjft6oPC0relV6IU4Y5laGKe-4NLB9WGcSirqgSTC6ArWL6I5egZsBQI0Se1rUm1McIrDDeRthzZYGgfmB7SP90tRL7uzoQmxv5tf2UXX8vLeBCKWTwwIz4OaRN-3tRaJ2p-6ly6bmtN-MUij5HuUy3-qEMEzkynh66guq2Zrf2WCOV6sxS8kgqSgpBV-lXNsBZEGOpW4EvxkYeghQruZXnyduaqxxzOYszjudfRYD5jTdIBL8bFV4wfaICacuqHGQ1L7rg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal