Abstract
Signal regulatory protein α (SIRPα) is a critical immune inhibitory receptor on macrophages, and its interaction with CD47, a ligand for SIRPα, prevents autologous phagocytosis. We hypothesized that interspecies incompatibility of CD47 may contribute to the rejection of xenogeneic cells by macrophages. Here, we show that pig CD47 does not interact with mouse SIPRα. Similar to CD47−/− mouse cells, porcine red blood cells (RBCs) failed to induce SIRPα tyrosine phosphorylation in mouse macrophages. Blocking SIRPα with antimouse SIRPα mAb (P84) significantly enhanced the phagocytosis of CD47+/+ mouse cells, but did not affect the engulfment of porcine or CD47−/− mouse cells by mouse macrophages. CD47-deficient mice, whose macrophages do not phagocytose CD47−/− mouse cells, showed markedly delayed clearance of porcine RBCs compared with wild-type mouse recipients. Furthermore, mouse CD47 expression on porcine cells markedly reduced their phagocytosis by mouse macrophages both in vitro and in vivo. These results indicate that interspecies incompatibility of CD47 contributes significantly to phagocytosis of xenogeneic cells by macrophages and suggest that genetic manipulation of donor CD47 to improve its interaction with the recipient SIRPα may provide a novel approach to prevent phagocyte-mediated xenograft rejection.
Introduction
The severe shortage of allogeneic donors currently limits the number of organ transplantations performed.1 This supply-demand disparity could be corrected by the ability to use organs from other species (xenografts). In view of the ethical issues and impracticalities associated with the use of nonhuman primates, pigs are considered the most suitable organ donor species for humans. In addition to organ size and physiologic similarities to humans, the ability to rapidly breed and inbreed pigs makes them particularly amenable to genetic modifications that could improve their ability to function as organ donors to humans.2,3 However, xenotransplantation from pigs is hampered by vigorous immunologic rejection. In addition to the adaptive immune responses, which play critical roles in both allograft and xenograft rejection, the innate immune system also mediates strong rejection of organs and cells from discordant xenogeneic donors.
Studies in various models have shown that macrophages contribute significantly to xenograft rejection. In xenotransplant recipients, macrophages are activated and recruited rapidly, and their responses to xenoantigens precede the activation of T cells.4 It has been reported that macrophages contribute significantly to the rejection of porcine hematopoietic cells5,6 and islet xenografts7-9 in both rodents and primates. Similarly, macrophages also mediate strong rejection of human hematopoietic cells10 and islets11 in mice. The rapid and refractory rejection of xenogeneic hematopoietic cells by macrophages greatly impedes the application of mixed chimerism, a means of tolerance induction, to xenotransplantation.
Macrophage activation is regulated by the balance between activating and inhibitory signals. CD47 (integrin-associated protein), a member of the Ig superfamily, is ubiquitously expressed in all tissues and serves as a ligand for signal regulatory protein α (SIRPα; also known as CD172a, SHPS-1), an immune inhibitory receptor on macrophages.12,13 Studies using CD47-deficient mice demonstrated that SIRPα on macrophages recognizes CD47 as a marker of “self.”14 CD47-SIRPα signaling prevents phagocytosis of normal hematopoietic cells by autologous macrophages14,15 and reduces the sensitivity of antibody- and complement-opsonized cells to phagocytosis.16,17 These results indicate that macrophages rely on CD47 expression to distinguish “self” from “nonself” and to set a threshold for macrophage-mediated phagocytosis of opsonized cells. Thus, donor cells would be highly susceptible to phagocytosis by recipient macrophages in a xenogeneic transplantation setting if donor CD47 fails to interact with recipient SIRPα. As a first step in investigating this question, in this study we assessed the role of CD47 in phagocytosis of xenogeneic cells in the setting of pig-to-mouse xenotransplantation. Our results indicate that the failure of pig CD47 to interact with mouse SIRPα makes porcine cells highly sensitive to phagocytosis by mouse macrophages. Furthermore, genetic manipulation of donor CD47 to improve its interaction with the recipient SIRPα is effective in preventing the rejection of porcine cells by macrophages in mice.
Materials and methods
Animals
C57BL/6 (B6) mice were purchased from the Jackson Laboratories (Bar Harbor, ME); CD47 gene knock-out (CD47 KO) mice on a B6 background were generated as previously described.14 We used inbred Massachusetts General Hospital miniature swine (kindly provided by Dr David H. Sachs) as porcine cell donors. Care of animals was in accordance with the Guide for the Care and Use of Laboratory Animals.18 Protocols involving animals were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
Antibodies
An anti-SIRPα antibody (P84)12 was used to block macrophage inhibitory receptor SIRPα. Fluorescein isothiocyanate (FITC)–conjugated anti–mouse CD47 (miap 301; Pharmingen, San Diego, CA) and R-phycoerythrin (R-PE)–conjugated anti-F4/80 (Caltag Laboratories, Burlingame, CA) were used for flow cytometry and immunohistology. In flow cytometric analyses, nonspecific binding of labeled mAbs was blocked with 2.4G2 (rat anti–mouse FCγR mAb); HOPC1 (murine IgG2a) and rat IgG (both from Pharmingen) were used as isotype controls.
Mouse macrophage preparation
Bone marrow–derived and splenic macrophages were prepared as previously described.14,16 To prepare peritoneal macrophages, peritoneal cells were harvested from B6 mice 4 days after intraperitoneal injection of 2% Bio-Gel polyacrylamide P 100 (1 mL/mouse; Bio-RAD Laboratories Hercules, CA) and cultured at 37°C for 2 hours. Macrophages were used after washing off the nonadherent cells.
Immunoprecipitation and Western blot analysis
Bone marrow–derived macrophages (2 × 106) were plated on 150 × 25-mm plastic Petri dishes (Becton Dickinson, Franklin Lakes, NJ) for 16 hours and then rinsed once with PBS prior to plating of mouse or porcine red blood cells (RBCs). The cultures were kept in a 37°C water bath for 30 minutes. After lysing RBCs in cold ACK lysing buffer (Cambrex Bio Science Walkersville, Walkersville, MD), macrophages were harvested, washed with PBS, and lysed in 0.4 mL lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1% protease inhibitor cocktail [Sigma, St Louis, MO], and 2 mM sodium pervanadate). The whole-cell lysates were assayed for protein quantity, using a Bio-RAD protein assay kit. For Western blot, 30 μg macrophage lysates was separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel and blotted onto nitrocellulose membrane. The membrane was stained with mouse antiactin mAb IgG (C-2; Upstate, Charlottesville, VA) followed by bovine anti–mouse IgG-HRP (Upstate), or with rabbit anti–phosphotyrosine IgG (Upstate) followed by goat anti–rabbit IgG-HRP (Santa Cruz Biotechnology, Santa Cruz, CA). For immunoprecipitation, 300 μg macrophage lysates was mixed with rat anti–mouse SIRPα antibody (P84)12 and a 50% slurry of protein G–Sepharose beads (Sigma) by rotation at 4°C for 2 hours. Precipitated proteins were separated on 10% SDS-PAGE and transferred to nitrocellulose membrane for Western blotting, in which rabbit immunoaffinity purified anti–phosphotyrosine IgG (Upstate) and goat anti–rabbit HRP-conjugated IgG (Santa Cruz Biotechnology) were used as primary and secondary antibodies, respectively.
Mouse CD47 cDNA plasmid construction and transfection
Mouse CD47-expressing plasmid (pCDNA3.1-mCD47) was prepared by inserting full-length mouse CD47 cDNA (kindly provided to us by Dr Tadashi Furusawa, National Institute of Animal Research Industry, Japan) into a eukaryotic expression vector pCDNA-3.1 (Invitrogen, Carlsbad, CA). LCL-13271 cells (a pig lymphoma-like cell line kindly provided by Dr Christene Huang)19 were transfected with pCDNA3.1-mCD47 or the empty plasmid (pCDNA3.1-neo) using the Effectene Transfection kit (Qiagen, Valencia, CA) and selected by incubation with 0.8 mg/mL G418 (Gibco, Carlsbad, CA).
In vitro phagocytic assay
Fluorescent labeling of cells with green fluorescent dye carboxyfluorescein diacetate succinimidyl ester (referred to as CFSE), red fluorescent dye PKH-26-GL (referred to as PKH-26), and blue fluorescent dye 7-amino-4-chloromethylcoumarin (referred to as CMAC) was performed according to the manufacturer's protocols (Molecular Probes, Eugene, OR). Fluorescent dye (CFSE or PKH-26)–labeled target cells were incubated with splenic or peritoneal macrophages. The cultures were harvested at various times and analyzed for numbers of viable target cells and phagocytosis by flow cytometry. The numbers of viable target cells were calculated as the product of the total number of viable cells (as counted by trypan blue exclusion) and the percentage of target cells (as measured by flow cytometry). To measure phagocytosis, CFSE-labeled target cells were incubated with macrophages; the cells were harvested at the indicated times and stained with anti–mouse Mac-1-PE prior to flow cytometric analysis. We also measured phagocytosis using fluorescence microscopy, in which target cells and macrophages were labeled with different fluorescent colors. At the indicated times after incubation, noningested target cells were washed off, or for RBCs, were lysed with ACK buffer, and wells were viewed under a Nikon Eclipse TE2000-U fluorescence microscope equipped with a 10×/0.3 numerical aperture (NA) or a 20×/0.45 NA objective lens, and were photographed using a DS-U1 digital camera (Nikon, Melville, NY) and Adobe Photoshop Elements software version 3.0 (Adobe Systems, San Jose, CA).
Transwell experiments
These experiments were performed using 24-well plates with transwell inserts (0.4-μm pore size; Costar, Cambridge, MA). A mixture (1:1 ratio) of unlabeled LCL-mCD47 and LCL-neo cells (1 × 105/well) was added to the lower chamber with or without mouse macrophages (1 × 106/well), and a mixture (1:1 ratio) of LCL-mCD47 and LCL-neo cells (1 × 105/well) labeled with different fluorescent colors (CFSE or PKH-26) was placed in the upper transwell chamber. The plates were then incubated at 37°C. At various times after incubation, the cultures were harvested and the numbers of LCL-mCD47 and LCL-neo porcine cells in the upper transwell chambers were determined by flow cytometry as described in “In vitro phagocytic assay.”
RBC clearance assay
The assay was performed as previously described.14 Briefly, fresh pig RBCs were labeled with CFSE and injected (intravenously) into WT or CD47 KO mice (2 × 108 RBCs per mouse). RBC clearance was measured by flow cytometric analysis of 5-μL blood samples collected at various times. In some experiments, recipient spleens were harvested at various times after pig RBC injection and stored at −70°C. Frozen sections (8 μm) were prepared, fixed in acetone for 10 minutes at 4°C, and stained with PE-labeled rat anti–mouse F4/80 (Caltag Laboratories) overnight at 4°C. After being washed and mounted, slides were viewed under a Nikon Eclipse TE2000 fluorescence microscope.
In vivo phagocytic assay
CFSE-labeled target cells were injected (intravenously) into mice. The recipient spleens were harvested at various times and stored at −70°C. Frozen sections were prepared, fixed in acetone for 10 minutes at 4°C, and stained with PE-labeled rat anti–mouse F4/80 (Caltag Laboratories) overnight at 4°C. After being washed and mounted, slides were viewed under a Nikon Eclipse TE2000-U fluorescence microscope equipped with a 10×/0.3 NA or a 20×/0.45 NA objective lens, and were photographed using a DS-U1 digital camera and Adobe Photoshop Elements version 3.0.
Statistical analysis
Significant differences between groups were determined using the Student t test. A P value of less than .05 was considered statistically significant.
Results
Pig CD47 does not interact with mouse SIRPα
SIRPα contains intracellular immune receptor tyrosine-based inhibitory motifs (ITIMs). SIRPα activation after binding to CD47 results in tyrosine phosphorylation of ITIMs, leading to the recruitment and activation of protein tyrosine phosphatases.20 To determine whether pig CD47 can interact with mouse SIRPα, we compared SIRPα tyrosine phosphorylation in bone marrow–derived macrophages after contact with porcine, CD47 KO, and wild-type (WT) mouse RBCs. Western blot revealed that incubation of WT mouse macrophages with WT mouse RBCs resulted in significant SIRPα tyrosine phosphorylation (Figure 1A, lane 3). However, similar to CD47 KO mouse RBCs, porcine RBCs failed to induce SIRPα tyrosine phosphorylation in WT mouse macrophages. Macrophages showed a similar low level of SIRPα tyrosine phosphorylation after incubation with CD47 KO mouse or porcine RBCs (Figure 1A, lanes 2 and 4), or in medium alone (Figure 1A, lane 1).
Pig CD47 does not interact with mouse SIRPα. (A) Western blot analysis of SIRPα tyrosine phosphorylation in WT mouse macrophages. Macrophages were incubated in medium alone (control; lane 1), or with CD47−/− mouse (lane 2), WT mouse (lane 3), or porcine (lane 4) RBCs for 30 minutes. (Rows 1-2) Macrophage lysates were used directly in Western blot with anti–β-actin (row 1, as a loading control) or with antiphosphotyrosine Ab (α-pTyr; row 2). (Row 3) Macrophage lysates were immunoprecipitated by anti-SIRPα mAb P84; precipitated proteins were then analyzed by Western blot with antiphosphotyrosine Ab (α-pTyr). A representative experiment of 3 is shown. (B) Blocking SIRPα by anti-SIRPα mAb (P84) augments phagocytosis of WT mouse, but not CD47−/− mouse or porcine RBCs. CFSE (green)–labeled splenic macrophages (5 × 105/well) were incubated with or without anti-SIRPα antibody (P84) in 96-well plate for 20 minutes; then PKH-26 (red)–stained WT mouse (WT), CD47 KO mouse (CD47−/−), untreated pig (pRBC), or opsonized pig (ops pRBC) RBCs (1 × 106/well) were added and phagocytosis was determined 1 hour after incubation using fluorescent microscope (engulfment was seen as a yellow event). The percent of macrophages engulfing target cells per well was calculated as follows: [number of yellow events/(number of yellow events + number of green nonengulfing macrophages)] × 100%. Data are presented as mean ± SDs (n = 10-12 wells per group). **P < .01.
Pig CD47 does not interact with mouse SIRPα. (A) Western blot analysis of SIRPα tyrosine phosphorylation in WT mouse macrophages. Macrophages were incubated in medium alone (control; lane 1), or with CD47−/− mouse (lane 2), WT mouse (lane 3), or porcine (lane 4) RBCs for 30 minutes. (Rows 1-2) Macrophage lysates were used directly in Western blot with anti–β-actin (row 1, as a loading control) or with antiphosphotyrosine Ab (α-pTyr; row 2). (Row 3) Macrophage lysates were immunoprecipitated by anti-SIRPα mAb P84; precipitated proteins were then analyzed by Western blot with antiphosphotyrosine Ab (α-pTyr). A representative experiment of 3 is shown. (B) Blocking SIRPα by anti-SIRPα mAb (P84) augments phagocytosis of WT mouse, but not CD47−/− mouse or porcine RBCs. CFSE (green)–labeled splenic macrophages (5 × 105/well) were incubated with or without anti-SIRPα antibody (P84) in 96-well plate for 20 minutes; then PKH-26 (red)–stained WT mouse (WT), CD47 KO mouse (CD47−/−), untreated pig (pRBC), or opsonized pig (ops pRBC) RBCs (1 × 106/well) were added and phagocytosis was determined 1 hour after incubation using fluorescent microscope (engulfment was seen as a yellow event). The percent of macrophages engulfing target cells per well was calculated as follows: [number of yellow events/(number of yellow events + number of green nonengulfing macrophages)] × 100%. Data are presented as mean ± SDs (n = 10-12 wells per group). **P < .01.
We also examined the effect of anti–mouse SIPRα-blocking mAb (P84) on phagocytosis of porcine cells by mouse macrophages using an in vitro phagocytic assay. Previous studies have shown that P84 blocks CD47-SIRPα interaction and thereby augments phagocytosis.14 P84 should not affect the phagocytosis of porcine RBCs by mouse macrophages if pig CD47 does not interact with murine SIRPα. In these experiments, WT mouse macrophages were incubated in medium with or without P84 for 20 minutes prior to the addition of target cells (ie, CD47 KO mouse, WT mouse, and porcine RBCs). As shown in Figure 1B, blocking SIRPα with P84 led to a significant increase in the engulfment of WT mouse RBCs, but had no effect on the higher baseline levels of ingestion of CD47 KO mouse or porcine RBCs (both untreated and antibody opsonized) by WT mouse macrophages. Together, these results indicate that pig CD47 cannot deliver inhibitory signals to mouse macrophages through the SIRPα receptor.
Delayed rejection of porcine cells in CD47 KO compared with WT mice
In CD47 KO mice, macrophages are adapted and do not phagocytose CD47−/− cells.14 CD47 KO cells were rapidly rejected after injection into syngeneic WT mice, but survived equivalently to WT mouse cells in CD47 KO mice (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). Thus, it is expected that porcine cells will be more rapidly eliminated by macrophages in WT mice than in CD47 KO mice if pig CD47 cannot interact with mouse SIRPα. To address this question, we compared the survival of porcine RBCs in WT and CD47 KO mice. CFSE-labeled porcine RBCs were injected into WT or CD47 KO mice; blood was collected from the recipient mice at various times and the levels of injected porcine RBCs were measured by flow cytometric analysis. While porcine RBCs were completely rejected in both WT and CD47 KO mice, the clearance of porcine RBCs from blood was significantly delayed in CD47 KO mice. As shown in Figure 2A, porcine cells were almost completely cleared from blood of WT mouse recipients by 2 hours, but remained detectable in CD47 KO mouse recipients 8 hours after cell transfer. We acknowledge that antipig xenoresponses by T cells, B cells, and natural killer (NK) cells may also contribute to the rejection of pig cells in the mouse recipients. However, the dramatic difference in the clearance of pig RBCs between WT and CD47 KO mice suggests that macrophages play an important role in the rejection of pig cells.
Delayed clearance of porcine RBCs in CD47 KO compared with WT mouse recipients. CFSE-stained pig RBCs (2 × 108) were intravenously injected into CD47 KO (n = 5) or WT (n = 5) mice. (A) Blood was collected at the indicated time points and the percentages of injected pig RBCs were analyzed by flow cytometry. (Top) Representative fluorescence-activated cell sorter (FACS) profiles showing percentages of porcine RBCs in the blood at the indicated times. Numbers indicate the percentages of CFSE+ porcine RBCs. (Bottom) Percentages (mean ± SDs) of porcine RBCs in blood, which were normalized with the levels at 15 minutes after injection as 100%. Results from 2 experiments are combined. *P < .01; **P < .001. (B) Spleens were harvested from CD47 KO (top row, × 100) and WT (middle row, × 100; bottom row, × 200) at 1 hour after injection of CFSE-stained pig RBCs (green), and frozen spleen sections were stained with anti-F4/80 mAb (red). Engulfment was seen as a yellow event after merging the green-filtered and red-filtered images (right column). Three mouse recipients from each group were examined and representative results are shown.
Delayed clearance of porcine RBCs in CD47 KO compared with WT mouse recipients. CFSE-stained pig RBCs (2 × 108) were intravenously injected into CD47 KO (n = 5) or WT (n = 5) mice. (A) Blood was collected at the indicated time points and the percentages of injected pig RBCs were analyzed by flow cytometry. (Top) Representative fluorescence-activated cell sorter (FACS) profiles showing percentages of porcine RBCs in the blood at the indicated times. Numbers indicate the percentages of CFSE+ porcine RBCs. (Bottom) Percentages (mean ± SDs) of porcine RBCs in blood, which were normalized with the levels at 15 minutes after injection as 100%. Results from 2 experiments are combined. *P < .01; **P < .001. (B) Spleens were harvested from CD47 KO (top row, × 100) and WT (middle row, × 100; bottom row, × 200) at 1 hour after injection of CFSE-stained pig RBCs (green), and frozen spleen sections were stained with anti-F4/80 mAb (red). Engulfment was seen as a yellow event after merging the green-filtered and red-filtered images (right column). Three mouse recipients from each group were examined and representative results are shown.
To further determine whether macrophages are responsible for the rapid clearance of porcine RBCs in WT recipients, frozen tissue sections were prepared from recipient spleens harvested 0.5, 1, and 2 hours after injection of CFSE-labeled porcine RBCs, and were analyzed by fluorescence microscopy. Substantially greater numbers of porcine RBCs were detected in the red pulp area of WT compared with CD47 KO mouse recipients (Figure 2B and data not shown). Immunofluorescence staining revealed that porcine cells detected in the red pulp were mainly engulfed by F4/80+ macrophages. Since WT and CD47 KO mice have a similar number of F4/80+ macrophages in the spleen (Figure 2B and Figure S2), these results suggest that the failure of pig CD47 to interact with mouse SIRPα may increase the susceptibility of porcine cells to phagocytosis by mouse macrophages.
Mouse CD47 expression on porcine cells reduces their susceptibility to phagocytosis by mouse macrophages
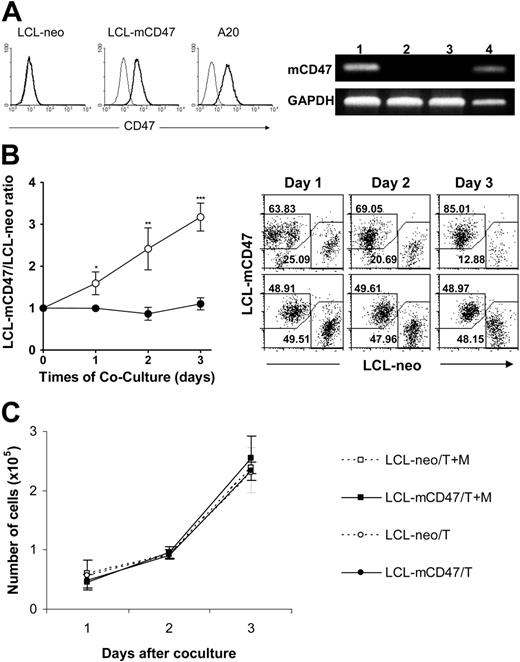
To further understand the role of CD47 in phagocytosis of xenogeneic cells and to determine whether expression of mouse CD47 on porcine cells could confer protection from phagocytosis by mouse macrophages, we generated mouse CD47–expressing (mCD47) porcine cell lines by transfection of porcine B lymphoma-like cells (LCL-13271)21 with a mouse CD47–expressing plasmid (Figure 3A). We compared the survival and expansion of mouse CD47–transfected (LCL-mCD47) and Neo-transfected (control) (LCL-neo) porcine cells in cultures containing mouse macrophages. LCL-mCD47 and LCL-neo cells were labeled with different fluorescent dyes (red or green) and cocultured at a 1:1 ratio in the presence and absence of mouse macrophages. The cultures were harvested daily for 3 days and the numbers of viable LCL-mCD47 and LCL-neo cells in the cultures were determined. As shown in Figure 3B, the ratio of viable LCL-mCD47 to LCL-neo cells was significantly increased in the presence of mouse macrophages but remained constant in the absence of macrophages. However, in the transwell experiments, LCL-mCD47 and LCL-neo cells grew equally in the upper transwell chambers regardless of whether the lower chambers contained LCL target cells alone or along with mouse macrophages (Figure 3C). These results imply that the increased expansion of LCL-mCD47 cells in the mixed cultures with mouse macrophages (Figure 3B) reflects a mouse CD47-induced protection against direct contact-mediated cytotoxicity of mouse macrophages.
Mouse CD47 expression reduces the susceptibility of porcine cells to cytotoxicity by mouse macrophages. (A) Expression of murine CD47 (mCD47) on transfected LCL-13271 pig tumor cell lines. (Left) Flow cytometric analysis. Thin and bold histograms represent staining with isotype control and anti–mouse CD47 mAb (miap301), respectively. Neo transfectant LCL cells (LCL-neo), a representative clone (no. 1007) of mCD47 transfectant LCL cells (LCL-mCD47), and mouse CD47+/+ A20 cells are shown. (Right) mCD47 RT-PCR. (Lane 1) LCL-mCD47 cells (clone no. 1007); (lane 2) LCL-neo cells; (lane 3) nontransfected LCL-13271 cells; (lane 4) CD47+/+ mouse cell line A20. GAPDH was used as a DNA loading control. (B) LCL-mCD47 and LCL-neo cells were stained with different colors (CFSE or PKH-26), mixed at a 1:1 ratio, and cultured in culture plate (2.5 × 104/well) with (○) or without (•) WT mouse intraperitoneal macrophages (5 × 105/well) for 3 days. Shown are ratios of viable LCL-mCD47 to LCL-neo cells (left) and representative flow cytometric profiles (right; the percentages of LCL-mCD47 and LCL-neo cells are indicated) at the indicated time points. Combined results (mean ± SDs) from 3 independent experiments are presented. *P < .05; **P < .01; ***P < .001. (C) Numbers of LCL-mCD47 (▪/•) and LCL-neo (□/○) cells in the upper transwell chambers (inside the transwells) in cultures, in which the lower chambers (outside transwells) contained either both target cells (ie, a 1:1 mixture of LCL-mCD47 and LCL-neo cells) and mouse macrophages (T + M) or target cells only (T). Results (mean ± SDs) from a representative experiment of 3 are shown.
Mouse CD47 expression reduces the susceptibility of porcine cells to cytotoxicity by mouse macrophages. (A) Expression of murine CD47 (mCD47) on transfected LCL-13271 pig tumor cell lines. (Left) Flow cytometric analysis. Thin and bold histograms represent staining with isotype control and anti–mouse CD47 mAb (miap301), respectively. Neo transfectant LCL cells (LCL-neo), a representative clone (no. 1007) of mCD47 transfectant LCL cells (LCL-mCD47), and mouse CD47+/+ A20 cells are shown. (Right) mCD47 RT-PCR. (Lane 1) LCL-mCD47 cells (clone no. 1007); (lane 2) LCL-neo cells; (lane 3) nontransfected LCL-13271 cells; (lane 4) CD47+/+ mouse cell line A20. GAPDH was used as a DNA loading control. (B) LCL-mCD47 and LCL-neo cells were stained with different colors (CFSE or PKH-26), mixed at a 1:1 ratio, and cultured in culture plate (2.5 × 104/well) with (○) or without (•) WT mouse intraperitoneal macrophages (5 × 105/well) for 3 days. Shown are ratios of viable LCL-mCD47 to LCL-neo cells (left) and representative flow cytometric profiles (right; the percentages of LCL-mCD47 and LCL-neo cells are indicated) at the indicated time points. Combined results (mean ± SDs) from 3 independent experiments are presented. *P < .05; **P < .01; ***P < .001. (C) Numbers of LCL-mCD47 (▪/•) and LCL-neo (□/○) cells in the upper transwell chambers (inside the transwells) in cultures, in which the lower chambers (outside transwells) contained either both target cells (ie, a 1:1 mixture of LCL-mCD47 and LCL-neo cells) and mouse macrophages (T + M) or target cells only (T). Results (mean ± SDs) from a representative experiment of 3 are shown.
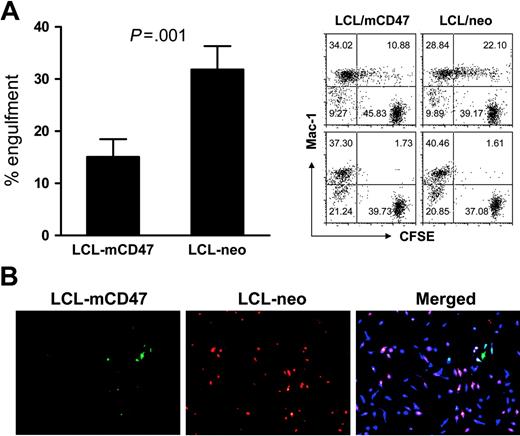
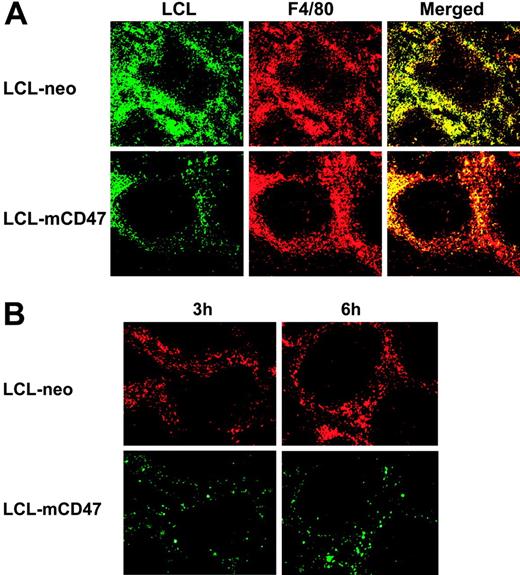
We further confirmed that mouse CD47 expression on porcine cells prevents their phagocytosis by mouse macrophages. In in vitro phagocytic assays, mouse macrophages were markedly less effective in engulfing porcine LCL-mCD47 cells than engulfing LCL-neo cells (Figure 4A). Mouse macrophages preferentially phagocytosed LCL-neo cells even when LCL-mCD47 and LCL-neo cells were both present, indicating that CD47 expression on individual target cells mediates this protection (Figure 4B). We also assessed the ability of mouse CD47 expression to prevent phagocytosis of porcine cells in vivo. Because red pulp macrophages in the spleen efficiently phagocytose CD47 KO mouse cells14,15 and porcine cells (Figure 2), we assessed phagocytosis of CFSE-labeled LCL-mCD47 and LCL-neo cells in the mouse spleen. We observed that more CFSE+ cells were detected in red pulp of the spleen in mice receiving LCL-neo cells than in those injected with LCL-mCD47 cells (Figure 5A). Staining of mouse macrophages revealed that most porcine cells trapped in red pulp of the spleen were engulfed by macrophages (Figure 5A). At 3 hours after cell infusion, almost all F4/80+ macrophages (stained red) in red pulp had engulfed porcine cells (appearing yellow in merged pictures) in mice injected with LCL-neo cells, whereas large numbers of red pulp macrophages showed no engulfment in mice that received LCL-mCD47 cells. Similar results were observed in mice injected with a mixture (1:1 ratio) of LCL-mCD47 and LCL-neo cells, in which more LCL-neo cells than LCL-mCD47 cells were detected in red pulp (ie, engulfed by macrophages) (Figure 5B).
Mouse CD47 expression attenuates phagocytosis of porcine cells in vitro by mouse macrophages. (A) CFSE-labeled LCL-mCD47 or LCL-neo cells (2.5 × 104/well) were incubated with mouse intraperitoneal macrophages (5 × 105/well) in 96-well plate at 37°C or 4°C (controls); cultures were harvested 3 hours later and phagocytosis was determined by flow cytometry. (Left panel) Percent engulfment in Mac-1+ cells (mean ± SDs of 4 experiments); (right panel) representative staining profiles showing engulfment (at 37°C, top) or background (4°C, bottom). (B) LCL-mCD47 and LCL-neo cells labeled with different colors (CFSE or PKH-26) were mixed at a 1:1 ratio (2.5 × 104 each) and cultured with 5 × 105 CMAC-labeled mouse intraperitoneal macrophages (blue) for 3 hours; then nonengulfed target cells were washed off and phagocytosis was assessed by fluorescence microscopy. Pictures shown are images taken from an experiment, in which LCL-mCD47 and LCL-neo cells were labeled with CFSE (green) and PKH-26 (red), respectively. Data are representative of 3 experiments.
Mouse CD47 expression attenuates phagocytosis of porcine cells in vitro by mouse macrophages. (A) CFSE-labeled LCL-mCD47 or LCL-neo cells (2.5 × 104/well) were incubated with mouse intraperitoneal macrophages (5 × 105/well) in 96-well plate at 37°C or 4°C (controls); cultures were harvested 3 hours later and phagocytosis was determined by flow cytometry. (Left panel) Percent engulfment in Mac-1+ cells (mean ± SDs of 4 experiments); (right panel) representative staining profiles showing engulfment (at 37°C, top) or background (4°C, bottom). (B) LCL-mCD47 and LCL-neo cells labeled with different colors (CFSE or PKH-26) were mixed at a 1:1 ratio (2.5 × 104 each) and cultured with 5 × 105 CMAC-labeled mouse intraperitoneal macrophages (blue) for 3 hours; then nonengulfed target cells were washed off and phagocytosis was assessed by fluorescence microscopy. Pictures shown are images taken from an experiment, in which LCL-mCD47 and LCL-neo cells were labeled with CFSE (green) and PKH-26 (red), respectively. Data are representative of 3 experiments.
Mouse CD47 expression attenuates in vivo phagocytosis of porcine cells. (A) LCL-mCD47 and LCL-neo cells were labeled with CFSE and injected intravenously (1 × 107/mouse) into C57BL/6 mice. At 3 hours after cell injection, spleens were harvested and stained with PE-conjugated anti–mouse F4/80 mAb. Engulfment was seen as a yellow event after merging the green-filtered and red-filtered images (right column). (B) Mice were injected intravenously with a 1:1 mixture of LCL-mCD47 and LCL-neo cells that were labeled with different colors (total 1 × 107 cells per mouse). Spleens were harvested at 3 and 6 hours after cell injection. Pictures shown are representative images taken from an experiment, in which LCL-neo and LCL-mCD47 cells were labeled with PKH-26 (red) and CFSE (green), respectively. Data presented in Figure 5 were representative of 3 or more experiments.
Mouse CD47 expression attenuates in vivo phagocytosis of porcine cells. (A) LCL-mCD47 and LCL-neo cells were labeled with CFSE and injected intravenously (1 × 107/mouse) into C57BL/6 mice. At 3 hours after cell injection, spleens were harvested and stained with PE-conjugated anti–mouse F4/80 mAb. Engulfment was seen as a yellow event after merging the green-filtered and red-filtered images (right column). (B) Mice were injected intravenously with a 1:1 mixture of LCL-mCD47 and LCL-neo cells that were labeled with different colors (total 1 × 107 cells per mouse). Spleens were harvested at 3 and 6 hours after cell injection. Pictures shown are representative images taken from an experiment, in which LCL-neo and LCL-mCD47 cells were labeled with PKH-26 (red) and CFSE (green), respectively. Data presented in Figure 5 were representative of 3 or more experiments.
Taken together, these results indicate that the lack of efficient interaction between pig CD47 and mouse SIRPα is an important factor contributing to the susceptibility of porcine cells to phagocytosis by mouse macrophages. Furthermore, mCD47 expression is effective in preventing the rejection of porcine cells by macrophages in mice.
Discussion
Although macrophage depletion has been shown to be effective in preventing cellular xenograft rejection, the rapid recovery of macrophages and associated graft destruction after withdrawal of treatment indicates that sustained macrophage depletion or adaptation may be required to maintain long-term xenograft survival.5,10,11,22 Because macrophages play a critical role in initiating immune responses against pathogens, the long-term use of macrophage-depleting reagents is unlikely to be clinically feasible, even if nontoxic drugs can be developed. Thus, the development of strategies for specifically suppressing xenogeneic cell–triggered macrophage activation may be essential to the maintenance of durably functional cellular xenografts, and may also be beneficial in solid organ xenotransplantation for which macrophages have also been implicated in rejection.23,24
SIRPα is an ITIM-bearing inhibitory receptor expressed on macrophages that plays an important role in controlling macrophage activation.12,13 It has been demonstrated that SIRPα on macrophages recognizes CD47 as a marker of “self” and that CD47-SIRPα signaling prevents autologous phagocytosis of normal cells.14,15 The present study shows that pig CD47 does not cross react with mouse SIRPα. Ligation of the mouse SIRPα by mouse CD47 induces tyrosine phosphorylation of ITIMs (Figure 1A), leading to the recruitment and activation of protein tyrosine phosphatases.20 However, SIRPα phosphorylation could not be induced in mouse macrophages after incubation with porcine RBCs that express pig CD47 (Figure 1A). Furthermore, blocking SIRPα with anti–mouse SIPRα mAb (P84) markedly augmented the engulfment of mouse cells, but did not affect the ingestion of porcine cells by mouse macrophages (Figure 1B). To further understand the role of CD47 incompatibility in phagocytosis of xenogeneic cells, we established mouse CD47-expressing porcine cell lines. Both in vitro and in vivo phagocytic assays showed that forced expression of mouse CD47 on porcine cells can significantly reduce their susceptibility to phagocytosis by mouse macrophages (Figures 3,Figure 4–5). To our knowledge, this study is the first to show that pig CD47 cannot deliver inhibitory signals to mouse macrophages via SIRPα, and that mouse CD47 expression prevents phagocytosis of porcine cells by mouse macrophages. While long-term in vivo experiments assessing xenograft survival are clearly needed to draw a final conclusion, the current data suggest that CD47 may provide a potential molecular target for inhibiting macrophage-mediated rejection of xenogeneic cells.
The species specificity of CD47 has also been demonstrated in other species,13,25,26 and there has been no evidence that a cross-species CD47-SIRPα interaction can occur in a highly disparate xenogeneic combination. A recent report indicates that human macrophages can phagocytose porcine cells in the absence of antibody or complement opsonization, and that removing α1,3-galactosyl xenoantigens from porcine cells failed to prevent phagocytosis.27 Considering the lack of cross reaction between CD47 and SIRPα in other species and the limited identity (73%) in amino acid sequences between pig and human CD47,28 the lack of cross reaction between pig CD47 and human SIRPα is likely also an important mechanism resulting in phagocytosis of porcine cells by human macrophages.
Mixed hematopoietic chimerism has been shown to induce tolerance across the MHC barrier.29 Previous studies using a transgenic nonobese diabetic–severe combined immunodeficient (NOD/SCID) mouse model suggested that mixed hematopoietic chimerism may also induce mouse and human T-cell tolerance to porcine xenografts.30,31 However, unlike bone marrow transplantation within the same species, the innate immune system poses a formidable obstacle to the establishment of donor hematopoiesis across discordant xenogeneic barriers.32 Macrophages are one of the factors mediating strong rejection of xenogeneic hematopoietic cells.5,6 The rejection of porcine hematopoietic cells by host macrophages developing de novo in porcine hematopoietic chimeras5 suggests that mixed chimerism may not overcome the macrophage barrier. Therefore, inhibition of donor hematopoietic cell rejection by macrophages is critical for xenotolerance induction through mixed chimerism. Studies in the CD47 KO mouse model have demonstrated that CD47 expression is critical for preventing phagocytosis of hematopoietic cells.14,15 The rapid and vigorous rejection of CD47 KO hematopoietic cells in syngeneic WT mouse recipients14,15 suggests that CD47 incompatibility alone is sufficient to cause rejection of donor hematopoietic cells in a xenogeneic recipient. Thus, genetic manipulation of donor CD47 to improve its interaction with recipient SIRPα is likely critical for achieving donor hematopoietic engraftment and hence chimerism in xenogeneic recipients.
Although CD47-SIRPα interaction has been proven to be essential for the protection of normal hematopoietic cells from phagocytosis, it is unclear whether this interaction pathway also plays an important role in protecting nonhematopoietic tissues or cells from destruction by macrophages. Recent studies have shown that lung collectins, surfactant-A (SP-A) and SP-D, also bind SIRPα on alveolar macrophages through their globular heads to initiate an inhibitory signaling that helps to maintain a noninflammatory or anti-inflammatory lung environment.33 These results suggest that the function of a porcine lung xenograft could also be severely compromised if porcine surfactants cannot bind human SIRPα. Among the other immune inhibitory receptors on macrophages, CD200 receptor (CD200R, also known as OX2R) has been shown to play a critical role in the regulation of tissue macrophage activation. The ligand for CD200R, CD200 (also known as OX2), is widely expressed throughout the body. Studies using CD200-deficient mice demonstrated that the absence of CD200-CD200R signaling leads to accelerated activation and expansion of tissue macrophages.34,35 In addition to SIRPα and CD200R, paired Ig-like receptor B (PIR-B),36 immunoglobulin-like transcript 3 (ILT3),37 and CD33-related receptors38 have also been shown to serve as inhibitory receptors for macrophages. Considering the possibility of functional overlap (or redundancy) among these receptors in the normal situation, macrophages may mediate more robust phagocytosis of xenogeneic cells if the donor and host are incompatible for multiple immune inhibitory receptor-ligand interactions. In this regard, identifying the cross reactivity of the major macrophage inhibitory receptors between pigs and humans should provide further insights into the understanding of the robust xenoreactivity of macrophages, and may lead to the development of approaches for attenuating macrophage-mediated xenograft rejection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: H.W. designed and performed research, analyzed data, and wrote the paper; J.V. and M.L.M. designed and performed research and analyzed data; S.X., S.W., and P.L. performed research; P.-A.O. contributed vital reagents and provided important advice on experimental design and data analysis; and Y.-G.Y. devised the study, designed experiments, analyzed data, and wrote the paper.
H.W., J.V., and M.L.M. contributed equally to this work.
The authors thank Drs Christian LeGuern and John Hanekamp for critical review of this paper; Gerald L. Waneck and Josie Han Lee for advice and technical help; Christene Huang for providing LCL 13271 cells; Tadashi Furusawa (National Institute of Animal Research Industry, Japan) for providing full-length mouse CD47 cDNA; and Ms Luisa Raleza for expert assistance with the paper.
This study was supported by grants from JDRF (1-2005-72), ROTRF (no. 848155553), NIH (GM57573-06), the Swedish Research Council (06P-14098, 31X-14286), and the Faculty of Medicine, Umeå University.

![Figure 1. Pig CD47 does not interact with mouse SIRPα. (A) Western blot analysis of SIRPα tyrosine phosphorylation in WT mouse macrophages. Macrophages were incubated in medium alone (control; lane 1), or with CD47−/− mouse (lane 2), WT mouse (lane 3), or porcine (lane 4) RBCs for 30 minutes. (Rows 1-2) Macrophage lysates were used directly in Western blot with anti–β-actin (row 1, as a loading control) or with antiphosphotyrosine Ab (α-pTyr; row 2). (Row 3) Macrophage lysates were immunoprecipitated by anti-SIRPα mAb P84; precipitated proteins were then analyzed by Western blot with antiphosphotyrosine Ab (α-pTyr). A representative experiment of 3 is shown. (B) Blocking SIRPα by anti-SIRPα mAb (P84) augments phagocytosis of WT mouse, but not CD47−/− mouse or porcine RBCs. CFSE (green)–labeled splenic macrophages (5 × 105/well) were incubated with or without anti-SIRPα antibody (P84) in 96-well plate for 20 minutes; then PKH-26 (red)–stained WT mouse (WT), CD47 KO mouse (CD47−/−), untreated pig (pRBC), or opsonized pig (ops pRBC) RBCs (1 × 106/well) were added and phagocytosis was determined 1 hour after incubation using fluorescent microscope (engulfment was seen as a yellow event). The percent of macrophages engulfing target cells per well was calculated as follows: [number of yellow events/(number of yellow events + number of green nonengulfing macrophages)] × 100%. Data are presented as mean ± SDs (n = 10-12 wells per group). **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/2/10.1182_blood-2006-04-019794/4/m_zh80020706720001.jpeg?Expires=1767753509&Signature=nbG1tMM62gyl5V-uPca0bQgj7UkxsHPOK~icZs0tYYEE9wHqgrW2OaE9BF-poSoVyd5kTaNohwGuBoZnCELBoSH82NdLHuWCeHIhHKyEW2Emb9WlyZeTnbgGQqJFC5GrbPM6nUldmzJyMgwq~SrQZ7btiNfkMvo8j4rkQjKInt6ZtHsqKoYvZYS8~U7Z3KaxHdaPvqUmB7V-bGNWIV0C2Zsa6myX2ISIR8wiolOTFHTLUgayRstv6uov-ymABWAiPUK~B7cItxp3PXlwdNMreHtKz3LxSJhMCiAX7cXBxjD5W6VoCB4f7ZlZfNccI0Ms8Hr3y0b5mJyJO9WU2EKuVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal