Abstract
Histone deacetylases (HDACs) play a critical role in regulating gene expression and key biological processes. However, how HDACs are involved in innate immunity is little understood. Here, in this first systematic investigation of the role of HDACs in immunity, we show that HDAC inhibition by a small-molecule HDAC inhibitor (HDACi), LAQ824, alters Toll-like receptor 4 (TLR4)–dependent activation and function of macrophages and dendritic cells (DCs). Surprisingly, pan-HDAC inhibition modulates only a limited set of genes involved in distinct arms of immune responses. Specifically, it inhibited DC-controlled T helper 1 (Th1) effector but not Th2 effector cell activation and migration. It also inhibited macrophage- and DC-mediated monocyte but not neutrophil chemotaxis. These unexpected findings demonstrate the high specificity of HDAC inhibition in modulating innate and adaptive immune responses, and highlight the potential for HDACi to alter the Th1 and Th2 balance in therapeutic settings.
Introduction
Histone-modifying enzymes, such as histone acetyltransferases (HATs) and histone deacetylases (HDACs), are critical in controlling the dynamics of chromatin structure and function by regulating histone acetylation. This process is essential in modulating gene transcription through chromatin organization, and plays an important role in many key biological processes.1 The perturbation of this process results in aberrant gene transcription and causes neoplasia and other diseases.2 In general, increased histone acetylation is associated with transcription activation through relaxed chromatin structure, whereas decreased histone acetylation is associated with repression of transcription via chromatin condensation. Recently, an increasing number of nonhistone proteins such as p53, HSP90, and STAT3, for example, have been identified as substrates of HDACs, indicating a novel mechanism of action of HDACs in modulating gene function.3
The classical HDAC gene family consists of 11 members, which fall into 2 classes. Class I HDACs (HDAC1, HDAC2, HDAC3, and HDAC8) are expressed ubiquitously, whereas the expression of class II HDACs (HDAC4, HDAC5, HDAC6, HDAC7, HDAC9, and HDAC10) is more restricted.4 Small molecules capable of inhibiting both class I and II HDAC activities, such as suberoylanilide hydroxamic acid (SAHA), trichostatin (TSA), trapoxin, and others, induce general histone acetylation, and are promising anticancer agents.3,5 In addition to their anticancer activities, several HDAC inhibitors (HDACi's) have been shown to be effective in selected immune disease models. For example, SAHA has been shown to reduce graft-versus-host disease (GVHD) in a murine model of bone marrow transplantation6 and block renal disease in MRL-lpr/lpr mice7 ; TSA suppressed adjuvant-induced rheumatoid arthritis (RA) in rats8 and reduced clinical symptoms in a murine model of multiple sclerosis (MS).9 However, little is known about the cellular and molecular mechanisms governing the in vivo effects of HDACi's in these autoimmune models. Therefore, we set out to systematically investigate the cellular effects of LAQ824, a novel hydroxamic acid derivative with demonstrated clinical efficacy as an HDAC pan-inhibitor,5,10 in immune responses, and to suggest a mechanistic rationale for using HDACi's in inflammatory diseases.
Chronic inflammation is a physiologic state that underlies many diseases.11 The inflammation cascade is usually initiated by immune or stress responses to environmental or endogenous stimuli, and it is tightly controlled by the activation and silencing of differentially expressed genes. Professional antigen-presenting cells (APCs), particularly macrophages and dendritic cells (DCs), are central players in the initiation of an inflammation cascade. They are the first line of defense in that they respond to diverse antigens, secrete cytokines and chemokines, modulate the expression of costimulatory molecules, and thereby instruct other innate and adaptive immune cells to mount appropriate responses.12,13 Depending on the nature of the antigen (eg, bacterial, viral or cell-associated antigen) and the tissue microenvironment where they are activated, APCs develop into functionally different subsets. These phenotypically distinct subsets can, in turn, promote specific immune processes, such as the activation of functionally distinct types of CD4+ T cells (T helper 1 [Th1], Th2, or T regulatory cells), and the activation and attraction of neutrophils, monocytes, and CD8 or B lymphocytes.13-17 Therefore, macrophages and DCs are critical in orchestrating the immune system and initiating appropriate immune responses.
The development of functionally distinct subsets of APCs is controlled through the stimulation of specific pattern-recognition receptors, particularly Toll-like receptors (TLRs), by pathogen components and endogenous antigens.18-20 Stimulation through TLRs in APCs is critical in regulating the activation signals leading to both innate and adaptive immune responses.18 To elucidate the role of HDACs in APC function and their contribution to shaping innate and adaptive immune responses, we decided to investigate the effects of LAQ824 on TLR-mediated APC activation. Because of its critical role in sensing microbial infection,21 we focused on TLR4-mediated activation of APCs upon recognition of lipopolysaccharide (LPS), a major component of the outer membrane of Gram-negative bacteria. Surprisingly, HDACi LAQ824 exhibited a highly selective effect on TLR4-mediated APC function, immune cell chemotaxis, and Th1/Th2 effector activation by differentially modulating the expression profile of a distinct set of genes in LAQ824-treated APCs.
Materials and methods
Reagents
HDACi NVP-LAQ82410 was dissolved in DMSO at 0.1 M and kept at −20°C. LPS was obtained from Sigma (Escherichia coli 055:B5; St Louis, MO) and used at 1 μg/mL in tissue culture. Ovalbumin (OVA) protein was obtained from Sigma, and OVA (323-329) peptide was purchased from Biosource (Camarillo, CA).
Cell lines and mice
THP-1 cells (American Type Culture Collection [ATCC], Manassas, VA) were maintained under standard conditions described in the ATCC instructions. Human macrophages and DCs were differentiated from elutriated monocytes (Advanced Biotechnologies, Columbia, MD) as described previously.22,23 Human neutrophils were obtained from AllCells (Berkeley, CA). Mouse bone marrow–derived DCs were prepared as described previously.24 Murine Th1 and Th2 effector cells were differentiated from DO11.10 mice as described before.25 Balb/C and DO11.10 T-cell receptor (TCR)–transgenic mice were obtained from the Jackson Laboratories (Bar Harbor, ME). All mice were manipulated according to NIBR animal guidelines. Human Th1 and Th2 effector cells were differentiated as follows. Naive CD4+ T cells (CD45RA+) were purified from peripheral blood mononuclear cells (PBMCs) by magnetic-activated cell-sorter (MACS) microbead sorting (Miltenyi, Bergish Gladbach, Germany). T cells were stimulated with anti-CD3/CD28–coated microbeads (Invitrogen, Carlsbad, CA) and 20 U/mL interleukin-2 (IL-2). For Th1-inducing conditions, the culture was supplemented with 20 ng/mL IL-12 and 1 μg/mL anti–IL-4. For Th2-inducing conditions, the culture was supplemented with 20 ng/mL IL-4, 2 μg/mL anti–IL-12, and 10 μg/mL anti–IFN-γ. The medium used was RPMI-1640 supplemented with 1% glutamine, 1% nonessential amino acids, 1% pyruvate, 50 U/mL penicillin, 50 μg/mL streptomycin, 5 × 10−5 M 2-mercaptoethanol, and 10% fetal bovine serum (Invitrogen, Carlsbad, CA). Proliferating cells were expanded in medium supplemented with 100 U/mL IL-2 only, and cells were used 12 to 15 days following stimulation. Cytokines were purchased from Peprotech (Rocky Hill, NJ), and antibodies were from BD Pharmingen (San Diego, CA).
ELISA and FACS
Secretion of human IL-12p40, IP10, macrophage-derived chemokine (MDC), mouse IFN-γ, and IL-4 in culture supernatants was measured using Duoset kits (R&D Systems, Minneapolis, MN) based on the manufacturer's instructions. Production of RANTES, IL-8, MIP1α, MIP1β, and MCP-1 was measured using the Cytometric Bead Array human chemokine kit (BD Pharmingen) based on the manufacturer's instructions. The surface expression of CD40, CD86, and CCR5 was determined by flow cytometry using PE- or PE-Cy5–labeled antibodies from BD Pharmingen (CD40 and CD86) and R&D Systems (CCR5).
Genomewide gene expression
Human monocyte-derived macrophages (107 cells/condition) were treated with various stimuli; then, cells were harvested at 0, 1, 3, 6, 12, and 24 hours. Total RNA was isolated and prepared for hybridization to U133A oligonucleotide arrays (Affymetrix, Santa Clara, CA). Data were collected and analyzed as described previously22 with a small modification. Specifically, genes regulated by any stimulus over media control time course were identified using a scoring system. For each donor, up-regulated genes for each treatment condition were selected when (1) Si22 was greater than or equal to 1.8 for any 2 consecutive time points or Si was greater than or equal to 5 for any single time point; and (2) increased gene expression included at least one present call (Affymetrix algorithm). Down-regulated genes were selected based on (1) Si of 2 or less for any 2 consecutive time points; and (2) control time course included at least one present call. The genes regulated in response to each treatment from 2 donors were compared, and the common set was selected as the final list. Genes up-regulated by stimulus A but not by B were identified as follows: (1) genes were up-regulated in response to stimulus A based on the above criteria for up-regulated genes; and (2) Si is less than or equal to 1.4 for less than 2 consecutive time points in condition B.
Antigen-specific Th cell activation
Bone marrow–derived DCs were seeded at 2 × 103/well in a 96-well round-bottom plate, and pulsed with antigen OVA or OVA peptide in the presence of LPS, LAQ824, or LPS with LAQ824 for 24 hours. After extensive washing, DO11.10 Th1 or Th2 effector cells were added at 5 × 104/well and incubated for 48 hours.
Chemotaxis assays
Human neutrophil, monocyte, Th1 cell, and Th2 cell chemotaxis assays were done using a transwell system (ChemoTx no. 106-5; Neuro Probe, Gaithersburg, MD). Conditioned culture medium (30 μL) from monocyte-derived macrophages and DCs treated with various stimuli was placed in the bottom chamber of each well. Either 5 × 104 of neutrophil, Th1 or Th2 cells, or 104 of LPS-treated monocytes were added to the upper chamber of each well in 25 μL. Transwell plates were incubated for 3 hours in a tissue-culture incubator at 37°C with 5% CO2. Migrated cells were collected from the lower chamber after 3 hours, and numbers were determined by CytotiterGlow (Promega, Madison, WI).
Results
HDAC inhibition by LAQ824 induces both up- and down-regulation of a limited set of genes in resting and LPS-stimulated macrophages
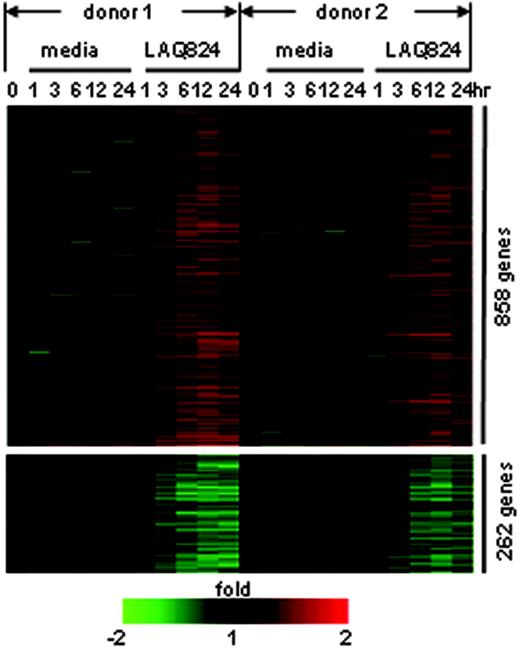
To systematically investigate the role of HDACs in APC activation, genomewide gene expression analysis was performed on macrophages treated with LPS and/or LAQ824. Human monocyte-derived macrophages were exposed to the TLR4 ligand LPS in the presence or absence of LAQ824 for 1, 3, 6, 12, or 24 hours. Total RNA was isolated at the indicated time points, and hybridized to Affymetrix U133A arrays. Each experimental condition was performed with macrophage cells derived from 2 independent donors. Data were analyzed using the score system described previously.22 LAQ824 treatment alone resulted in the induction of 858 genes or repression of 262 genes in resting macrophages from 2 donors (Figure 1). The relatively small number of LAQ824-regulated genes (less than 5% of all the elements on Affymetrix U133A) indicates a specificity of global HDAC inhibition in modulating gene expression in resting macrophages.
LAQ824 modulates gene expression in resting human monocyte-derived macrophages from 2 donors. Each gene is represented by a single row of color bars, and each time point is represented by a single column. Color bars represent the ratio of hybridization signals between corresponding time points in media control or LAQ824 condition and time 0 in each donor, according to the scale shown.
LAQ824 modulates gene expression in resting human monocyte-derived macrophages from 2 donors. Each gene is represented by a single row of color bars, and each time point is represented by a single column. Color bars represent the ratio of hybridization signals between corresponding time points in media control or LAQ824 condition and time 0 in each donor, according to the scale shown.
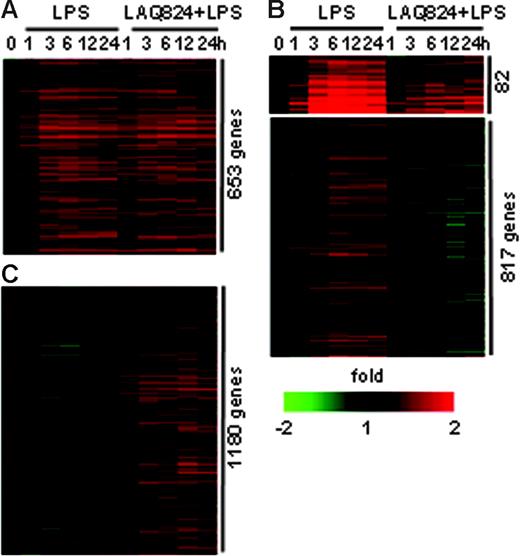
LAQ824 also altered gene expression in LPS-stimulated macrophages. In donor 1, there were 1618 genes up-regulated in response to LPS (Table S2, available on the Blood website; see the Supplemental Materials link at the top of the online article). Of these, 653 were unaffected by LAQ824 treatment (Figure 2A). HDACs appeared to play no role in the induction of these genes by LPS. The induction of 82 LPS-stimulated genes was greatly reduced or delayed in the presence of LAQ824 (Figure 2B), and the expression of 817 LPS-stimulated genes was completely inhibited by LAQ824 (Figure 2B). Therefore, these 2 sets of genes (899 total) require HDAC activities for LPS-induced transcriptional activation. Last, there were 1180 genes induced in response to LPS plus LAQ824, but not to LPS alone (Figure 2C). As expected, a large portion of genes in this group (742) were also induced in response to LAQ824 alone (Figure S1), suggesting that HDACs act as transcriptional repressors for this set of genes. Similar results were found with cells from donor 2 (Table S3), demonstrating that LAQ824 alters the LPS-induced gene expression profile in macrophages. In summary, our data indicate that LAQ824 has differential gene-specific effects by acting as a repressor or activator via HDAC inhibition in macrophages.
LAQ824 alters LPS-induced gene expression profiles in human monocyte-derived macrophages. (A) Genes induced at the same level in response to LPS alone or LPS with LAQ824 simultaneously. (B) Genes with decreased expression in the presence of LAQ824. (C) Genes with induced expression in the presence of LAQ824.
LAQ824 alters LPS-induced gene expression profiles in human monocyte-derived macrophages. (A) Genes induced at the same level in response to LPS alone or LPS with LAQ824 simultaneously. (B) Genes with decreased expression in the presence of LAQ824. (C) Genes with induced expression in the presence of LAQ824.
HDAC inhibition by LAQ824 differentially regulates specific proinflammatory genes and inhibits APC-mediated monocyte but not neutrophil chemotaxis
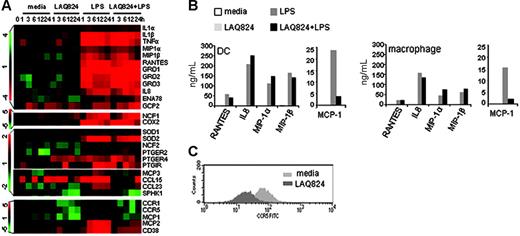
Recognition of LPS by TLR4 in macrophages and DCs activates multiple signaling pathways such as NF-κB and MAPK, and initiates an inflammatory response characterized by the rapid induction of a group of proinflammatory cytokines and chemokines.26 The expression of these proinflammatory mediators serves to activate and attract neutrophils, monocytes, natural killer cells, and other leukocytes to the site of inflammation, thereby initiating an orchestrated innate immune response to invading pathogens.27 To identify the effects of LAQ824 in modulating innate immune cell activation, LPS-induced proinflammatory genes in macrophages were grouped according to function. Expression of LPS-induced acute proinflammatory mediators, such as TNFα, IL-1β, MIP1α, MIP1β, RANTES, and prostaglandin synthase and receptors (Figure 3A) remained unchanged with the addition of LAQ824. Furthermore, expression of LPS-induced effector genes known to lyse pathogens, such as NCF28 and SOD,29 were also unchanged in the presence of LAQ824 (Figure 3A). Therefore, HDACs appeared to play no role in the induction of these genes by LPS.
Effects of LAQ824 on the expression of LPS-induced proinflammatory genes in human monocyte-derived macrophages and DCs. (A) mRNA levels of proinflammatory genes, lipid mediators, and chemotactic factors in macrophages treated with indicated stimuli. Color bars represent the ratio of hybridization signals between corresponding time points and time 0, according to the scale shown on the left. (B) Protein secretion of selected proinflammatory genes from macrophages and DCs. Expression levels are determined by Cytometric Bead Array chemokine kit (BD Pharmingen). Representative of 4 independent experiments. (C) CCR5 surface expression in DCs incubated with indicated stimuli. Expression level is determined by flow cytometry.
Effects of LAQ824 on the expression of LPS-induced proinflammatory genes in human monocyte-derived macrophages and DCs. (A) mRNA levels of proinflammatory genes, lipid mediators, and chemotactic factors in macrophages treated with indicated stimuli. Color bars represent the ratio of hybridization signals between corresponding time points and time 0, according to the scale shown on the left. (B) Protein secretion of selected proinflammatory genes from macrophages and DCs. Expression levels are determined by Cytometric Bead Array chemokine kit (BD Pharmingen). Representative of 4 independent experiments. (C) CCR5 surface expression in DCs incubated with indicated stimuli. Expression level is determined by flow cytometry.
Some of these inflammatory mediators are chemokines, which are critical in directing leukocyte trafficking and necessary for neutrophil and monocyte recruitment to the site of infection. As shown in Figure 3A, LPS-induced neutrophil-attracting chemokines,30 including IL-8, GRO1, GRO2, GRO3, ENA78, and GCP2, were unchanged or further induced by LAQ824 treatment. In contrast, LAQ824 strongly inhibited the expression of chemokines and receptors predominately involved in monocyte/macrophage/DC migration,30 such as MCP-1, MCP-2, MCP-3, CCL15, CCL23, CCR1, CCR5, CD38,31 and sphingosine kinase 1 (SPHK1)32 (Figure 3A). Protein measurements confirmed the mRNA results, as there was little effect of LAQ824 on LPS-induced RANTES, IL-8, MIP1α, and MIP1β secretion, but a strong inhibition on MCP-1 (Figure 3B) and CCR5 expression (Figure 3C) in macrophages and DCs. These results demonstrate that LAQ824 differentially regulates proinflammatory gene expression and that there are discernible differences, at the transcriptional level, in how distinct arms of the innate immune responses are affected by LAQ824.
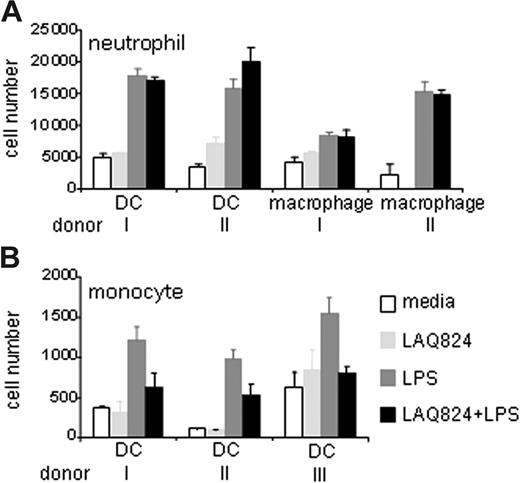
To determine whether LAQ824-induced gene expression changes observed from the array data had any functional consequences, neutrophil and monocyte chemotaxis assays were performed. In these assays, supernatant from LPS and/or LAQ824-treated macrophage and DC cultures were placed in the bottom migration chamber, and primary human neutrophils or LPS-stimulated monocytes were placed in the top chamber. As shown in Figure 4A, neutrophils migrated equally well toward the supernatant from LPS or LPS plus LAQ824–treated macrophage and DC cultures. However, monocyte migration was impaired when using supernatant from LPS plus LAQ824–treated cultures compared with supernatant from LPS-treated cultures (Figure 4B). These data demonstrate that LAQ824 differentially modulates chemokine expression in activated APCs, thereby affecting their ability to attract neutrophils and monocytes. Having coupled our gene expression analysis to a functional chemotaxis assay, our data demonstrate that HDAC inhibition in APCs has specific effects on proinflammatory gene expression and regulates the recruitment of distinct arms of the innate immune system.
LAQ824 inhibits LPS-induced monocyte but not neutrophil chemotaxis. Migration of human neutrophil (A) and LPS-stimulated monocytes (B) toward the supernatant from macrophage and DC cultures treated with indicated stimuli for 24 hours. Representative of 3 independent experiments.
LAQ824 inhibits LPS-induced monocyte but not neutrophil chemotaxis. Migration of human neutrophil (A) and LPS-stimulated monocytes (B) toward the supernatant from macrophage and DC cultures treated with indicated stimuli for 24 hours. Representative of 3 independent experiments.
HDAC inhibition by LAQ824 differentially regulates the expression of LPS-induced genes involved in Th1/Th2 cell activation and chemotaxis
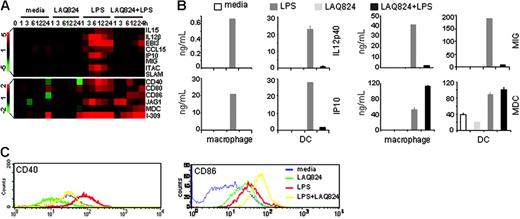
The generation of adaptive immunity depends on the activation states of APCs, particularly in the activation of naive and effector CD4+ and CD8+ T cells.14,17,18 Specifically, 3 signals generated from TLR-activated APCs direct the differentiation and activation of Th1 or Th2 subsets, including the dose of antigen presented (signal 1), the type and degree of costimulatory molecule expressed (signal 2), and the type of cytokine secreted (signal 3).33-37 LPS activates APCs via TLR4 generating both signals 2 and 3, allowing them to induce T-cell differentiation and activation of both Th1 and Th2 responses.15,33,38-40 Analysis of the global gene expression pattern in LPS-activated macrophages revealed that LAQ824 modulates APC-controlled CD4+ T-cell activation. Expression of LPS-induced Th1 activation signals, such as costimulatory molecules CD40 and SLAM,41 polarization cytokines IL-12, IL-15, and EBI3,42 and Th1-attracting chemokines ITAC, MIG, and IP1043 were inhibited by LAQ824 (Figure 5A). Protein expression of IL-12p40, IP10, MIG, and CD40 in both LPS-treated macrophages and DCs were also significantly inhibited in response to LAQ824 treatment (Figure 5B-C). In contrast, LPS-induced signals involved in effector Th2 activation like CD86,44 Jagged 1,45 MDC,46 and I-30947 were unchanged or further induced by LAQ824 at both the transcriptional and protein production levels (Figure 5A-C). Therefore, LAQ824 differentially modulated the expression of signals 2 and 3 in APC required for Th cell activation, indicating that LAQ824 might alter the Th1/Th2 balance.
LAQ824 modulates the expression of genes involved in CD4+ T-cell activation in human monocyte-derived macrophages and DCs. (A) mRNA levels of genes involved in Th1/Th2 activation and migration in macrophages. (B) Protein production of IL-12p40, IP10, MIG, and MDC from macrophages and DCs after 24 hours of treatment with indicated stimuli. Representative of 4 independent experiments. (C) CD40 and CD86 surface expression (flow cytometry) in DCs after 24 of hours treatment with indicated stimuli.
LAQ824 modulates the expression of genes involved in CD4+ T-cell activation in human monocyte-derived macrophages and DCs. (A) mRNA levels of genes involved in Th1/Th2 activation and migration in macrophages. (B) Protein production of IL-12p40, IP10, MIG, and MDC from macrophages and DCs after 24 hours of treatment with indicated stimuli. Representative of 4 independent experiments. (C) CD40 and CD86 surface expression (flow cytometry) in DCs after 24 of hours treatment with indicated stimuli.
HDAC activities are required for TLR4-dependent DC-mediated Th1 effector cell, but not for Th2 effector cell activation and migration
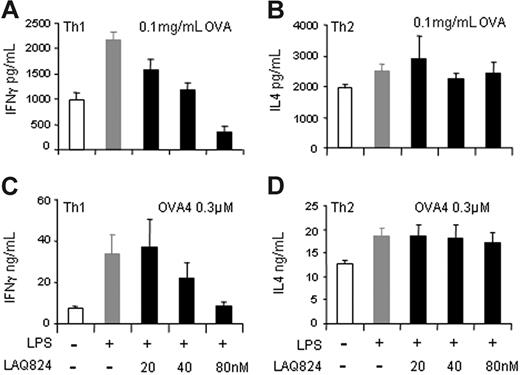
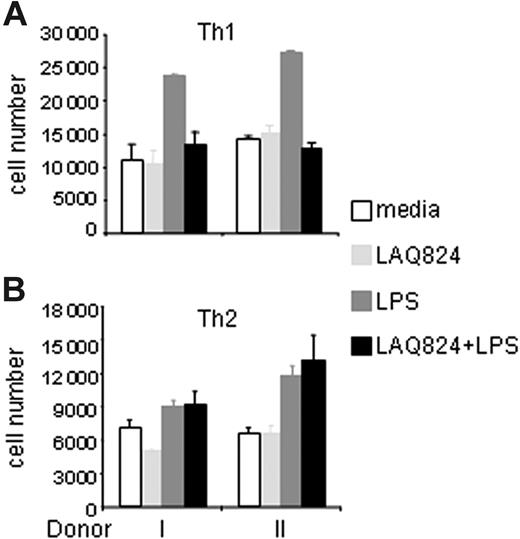
To test whether the effects of LAQ824 on the expression of genes involved in CD4+ T-cell activation had any functional consequences, we measured DC-mediated Th1 and Th2 effector activation in an antigen-specific coculture system. We used DO11.10 CD4+ T cells, which recognize an OVA-derived peptide presented on MHC II molecules on APCs. Specifically, bone marrow–derived DCs were generated from Balb/c mice, incubated with OVA protein, and treated with LPS and/or LAQ824. DCs were washed extensively before being cultured with effector Th1 or Th2 cells derived from DO11.10 mice. Coculture was carried out for 48 hours, and IFN-γ or IL-4 secretion was measured to monitor T-cell activation. As shown in Figure 6A, LPS-treated DCs, with increased expression of Th1 activation signals, induced more IFN-γ secretion from Th1 cells compared with unstimulated DCs. However, IFN-γ secretion by Th1 cells was significantly blocked in response to LAQ824 treatment of LPS-stimulated DCs in a dose-dependent manner. In contrast, when Th2 effector cells were incubated with stimulated DCs, IL-4 secretion was unchanged in the presence of LAQ824, regardless of LAQ824 concentration (Figure 6B). These data demonstrate that LAQ824 selectively modulates DC function to inhibit Th1 but not Th2 effector cell activation.
LAQ824 inhibits DC-mediated IFN-γ secretion from Th1 but not IL-4 production from Th2 cells. (A-B) IFN-γ and IL-4 secretion from DO11.10 Th1 (A) or Th2 (B) effector cells cocultured with bone marrow–derived DCs incubated with 0.1 mg/mL OVA and indicated stimuli. (C-D) IFN-γ and IL-4 secretion from DO11.10 Th1 (C) or Th2 (D) effector cells cocultured with bone marrow–derived DCs pulsed with 0.3 μM OVA peptide and indicated stimuli. Representative of 3 independent experiments.
LAQ824 inhibits DC-mediated IFN-γ secretion from Th1 but not IL-4 production from Th2 cells. (A-B) IFN-γ and IL-4 secretion from DO11.10 Th1 (A) or Th2 (B) effector cells cocultured with bone marrow–derived DCs incubated with 0.1 mg/mL OVA and indicated stimuli. (C-D) IFN-γ and IL-4 secretion from DO11.10 Th1 (C) or Th2 (D) effector cells cocultured with bone marrow–derived DCs pulsed with 0.3 μM OVA peptide and indicated stimuli. Representative of 3 independent experiments.
Our data suggest that antigen processing and presentation (signal 1) were not affected in DCs by LAQ824 treatment, since DC-mediated Th2 activation was unchanged when pulsed with OVA protein. Thus, the impairment of DC-mediated Th1 activation may be due to a decreased signal 2 or 3 in response to LAQ824 treatment, as indicated from the gene expression analysis and IL-12 production in bone marrow–derived DCs (Figure S2). To further analyze this observation, we tested Th1 and Th2 activation using DCs pulsed with OVA peptide. As shown in Figure 6C, LPS-stimulated DCs induced IFN-γ secretion from effector Th1 cells when pulsed with 0.3 μM OVA peptide; the induction was significantly inhibited when DCs were incubated with an increasing dose of LAQ824. In contrast, LAQ824 did not have any effects on DC-mediated IL-4 secretion from effector Th2 cells, even when present at doses as high as 80 nM (Figure 6D). This demonstrates that LAQ824 blocks signals 2 and 3 in DCs that are important for effector Th1 activation, but have no effect on Th2 activation. These results confirmed our prediction based on gene expression analysis that LAQ824 would reduce Th1 effector function by blocking LPS-induced Th1 activation signals in APCs.
LAQ824 not only differentially modulated the expression of genes involved in Th1/Th2 effector activation, but also the chemokines directing Th1/Th2 cell migration. As shown in Figure 5, LPS highly induced expression of both Th1- and Th2-attracting chemokines in DCs and macrophages. However, LAQ824 completely blocked the mRNA expression of Th1 chemokine (IP10, MIG, and ITAC) and the secretion of IP10 and MIG protein, but had little effect on the expression of Th2 chemokines MDC and I-309 (Figure 5A-B). To test the functional consequences of this observation, we performed transwell migration assays as before. As shown in Figure 7A, far fewer effector Th1 cells migrated to LPS plus LAQ824–treated DC supernatant than to LPS-treated supernatant. In contrast, effector Th2 cells migrated equally well to both types of supernatant (Figure 7B). This result demonstrates differential activities of LAQ824 in modulating APC function to attract Th1 and Th2 cells by HDAC inhibition. Therefore, not only was effector Th1 activation inhibited, but its migration was also impaired. In contrast, neither Th2 effector cell activation nor migration was significantly affected by LAQ824 treatment of APCs.
LAQ824 inhibits LPS-induced Th1 but not Th2 chemotaxis. Migration of human Th1 (A) and Th2 (B) effector cells toward the supernatant from DC cultures stimulated with indicated stimuli for 24 hours. Representative of 3 independent experiments.
LAQ824 inhibits LPS-induced Th1 but not Th2 chemotaxis. Migration of human Th1 (A) and Th2 (B) effector cells toward the supernatant from DC cultures stimulated with indicated stimuli for 24 hours. Representative of 3 independent experiments.
Discussion
We carried out the first systematic investigation of the role of HDACs in TLR4-dependent innate and adaptive immune responses. Using the pan-HDACi LAQ824, genomewide gene expression analysis of LAQ824-treated APCs revealed unexpected and immunologically important findings. We determined that the effects of HDAC inhibition on gene expression in APCs are selective and characterized by (1) a relatively small number of genes regulated by LAQ824; (2) the existence of both up-regulated and down-regulated gene expression in response to HDAC inhibition; and (3) distinct functional (ie, immunologic) consequences.
Studies have shown that approximately 2% to 40% of genes were up- or down-regulated in transformed cells in response to various HDACi's.48-50 Many of these genes are involved in apoptosis, cell growth, and survival pathways.48,51,52 In this study, we measured gene expression in response to an HDACi, LAQ824, in monocyte-derived macrophages from healthy donors. Our data showed that LAQ824 treatment did not induce a global gene expression change, as one might expect from a pan-HDACi. Surprisingly, only approximately 5% of genes had altered expression in the presence of LAQ824 over 24 hours in resting macrophages. This suggests that most endogenous promoters require other factors for activation or repression. Indeed, histone tails are subject to other modifications such as phosphorylation, methylation, and sumoylation, and the combination of these modifications determine transcriptional activity.53,54 At the concentrations of HDACi's used throughout our studies, it appears that HDAC activities are essential in activating or repressing only 5% of the promoters in macrophages.
We also observed that the effect of HDAC inhibition on transcription is gene specific. In both resting and activated macrophages, the expression of some genes was induced while that of others was repressed by LAQ824 (Figures 1–2). This suggests that HDACs not only can function as transcription repressors as one would expect, but also as transcription activators depending on the specific promoters. The role of HDACs as repressors is understood as their recruitment to a promoter leads to histone deacetylation and chromatin condensation, a series of events which are favorable for transcriptional silencing.55,56 However, it is still unclear how HDACs function as activators. It may be due to a secondary effect of HDACi treatment, as HDAC inhibition may activate a transcription repressor which functions to silence the expression of targeted genes.57 It is also possible that HDAC inhibition modifies the acetylation state of signaling proteins or transcription factors and affects their ability to activate specific promoters. These hypotheses are currently under investigation.
Our results also demonstrated that the effect of HDACi's on gene expression can be cell-state dependent. LAQ824 modulated the expression of some genes only in activated but not in resting macrophages (Figures 3A, 5A). In addition, cell type seems to determine the response of specific genes to HDAC inhibition. For example, CD86 expression was induced by LAQ824 treatment in immature DCs (Figure 5C) but not in monocytic THP-1 cells (Figure S3). We have also observed some variation in the expression of IL-6, TNFα, and CD40 by HDAC inhibition with previously reported data,58-60 which could be due to the differences in cell types. These data demonstrate that the effects of HDAC inhibition on gene expression are highly selective and dependent on the cell type and activation state, and are restricted to a specific set of genes. Therefore, the epigenetic state of cells determines their response to HDAC inhibition, which provides a potential mechanism for the specific effects of HDACi's on malignant cells versus normal cells and activated immune cells versus resting cells.
Our systematic gene expression analysis revealed interesting cellular and molecular effects of an HDACi on TLR4-mediated immune responses. When LAQ824-regulated genes were categorized according to their functions in specific immunologic processes, particularly APC-mediated Th1 versus Th2 activation and migration, as well as neutrophil versus monocyte migration, we uncovered some rather surprising relationships.
The expression pattern of LPS-induced genes involved in Th1/Th2 activation (Figure 5) and the APC-mediated Th1/Th2 effector activation and migration experiments (Figures 6–7) demonstrated that LAQ824 altered LPS-stimulated APC function by reducing their capacity to activate and attract Th1 effector cells while preserving Th2 activation and migration signals. Given that LAQ824 is a pan-HDACi, its high degree of selectivity on Th1 but not Th2 effector cell function is interesting both mechanistically and from a therapeutic standpoint. A balanced Th1 and Th2 response is critical to maintain immune homeostasis, and dysregulation of Th1 and Th2 activation leads to pathologic immune responses.61 There has been intense effort to target Th1 and Th2 cells directly to treat autoimmune inflammation and asthma. This is the first study to demonstrate the potential to target APCs by a small molecule and thereby reset the Th1/Th2 balance, suggesting that LAQ824 might be useful in treating Th1-mediated autoimmune inflammation but not in Th2-mediated asthma and allergy. This is also specifically supported by the finding that LAQ824 is a potent inhibitor of IL-12p40 (Figure 5), a common subunit for IL-12 and IL-23, in both DCs and macrophages. These data are consistent with previous findings on the inhibition of IL-12p40 expression by other HDACi's.62,63 IL-12 plays a central role in Th1 development and is the therapeutic target in the treatment of various autoimmune diseases.64 Recently, IL-23 has been shown to significantly contribute to inflammatory diseases in models of multiple sclerosis (MS), arthritis, and inflammatory bowel disease.65 Therefore, LAQ824 may show efficacy in these autoimmune inflammation models. Our results may also provide a mechanistic model for elucidating the activities of HDACi's in RA, GVHD, and Crohn disease models in which overexpression of inflammatory genes plays an important role.
In addition to altering the chemokine profile for attracting Th1 and Th2 cells, LAQ824 treatment of APCs also modulated the expression of chemokines important for neutrophil and monocyte migration. Both gene expression profiles (Figure 3) and migration assays (Figure 4) demonstrated a reduction of monocyte- but not neutrophil-attracting signals in macrophages and DCs treated with LAQ824. Therefore, HDAC inhibition in APCs leads to differential effects on distinct arms of the innate immune responses.
While modifying the chemotactic profiles of neutrophils, monocytes, and T cells, LAQ824 treatment may also affect the migration of APCs themselves. LAQ824 blocked the expression of 2 key mediators in macrophage and DC chemotaxis, CD38 and SPHK1 (Figure 3A). Recently, CD38 has been shown to play a critical role in triggering chemokine-mediated DC migration by inducing calcium-mobilization metabolites ADPR and cADPR.31 In our study, LPS-induced CD38 expression was significantly blocked in the presence of LAQ824. This indicates that LAQ824 might inhibit a key step in DC maturation by interfering with its chemotaxis. The synthesis of another important inflammatory mediator, sphingosine-1 phosphate (S1P), is rapidly increased through the stimulation of SPHK1. SPHK1 is also critical in C5a-triggered calcium flux, cytokine production, and migration in macrophages.32 We found that LPS-induced SPHK1 expression was significantly reduced under LAQ824 treatment (Figure 3A). Taken together, LAQ824 inhibited key genes involved in APC chemotaxis. Combined with the reduced migration of monocytes (Figure 4), our data suggest that inhibition of HDAC activities could be an effective approach to treat inflammatory conditions in which dysregulated trafficking of cells of monocytic lineage plays an important role, as in atherosclerosis.66
Targeting chemokine networks represents a promising approach for anti-inflammatory drugs.67 However, the redundancy of different chemokines in various autoimmune conditions makes it challenging to develop effective therapies by targeting a single chemokine or receptor. Here, we show that LAQ824 is able to selectively inhibit a group of chemokines and receptors that are involved in similar processes. To our knowledge, LAQ824 is the first small molecule shown to have this type of broad-spectrum activity against multiple chemokines. Based on our data, we suggest that targeting HDACs with small-molecule inhibitors opens a new avenue for modulating the chemokine network in inflammatory settings.
We showed that LAQ824 modulated gene expression and function in macrophages and DCs at concentrations below 80 nM. DCs were viable at this concentration (Figure S4), suggesting that LAQ824's immune modulatory effects can be uncoupled from its antiproliferative effects. Indeed, in THP-1 cells, the concentration of LAQ824 that inhibited proliferation was found to be approximately 0.25 μM following 48 hours of treatment (Figure S5). However, a much lower concentration was required for modulating inflammatory gene expression in these cells. At less than 20 nM, LAQ824 completely blocked LPS-induced IL-12p40 secretion in THP-1 cells within 24 hours, and 10 nM LAQ824 decreased 50% of LPS-induced CD40 surface expression (Figure S6). These data demonstrate that LAQ824 exerts its anti-inflammatory effects at concentrations much lower than those needed to suppress tumor cell growth. Another HDACi, SAHA, also displayed similar effects on tumor cell killing versus anti-inflammation.62 This suggests that at low doses HDACi's are useful as anti-inflammatory agents.
Despite the success of available immunosuppressants, there remains a great need for specific and effective anti-inflammatory drugs for many indications. Here we show that HDAC inhibition by LAQ824 in macrophages and DCs displayed selective and desirable anti-inflammatory effects. Our results provide novel evidence for targeting the epigenetic machinery to modulate specific innate and adaptive immune responses, and thereby define a new molecular basis for developing specific immune modulators. We believe the HDAC family of enzymes and perhaps other epigenetic modulators like histone and DNA methyltransferases will provide a wealth of targets for pharmacologic intervention in inflammatory diseases.
Authorship
J.L.B. and Y.X. contributed equally to this work.
Conflict-of-interest statement: All of the authors are employed by Novartis Institute for Biomedical Research, whose potential product was studied in the present work.
D.C.'s current affiliation is Rosetta Genomics, Inc, North Brunswick, NJ 08902.
Correspondence: Qian Huang, Novartis Institute for BioMedical Research, 250 Massachusetts Ave, Rm 5B-122, Cambridge, MA 02138; e-mail: qian.huang@novartis.com.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Wendy Marston and Sharon Hunter for isolating bone marrow cells from mice; we also thank Eddie Adams and Daniel Thomis for critical reading and suggestions for the improvement of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal