Abstract
The malignant phenotype of chronic myeloid leukemia (CML) is due to the abnormal tyrosine kinase activity of the Bcr-Abl oncoprotein. We have previously reported that expression of the Bach2 transcription factor, which induces apoptosis in response to oxidative stress, is greatly reduced in CML cells. Because these cells are resistant to apoptosis, we tested whether Bach2 could also be regulated through posttranslational mechanisms that promote inhibition of the apoptotic response to mutagenic stimuli in CML. We found that Bach2 is phosphorylated on S521 via the phosphatidylinositol-3/S6 kinase pathway, and substitution of this site to alanine leads to nuclear accumulation of the protein, indicating that this phosphorylation is important for its subcellular localization. Ectopic expression of the S521 mutant imparts greater impairment to CML cell growth than the wild-type factor. Furthermore, we showed that Bach2 transcriptionally represses heme oxygenase-1, an antiapoptotic factor up-regulated in CML. Because CML cells are known to produce high levels of intracellular reactive oxygen species, overexpression of heme oxygenase-1 resulting from inhibition of Bach2 activity may contribute to their genomic instability and leukemic phenotype.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative disorder of the hematopoietic stem cell caused by a t(9;22)(q34;q11) translocation that generates the Philadelphia (Ph) chromosome. This genetic defect, also found in 20% to 30% of adult acute lymphoblastic leukemias, results in the expression of Bcr-Abl, a fusion oncoprotein with uncontrolled tyrosine kinase activity. Imatinib mesylate inhibits the Bcr-Abl tyrosine kinase, suppresses the proliferation of CML progenitor cells, and induces apoptosis of Ph-positive cell lines. It is a highly effective drug and has become the first-choice treatment of CML. However, resistance to the inhibitor emerges in some patients, especially in advanced phases of CML (reviewed by Yoshida and Melo1 and Deininger et al2 ).
Bcr-Abl phosphorylates several substrates that activate multiple signaling pathways, including Ras, signal transducer and activator of transcription-5 (STAT-5), extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK), Janus kinase 2 (Jak-2), phosphatidylinositol-3 kinase (PI-3K), and others.3 This abnormal signaling leads to the malignant cellular phenotype of CML, including increased proliferation, reduction of adhesion to the bone marrow stroma and extracellular matrix, and inhibition of the apoptotic response to mutagenic stimuli.4 DNA damage can be caused by oxidative stress arising when reactive oxygen species (ROSs) are not adequately removed from cells.5 Interestingly, it has been suggested that ROSs are increased in CML cells.6-8 Because a persistent increase of ROSs can lead to accumulation of DNA mutations, it may induce genomic instability as a long-term consequence.9,10 Thus, BCR-ABL–positive cells can survive under levels of oxidative stress that normally induce an apoptotic response in nonleukemic cells. However, the factors and mechanisms responsible for resistance to ROS-induced cell death in CML are largely unknown. Understanding these mechanisms may lead to insights on CML transformation and its prevention or treatment.
Bach2 is a transcription factor containing a basic leucine zipper, a BTB (broad complex Tramtrack bric-a-brac) domain, and a cytoplasmic localization signal (CLS).11,12 In the hematopoietic lineage, it is expressed mainly in B cells.13 Bach2 binds to the Maf recognition element (MARE), which contains the AP-1 binding sequence, as a heterodimer with small Maf proteins and usually inhibits target genes. In Bach2-deficient mice, Ig class switch recombination, somatic hypermutation, and germinal center formation are severely impaired,14 indicating that Bach2 is important for the antibody response of B cells. The human BACH2 gene was cloned via a differential display screening aimed at identifying transcriptional targets of Bcr-Abl.15,16 It is inhibited by Bcr-Abl, induced by imatinib, and down-regulated in CD34+ cells from CML patients as compared with those from healthy individuals. In diffuse large B-cell lymphoma, the expression level of Bach2 has been recently reported to be a useful prognostic marker.17 Bach2 localizes mainly in the cytoplasm in normal conditions but migrates to the nucleus and functions as a proapoptotic factor in response to oxidative stress.18 Overexpression of human Bach2 can potentiate apoptosis of B cells treated with chemotherapeutic drugs that induce intracellular ROSs and nuclear accumulation of Bach2.19 Accordingly, it is possible that inhibition of Bach2 may contribute to reduction of the apoptotic response to oxidative stress in Ph-positive cells.
Despite its down-regulation, Bach2 is still detectable at the protein level in Ph-positive cells.16 Therefore, it is possible that Bcr-Abl regulates it not only at the expression level but also through posttranslational mechanisms that promote inhibition of the apoptotic response to mutagenic stimuli. In this study, we show that Bcr-Abl exerts this function by preventing Bach2 dephosphorylation and consequent translocation to the cell nucleus in response to oxidative stress. We further identified a possible target gene of this transcription factor, heme oxygenase-1 (HO-1), which may be related to the antiapoptotic phenotype of CML cells and to imatinib resistance.
Materials and methods
Plasmids and in vitro mutagenesis
A cDNA fragment encoding human Bach2 was isolated by polymerase chain reaction (PCR) and subcloned into BamHI and EcoRI sites of pcDNA3.1 (Invitrogen, Carlsbad, CA), resulting in pcDNA3.1Bach2. pcDNA3.1HABach2 was constructed by insertion of annealed oligonucleotides 5′-AGCTTGCGGCCGCGCTAGCGCCGCCACCATGTACC-CATACGATGTTCCAGATTACGCTCTTG-3′ encoding the influenza virus hemagglutinin (HA) into the HindIII and BamHI sites of pcDNA3.1Bach2. pcDNA3.1HABach2T519A, pcDNA3.1HABach2S521A, and pcDNA3.1HABach2T817A were constructed by site-directed mutagenesis and express Bach2 mutant proteins. pEGFPBach2 and pEGFPBach2S521A express GFP-Bach2 and GFP-Bach2S521A fusion protein, respectively. pBSBach2 vectors were constructed by insertion of a BamHI-EcoRI fragment of pcDNA3.1HABach2 into pBlueScriptKS+ vector (Stratagene, La Jolla, CA) followed by site-directed mutagenesis and recloning of the mutated BamHI-EcoRI fragments into pcDNA3.1HABach2. Mutagenesis primers were as follows: 5′-GGAGACCAGGACCAGGGCTTCCAGCTCCTGCTC-3′ for T519A (pcDNA3.1HABach2T519A), 5′-GGAGACCAGGACCAGGACTTCCGCCTCCTGCTC-3′ for S521A (pcDNA3.1HABach2S521A), and 5′-GAACCCAGGAGCCAAGCAGTGACCGTGG-3′ for T817A (pcDNA3.1HABach2T817A). These mutations were confirmed by sequencing. pEGFPBach2 was constructed by insertion of a BamHI and XbaI fragment of pcDNA3.1Bach2 into the same sites of pEGFP-C1 (Promega, Madison, WI). MIGRBach2 and MIGRBach2S521A were constructed by insertion of a BamHI (blunt ended)–EcoRI fragment of pcDNA3.1HABach2 or pcDNA3.1HABach2S521A into the XhoI (blunt ended)–EcoRI cloning sites of MIGR1. The retrovirus vector MIGR1 encoding IRES-GFP was kindly provided by Dr W. Pear (University of Pennsylvania, Philadelphia). MIGRBach2 and MIGRBach2S521A encode BACH2-IRES-GFP and BACH2S521A-IRES-GFP, respectively. pCLAmpho was described previously.20 pcDNA3.1S6K2T401D and pcDNA3.1S6K1T412D were kindly donated by Dr O. Pardo (Cancer Research UK, London) and pRK7/HAS6K1E389D3E21 and pcDNA3HAS6K2ΔCT22 by Dr J. Blenis (Harvard Medical School, Boston, MA). pcDNA3.1HAS6K1E389D3E was constructed by insertion of an XbaI (blunt ended)–EcoRI fragment of pRK7/HAS6K1E389D3E into the BamHI (blunt ended)–EcoRI sites of pcDNA3.1. pGL3basic was from Promega. hHO4.9Luc, hHO4.9_M1Luc, hHO4.9_M2Luc, and hHO4.9_M1+M2Luc23 were gifts from Drs G. Kronke and N. Leitinger (Medical University of Vienna, Austria, and University of Virginia, Charlottesville). pEF-SP encodes a Renilla luciferase.13
Cell culture and transfection
BV173, Ramos, Namalwa, and Jurkat human lymphoid cell lines were cultured in RPMI 1640 medium (Gibco/Life Technologies, Paisley, United Kingdom) supplemented with 10% fetal calf serum (FCS), 200 mM l-glutamine, 5000 IU/mL penicillin, and 5000 μg/mL streptomycin. 3T3 and 293T cells were cultured in Dulbecco modified Eagle medium (DMEM) (Gibco/Life Technologies) supplemented as for human lymphoid cell lines. 3T3 cells expressing Bcr-Abl, HAS6K1E389D3E, S6K2T401D, or vehicle (pcDNA3.1) were established by transfection of these expression plasmids by calcium phosphate or FuGENE6 (Roche Diagnostics, Lewes, United Kingdom) according to the manufacturer's protocol, followed by selection and maintenance on 600 ng/mL G-418 sulfate (Gibco/Life Technologies).
Reagents
Imatinib mesylate was kindly provided by Dr E. Buchdunger (Novartis Pharma, Basel, Switzerland). LY294002 was purchased from Promega, wortmannin and diethyl maleate (DEM) from Sigma (Poole, United Kingdom), rapamycin from Cell Signaling Technology (Beverly, MA), and alkaline phosphatase from Roche Diagnostics. Monoclonal (2G11) and polyclonal (F69-2) anti-Bach2 antibodies were described previously.13 The antiphosphotyrosine antibody (4G10) was a kind gift from Dr B. Druker (Oregon Health & Science University, Portland, OR). Anti-AKT (9272), phospho-AKT (Ser473) (9271), p70 S6 kinase (9202), phospho-p70 S6 kinase (Thr389) (9205), S6 ribosomal protein (2212), and phospho-S6 ribosomal protein (Ser235/236) (2211) antibodies were obtained from Cell Signaling Technology; antiactin antibody (A-2066) from Sigma; and the anti–HO-1 antibody from Stressgen Bioreagents (SAP-896; Victoria, BC, Canada). Horseradish peroxidase (HRP) swine anti–rabbit Ig (P0217) and anti–rat IgG (NA935) antibodies were purchased from DakoCytomation (Ely, United Kingdom) and Amersham Biosciences (Little Chalfont, United Kingdom), respectively.
Immunoblotting
Cells were lysed in 1% Triton X-100, 20 mM Tris-HCl [pH 8.2], 150 mM NaCl, 1 mM sodium metavanadate, 1 mM phenylmethylsulfonylfluoride, and 100 nM okadaic acid, and the protein lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). After transfer to membranes, the proteins were immunoblotted with primary and HRP-conjugated secondary antibodies and detected by enhanced chemiluminescence (Amersham Biosciences). Densitometry analysis was performed using Scion image software (Scion, Frederick, MD).
Transduction of cells and fluorescence-activated cell sorter (FACS) analysis
The 293T cells were transfected with the retrovirus vectors and pCLAmpho by calcium phosphate; the viral supernatants were harvested after 2 days and concentrated × 10 by centrifugation. BV173 cells were infected with the concentrated virus particles and 4 μg/mL Polybrene. The percentage of GFP-positive cells was measured by flow cytometry (FACScalibur; Becton Dickinson, Franklin Lakes, NJ). The collected data were analyzed by CellQuest software (Becton Dickinson).
Reporter assay
Jurkat cells were transfected with the indicated plasmids and pEF-CP with FuGENE6 (Roche Diagnostics), and after 24 hours the reporter gene activity was determined using the dual luciferase reporter assay system (Promega) and normalized for transfection efficiency using the activity of Renilla luciferase as the standard internal control.
Fluorescence microscopy
The 3T3 cells were transiently transfected with the indicated plasmid by calcium phosphate and trypsinized from the plates 2 days later for seeding onto coverslips coated with 0.1% gelatin (Sigma). After 1 or 2 additional days, they were treated with various drugs when indicated and fixed with 4% paraformaldehyde (Sigma). The coverslips were mounted in DAPI containing Vectashield (Vector Laboratories, Burlingame, CA). Images were captured through an Olympus BX-41 fluorescence microscope (Tokyo, Japan). Suspension cells were stained with anti-Bach2 polyclonal antibody as previously reported18 and examined under a Zeiss LSM510 confocal laser scanning microscope (Zeiss, Tokyo, Japan).
Results
Bach2 is phosphorylated in BCR-ABL–positive cells in vivo
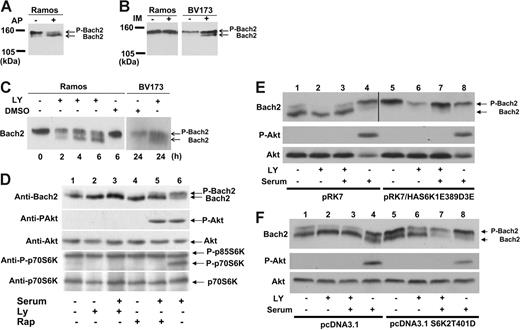
Immunoblotting of total cell lysate from Ramos, a human Burkitt lymphoma cell line, with anti-Bach2 antibody after treatment with alkaline phosphatase showed the appearance of a faster-migrating Bach2 band (Figure 1A). This demonstrates that Bach2 is phosphorylated in B cells in vivo and that the phosphorylated and unphosphorylated protein can be resolved via SDS-PAGE. To determine whether Bach2 was phosphorylated by Bcr-Abl, BV173, a CML lymphoid blast crisis cell line, was treated with imatinib. In this case, Bach2 was dephosphorylated in BV173 but not in Ramos cells (Figure 1B), indicating that Bach2 is phosphorylated in the presence of an active Bcr-Abl kinase in Ph-positive cells.
Bach2 is phosphorylated via the PI-3K/S6K pathway in vivo. (A) Lysates of Ramos cells were exposed (+) or not (-) to 20 units of alkaline phosphatase (AP) for 30 minutes. (B) BV173 and Ramos cells were exposed (+) or not (-) to 1 μM imatinib (IM) for 24 hours. (C) Ramos cells were either treated (+) or not (-) with 25 μM LY294002 (LY) or DMSO for the indicated time. BV173 cells were either treated (+) or not (-) with 50 μM LY or DMSO for 24 hours. (D) 3T3 cells were transiently transfected with pcDNA3.1Bach2. After serum starvation for 6 hours, the cells were either treated (+) or not (-) with 50 μM LY or 80 nM rapamycin (Rap) for 30 minutes and then stimulated (+) or not (-) with serum for 30 minutes. (E-F) 3T3 cells were transiently transfected with pcDNA3.1haBach2 together with pRK7, pRK7/HAS6K1E389D3E, pcDNA3.1, or pcDNA3.1S6K2T401D. After serum starvation for 6 hours, the cells were either treated (+) or not (-) with 50 μM LY for 30 minutes and then stimulated (+) or not (-) with serum for 30 minutes. (A-F) Total cell lysates were separated on SDS-PAGE and immunoblotted with a Bach2 monoclonal antibody, phospho-Akt (S473) (P-Akt), Akt, phosho-p70S6K (T389), or p70S6K antibodies. Phosphorylated (P-Bach2) and unphosphorylated Bach2 are indicated by the arrows. In panel E, the Bach2 strip for lanes 1 to 4 was taken from a 20-minute autoradiograph exposure and, for lanes 5 to 8, from a 5-minute exposure to compensate for different strengths of the Bach2 signal resulting from different efficiencies of cotransfection with the empty vector (pRK7) or the S6-K1 construct (pRK7/HAS6K1E389D3E). All images are representative of at least 3 independent experiments.
Bach2 is phosphorylated via the PI-3K/S6K pathway in vivo. (A) Lysates of Ramos cells were exposed (+) or not (-) to 20 units of alkaline phosphatase (AP) for 30 minutes. (B) BV173 and Ramos cells were exposed (+) or not (-) to 1 μM imatinib (IM) for 24 hours. (C) Ramos cells were either treated (+) or not (-) with 25 μM LY294002 (LY) or DMSO for the indicated time. BV173 cells were either treated (+) or not (-) with 50 μM LY or DMSO for 24 hours. (D) 3T3 cells were transiently transfected with pcDNA3.1Bach2. After serum starvation for 6 hours, the cells were either treated (+) or not (-) with 50 μM LY or 80 nM rapamycin (Rap) for 30 minutes and then stimulated (+) or not (-) with serum for 30 minutes. (E-F) 3T3 cells were transiently transfected with pcDNA3.1haBach2 together with pRK7, pRK7/HAS6K1E389D3E, pcDNA3.1, or pcDNA3.1S6K2T401D. After serum starvation for 6 hours, the cells were either treated (+) or not (-) with 50 μM LY for 30 minutes and then stimulated (+) or not (-) with serum for 30 minutes. (A-F) Total cell lysates were separated on SDS-PAGE and immunoblotted with a Bach2 monoclonal antibody, phospho-Akt (S473) (P-Akt), Akt, phosho-p70S6K (T389), or p70S6K antibodies. Phosphorylated (P-Bach2) and unphosphorylated Bach2 are indicated by the arrows. In panel E, the Bach2 strip for lanes 1 to 4 was taken from a 20-minute autoradiograph exposure and, for lanes 5 to 8, from a 5-minute exposure to compensate for different strengths of the Bach2 signal resulting from different efficiencies of cotransfection with the empty vector (pRK7) or the S6-K1 construct (pRK7/HAS6K1E389D3E). All images are representative of at least 3 independent experiments.
Bach2 is phosphorylated via the PI-3K/S6 kinase (S6K) pathway
Because Bcr-Abl is a tyrosine kinase, we assessed whether Bach2 is phosphorylated on tyrosine residues. Immunoblotting of immunoprecipitated Bach2 protein from BV173 cells with antiphosphotyrosine antibody did not reveal any bands (data not shown), suggesting that Bach2 is phosphorylated on serine (S) or threonine (T) residues by kinases downstream of Bcr-Abl. To determine the signaling pathways involved in Bach2 phosphorylation, Ramos and BV173 cells were treated with inhibitors of MEK (PD98059, U0126), Jak2 (AG490), Ras (L-744832), Src (PD166326), p38MAPK (SB203580), and PI-3K (LY294002). Immunoblotting with anti-Bach2 antibody showed dephosphorylation of Bach2 in both BCR-ABL–positive and –negative cells only upon treatment with LY294002 but not with the other kinase inhibitors (Figure 1C and Figure S2, which is available on the Blood website; see the Supplemental Figures link at the top of the online article). These results indicated that Bach2 is phosphorylated via PI-3K signaling pathways. Mammalian target of rapamycin (mTOR) is a downstream effector of PI-3/Akt and is inhibited by rapamycin. However, treatment with this kinase inhibitor showed only partial Bach2 dephosphorylation in 3T3 cells (Figure 1D, lane 5) compared with nearly complete dephosphorylation caused by LY294002 (Figure 1E, lane 3). Similar data were obtained in Ramos. These results suggested that Bach2 can be phosphorylated by a common kinase downstream of PI-3K and mTOR. However, no dephosphorylation of Bach2 was observed in BV173 cells treated with rapamycin (data not shown). This suggests that in Ph-positive cells, sole inhibition of mTOR may not be enough to repress phosphorylation of Bach2 because the Bcr-Abl–driven signaling of PI-3K may bypass mTOR and constitutively activate kinases further downstream, such as PDK1.24,25
The S6 kinases, S6K1 and S6K2, are known downstream substrates of Akt, PDK1, and mTOR. They each have 2 isoforms, p70α1 and p85α2 for S6K1 and p70β1 and p54β2 for S6K2, which are generated by alternative splicing.26-29 We therefore examined whether S6Ks could phosphorylate Bach2 by using cells expressing constitutively active forms of S6K1 and S6K2 that have S and/or T residues substituted by acidic amino acids (aa's) to mimic S6K phosphorylation.21,30 3T3 cells were transfected with Bach2 and constitutively active S6K1α2 (S6K1E389D3E) expression plasmids and serum starved. This resulted in phosphorylation of Bach2 even upon LY294002 treatment (Figure 1E). A similar result was observed with ectopic expression of constitutively active S6K2β1 (S6K2T401D) (Figure 1F). Although the phosphorylation in the latter case seems to be weaker than under the expression of S6K1E389D3E, this may be due to possible differences between the 2 mutants in their level of resistance to LY294002 rather than their specificity of interaction with Bach2. Similar data were obtained from ectopic expression of S6K1α1 (S6K1T412D) and S6K2β2 (S6K2ΔCT) (data not shown). Because Bcr-Abl is known to activate S6K constitutively,31 these experiments indicate that Bcr-Abl regulates phosphorylation of Bach2 via the PI-3K/S6K pathway.
Identification of Bach2 phosphorylation sites
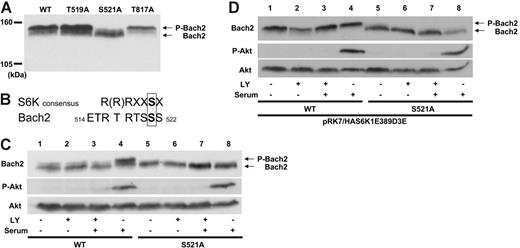
Candidate S6K phosphorylation sites on Bach2 were identified by Scansite32 screening for the Akt (R-X-R-X-X-S/T-X) phosphorylation consensus sequence because this is similar to that of S6K(R-(R)-R-X-X-S-X),33,34 which was not available in the software. Three candidate aa's, T519, S521, and T817, were thus identified, and mutagenesis of these sites was then carried out by their individual substitution to alanine (A).
Immunoblotting of cell lysates from 3T3 cells expressing each of the Bach2 mutants demonstrated that the Bach2S521A protein, but not the others, resolved on SDS-PAGE with no apparent phosphorylated band (Figure 2A). The aa sequence around S521 is consistent with the S6K consensus phosphorylation sequence, which is conserved between humans, mice, and rats (Figure 2B and data not shown). To confirm whether S521 is phosphorylated downstream of the PI-3K pathway, 3T3 cells expressing wild-type Bach2 or the S521A mutant were serum starved, treated with LY294002, and activated by serum addition. This elicited phosphorylation of wild-type but not mutant Bach2 under conditions of Akt activation (Figure 2C, lanes 4 and 8, respectively). Similar results were obtained when the cells were exposed to rapamycin (data not shown). Furthermore, the constitutively active form of S6K1 failed to phosphorylate the Bach2S521A mutant protein even in the absence of kinase inhibitors and in the presence of serum (Figure 2D). These data show that Bach2 is phosphorylated on S521 via the PI-3K/ S6K pathway.
Bach2 is phosphorylated on S521 by the PI-3K/S6K pathway. (A) 3T3 cells were transfected with expression vectors expressing wild-type or mutants of Bach2. Total cell lysates were separated on 5% SDS-PAGE and immunoblotted with a Bach2 monoclonal antibody. (B) Comparison between the consensus aa sequence for S6K phosphorylation and Bach2 aa's 514 to 522. (C) 3T3 cells were transiently transfected with pcDNA3.1HABach2 (lanes 1 to 4) or pcDNA3.1HABach2S521A (lanes 5 to 8) and (D) were also cotransfected with pRK7/HAS6K1E389D3E. After serum starvation for 6 hours, the cells were either treated (+) or not (-) with 50 μM LY294002 (LY) for 30 minutes and then either stimulated (+) or not (-) with serum for 30 minutes. Total cell lysates were separated on SDS-PAGE and immunoblotted with a Bach2 monoclonal antibody, antiphospho-Akt (S473), or Akt antibodies. Phosphorylated (P-Bach2) and unphosphorylated Bach2 are indicated by the arrows. All images are representative of at least 3 independent experiments.
Bach2 is phosphorylated on S521 by the PI-3K/S6K pathway. (A) 3T3 cells were transfected with expression vectors expressing wild-type or mutants of Bach2. Total cell lysates were separated on 5% SDS-PAGE and immunoblotted with a Bach2 monoclonal antibody. (B) Comparison between the consensus aa sequence for S6K phosphorylation and Bach2 aa's 514 to 522. (C) 3T3 cells were transiently transfected with pcDNA3.1HABach2 (lanes 1 to 4) or pcDNA3.1HABach2S521A (lanes 5 to 8) and (D) were also cotransfected with pRK7/HAS6K1E389D3E. After serum starvation for 6 hours, the cells were either treated (+) or not (-) with 50 μM LY294002 (LY) for 30 minutes and then either stimulated (+) or not (-) with serum for 30 minutes. Total cell lysates were separated on SDS-PAGE and immunoblotted with a Bach2 monoclonal antibody, antiphospho-Akt (S473), or Akt antibodies. Phosphorylated (P-Bach2) and unphosphorylated Bach2 are indicated by the arrows. All images are representative of at least 3 independent experiments.
The subcellular localization of Bach2 is regulated at the phosphorylation level
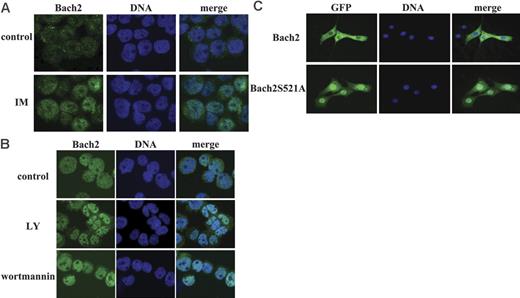
Because the subcellular localization of Bach2 is crucial for its activity,12,35 we examined whether this phenomenon is also regulated by Bcr-Abl. First, the intracellular localization of endogenous Bach2 in BV173 cells was assessed by indirect immunofluorescence with an anti-Bach2 polyclonal antibody. This showed that the little Bach2 expressed in these cells was localized predominantly in the cytoplasm and nucleus but translocated to the nucleus when the cells were treated with imatinib (Figure 3A), suggesting that the Bcr-Abl kinase activity does regulate the subcellular localization of Bach2.
The subcellular localization of Bach2 is regulated by the PI-3K/S6K pathway. Representative images of Bach2 (left panels), DNA (middle panels), and merged images (right panels) are shown for each experiment. (A) BV173 cells were either exposed or not (control) to 10 μM imatinib (IM) for 24 hours. (B) Namalwa cells were untreated (control) or treated with 100 μM LY294002 (LY) or 1 μM wortmannin for 2 hours. (A-B) Cells were fixed and immunostained with anti-Bach2 polyclonal antibody and Hoechst 33342, the coverslips were mounted in DAPI-containing Vectashield (Vector Labs, Burlingame, CA), and the slides were observed under a 40×/1.0 NA oil objective through a Zeiss LSM510 confocal laser scanning microscope. (C) 3T3 cells were transiently transfected with pEGFPBach2 or pEGFPBach2S521A. The coverslips were mounted in DAPI-containing Vectashield, and the slides were examined under a 40×/1.0 NA oil objective through an Olympus BX-41 fluorescence microscope (Olympus, Tokyo, Japan). Images were captured using an Orca AG digital camera (Hamamatsu Photonics UK, Hertfordshire, United Kingdom) and Smartcapture X software (Digital Scientific, Cambridge, United Kingdom) and were processed with Adobe Photoshop CS (Adobe Systems, San Jose, CA).
The subcellular localization of Bach2 is regulated by the PI-3K/S6K pathway. Representative images of Bach2 (left panels), DNA (middle panels), and merged images (right panels) are shown for each experiment. (A) BV173 cells were either exposed or not (control) to 10 μM imatinib (IM) for 24 hours. (B) Namalwa cells were untreated (control) or treated with 100 μM LY294002 (LY) or 1 μM wortmannin for 2 hours. (A-B) Cells were fixed and immunostained with anti-Bach2 polyclonal antibody and Hoechst 33342, the coverslips were mounted in DAPI-containing Vectashield (Vector Labs, Burlingame, CA), and the slides were observed under a 40×/1.0 NA oil objective through a Zeiss LSM510 confocal laser scanning microscope. (C) 3T3 cells were transiently transfected with pEGFPBach2 or pEGFPBach2S521A. The coverslips were mounted in DAPI-containing Vectashield, and the slides were examined under a 40×/1.0 NA oil objective through an Olympus BX-41 fluorescence microscope (Olympus, Tokyo, Japan). Images were captured using an Orca AG digital camera (Hamamatsu Photonics UK, Hertfordshire, United Kingdom) and Smartcapture X software (Digital Scientific, Cambridge, United Kingdom) and were processed with Adobe Photoshop CS (Adobe Systems, San Jose, CA).
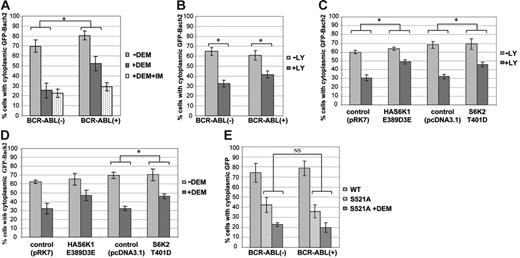
However, because expression of Bach2 is induced by treatment with imatinib,16 it was difficult to discriminate between the effect of this treatment on nuclear translocation due to overexpression or to inhibition of phosphorylation of the endogenous protein. Therefore, we established 3T3 cells stably expressing BCR-ABL or control and transiently transfected with a vector expressing Bach2 fused to GFP. The subcellular localization of the GFP-Bach2 protein was visualized and categorized as shown in Figure S_, and the results are presented as the percentage of cells in which GFP-Bach2 localizes predominantly in the cytoplasm (C>N). To evaluate whether Bcr-Abl could retain GFP-Bach2 in the cytoplasm under conditions of oxidative stress, the cells were treated with diethyl maleate (DEM), a glutathione-depleting agent that increases ROS concentration in cells. In BCR-ABL–negative cells, GFP-Bach2 was localized predominantly in the cytoplasm and migrated to the nucleus when the cells were treated with DEM (Figure 4A), in agreement with previous reports.12 In contrast, in Bcr-Abl–expressing cells, GFP-Bach2 was partially retained in the cytoplasm even in the presence of DEM. This retention was reversed when the cells were treated with imatinib for inhibition of Bcr-Abl kinase activity. These data suggest that the subcellular localization of Bach2 is regulated by its phosphorylation downstream of by Bcr-Abl.
The fraction of cells with cytoplasmic retention of Bach2 varies in response to PI-3K/S6K signaling and oxidative stress. 3T3 cells were transiently transfected with pEGFPBach2 plasmids, and the subcellular localization of GFP-Bach2 was observed through a fluorescence microscope. (A) Percentage of BCR-ABL–positive (+) or –negative (−) 3T3 cells with cytoplasmic GFP-Bach2 when exposed to 0.5 μM imatinib (+IM) for 24 hours, as indicated, and to 300 μM DEM (+DEM) or not (-DEM) for the last 3 hours before fixation. (B) Percentage of BCR-ABL–positive (+) or –negative (−) 3T3 cells with cytoplasmic GFP-Bach2 exposed to 25 μM LY294002 (+LY) or DMSO (-LY) for 2 hours. (C-D) Percentage of 3T3 cells expressing HAS6K1E389D3E, S6K2T401D, or vehicle (pRK7 or pcDNA3.1) with cytoplasmic GFP-Bach2 when treated with 5 μM LY294002 (+LY) or DMSO (-LY) for 2 hours (C) or treated (+DEM) or not (-DEM) with 300 μM DEM for 3 hours (D). (E) Percentage of cells stably expressing Bcr-Abl (+) or vehicle (-) with cytoplasmic GFP-Bach2 (WT) or GFP-Bach2S521A (S521A) treated or not with 300 μM DEM (+DEM) for 3 hours before fixation. At least 100 cells expressing GFP-Bach2 were counted in each experiment. Results are average ± standard deviation of 3 independent experiments. Comparison between the indicated groups shows a significant difference (*) or no significant difference (NS) by the Mann-Whitney U test (P < .05).
The fraction of cells with cytoplasmic retention of Bach2 varies in response to PI-3K/S6K signaling and oxidative stress. 3T3 cells were transiently transfected with pEGFPBach2 plasmids, and the subcellular localization of GFP-Bach2 was observed through a fluorescence microscope. (A) Percentage of BCR-ABL–positive (+) or –negative (−) 3T3 cells with cytoplasmic GFP-Bach2 when exposed to 0.5 μM imatinib (+IM) for 24 hours, as indicated, and to 300 μM DEM (+DEM) or not (-DEM) for the last 3 hours before fixation. (B) Percentage of BCR-ABL–positive (+) or –negative (−) 3T3 cells with cytoplasmic GFP-Bach2 exposed to 25 μM LY294002 (+LY) or DMSO (-LY) for 2 hours. (C-D) Percentage of 3T3 cells expressing HAS6K1E389D3E, S6K2T401D, or vehicle (pRK7 or pcDNA3.1) with cytoplasmic GFP-Bach2 when treated with 5 μM LY294002 (+LY) or DMSO (-LY) for 2 hours (C) or treated (+DEM) or not (-DEM) with 300 μM DEM for 3 hours (D). (E) Percentage of cells stably expressing Bcr-Abl (+) or vehicle (-) with cytoplasmic GFP-Bach2 (WT) or GFP-Bach2S521A (S521A) treated or not with 300 μM DEM (+DEM) for 3 hours before fixation. At least 100 cells expressing GFP-Bach2 were counted in each experiment. Results are average ± standard deviation of 3 independent experiments. Comparison between the indicated groups shows a significant difference (*) or no significant difference (NS) by the Mann-Whitney U test (P < .05).
The cells were next treated with LY294002 to test whether PI-3K mediates localization of Bach2: as shown in Figure 4B, inhibition of this kinase allowed translocation of GFP-Bach2 from the cytoplasm to the nucleus both in BCR-ABL–positive and –negative cells. This phenomenon was confirmed in B cells (which express endogenous Bach2) treated with LY94002 or with wortmannin, another PI-3K inhibitor (Figure 3B). Moreover, ectopic expression of S6K1 (S6K1E389D3E) and S6K2 (S6K2T401D) in 3T3 cells ensured that GFP-Bach2 was retained in the cytoplasm even in the presence of LY294002 (Figure 4C). These experiments demonstrate that the subcellular localization of Bach2 is regulated by both PI-3K and S6Ks.
We next examined whether the phosphorylation status of Bach2 could also influence its subcellular localization in response to oxidative stress. When cells expressing the active forms of S6Ks were treated with DEM, there was less nuclear translocation of Bach2 than in the control cells (Figure 4D). These data suggest that the mechanism by which Bcr-Abl forces the retention of Bach2 in the cytoplasm in the presence of oxidative stress (Figure 4A) is its constitutive activation of the PI-3/S6K pathway.31
S521 is important for the regulation of Bach2 subcellular localization
To confirm that the S6K target phosphorylation site is important for regulating Bach2 traffic in the cell, we examined the subcellular localization of the GFP-Bach2S521A mutant. The phosphorylation-defective protein was localized mainly in the nucleus in the absence of any treatment, in contrast to the wild-type Bach2, which was confined predominantly to the cytoplasm in both BCR-ABL–positive and –negative cells (Figures 3C and 4E). Furthermore, Bcr-Abl failed to retain the mutant in the cytoplasm when cells were treated with DEM, in contrast to the retention of the wild-type protein (Figure 4A). These data strongly indicate that the subcellular localization of Bach2 is regulated by the phosphorylation of S521 and that this mechanism is activated by Bcr-Abl.
The Bach2S521A mutant induces poorer survival of CML cells than the wild-type protein
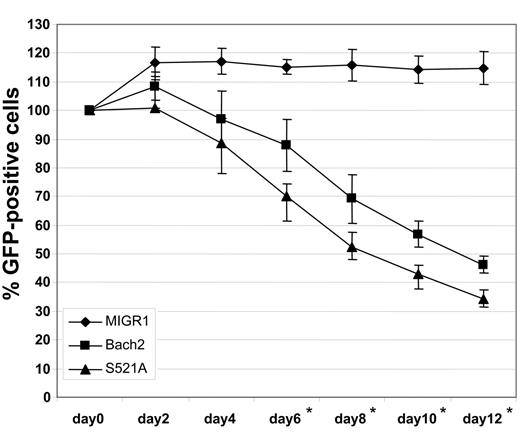
Bach2 has been previously shown to negatively regulate proliferation and induce apoptosis of BCR-ABL–negative cells.18 To examine whether the same phenomena could be observed in CML cells, BV173 cells were transduced with retroviruses expressing Bach2/IRES-GFP, Bach2S521A/IRES-GFP, or IRES-GFP (control) only, and the percentage of cells expressing GFP was measured every 2 days, starting 3 days after transduction (Figure 5). In control cells, this percentage remained roughly constant throughout the culture period. In contrast, the fraction of cells expressing Bach2 progressively decreased, and this was even more marked for cells carrying the Bach2S521A mutant. This demonstrates that Bach2 can impart a growth disadvantage to CML cells and that this is at least partly mediated by its phosphorylation status. Altogether, the data indicate that the mutant Bach2 causes greater impairment to cell survival than the wild-type protein, because the Bcr-Abl–induced phosphorylation cascade cannot ensure its retention in the cytoplasm. The constitutive migration to the nucleus in the presence of a Bcr-Abl–induced high level of ROSs6 may lead to increased cell death.
The Bach2S521A mutant induces poorer survival of CML cells than the wild type. BV173 cells were transduced with retroviruses encoding BACH2/IRES-GFP (WT), BACH2S521A/IRES-GFP (S521A), or IRES-GFP (control). GFP-positive cells were examined by FACS every 2 days. Results are presented as the average percentage of GFP-positive cells normalized as 100% on day 0 ± standard deviations from 3 independent experiments. *Comparison between the 3 types of cells shows a significant difference by the Mann-Whitney U test (P < .05).
The Bach2S521A mutant induces poorer survival of CML cells than the wild type. BV173 cells were transduced with retroviruses encoding BACH2/IRES-GFP (WT), BACH2S521A/IRES-GFP (S521A), or IRES-GFP (control). GFP-positive cells were examined by FACS every 2 days. Results are presented as the average percentage of GFP-positive cells normalized as 100% on day 0 ± standard deviations from 3 independent experiments. *Comparison between the 3 types of cells shows a significant difference by the Mann-Whitney U test (P < .05).
Bach2 regulates the HO-1 promoter
Although Bach2 has been shown to function as a proapoptotic factor, the target genes that effect this function have not been previously identified. Interestingly, it was recently reported that Bcr-Abl transcriptionally activates HO-1 expression, which has an antiapoptotic function and contributes to survival of BCR-ABL–positive cells.36 The expression of HO-1 is up-regulated by Bcr-Abl at the transcriptional level and is inhibited by treatment with imatinib. It is known that the promoter region of human HO-1, which is also recognized as an enhancer region in the murine gene, contains MARE sites regulated by Bach1, a close homolog of Bach2.37,38 Bach1 works as a transcriptional repressor and inhibits HO-1 expression but, in contrast to Bach2, does not possess proapoptotic function.18 Taken together, it is possible that Bach2 could inhibit HO-1 expression by binding to MAREs in its promoter region. Thus, in Ph-positive cells treated with imatinib, induction and dephosphorylation of Bach2 allowing it to translocate to the nucleus would result in repression of HO-1 and promotion of apoptosis. To test whether Bach2 regulates HO-1 expression, we ectopically expressed it in 293T cells, which normally do not express Bach2. Immunoblotting of total cell lysates with anti–HO-1 antibody showed that expression of HO-1 was clearly inhibited by Bach2, compared with the pattern in control vehicle-vector transfected cells (Figure 6A). Next, we determined whether Bach2 could transcriptionally inhibit HO-1 promoter activity by carrying out a reporter assay with a luciferase gene construct containing a 4.9 kb human HO-1 promoter region. This region contains 2 MARE sites, one of which is also referred to as a CRE site,23 homologous to 2 MARE sites previously identified in the murine HO-1 enhancer region.37 As shown in Figure 6B, Bach2 expression significantly repressed the HO-1 promoter-dependent reporter gene activity (lanes 3 and 4). Cotransfection of a promoterless reporter plasmid did not elicit any effect, indicating that the Bach2-induced luciferase repression was mediated via the HO-1 promoter (lanes 1 and 2). Furthermore, either of 2 luciferase constructs each containing a single mutant MARE (M1 or M2) showed less inhibition than the 4.9 kb wild-type promoter fragment (lanes 5 to 7), and there was no repression by Bach2 of a promoter possessing double mutants (M1+M2) or of a deleted construct containing only a 3.8 kb promoter region devoid of any MAREs (lanes 8 to 12). Transfection with Bcr-Abl alone was found to affect the basal activity of the HO-1 promoter (data not shown), as had been previously shown by Mayerhofer et al.36 For this reason, it was not possible to identify the role of Bach2, under the influence of Bcr-Abl, on the HO-1 promoter. These data demonstrate that Bach2 may inhibit HO-1 expression via the 2 MAREs in the HO-1 promoter, suggesting that HO-1 is a transcriptional target of Bach2.
Bach2 regulates the HO-1 promoter. (A) 293T cells were transiently transfected with vectors encoding BACH2 or vehicle (control) and harvested after 2 days. Cell lysates were separated on 10% SDS-PAGE and immunoblotted with anti–HO-1, anti-Bach2, and antiactin antibodies. The bar graph shows the densitometry of the bands indicating the HO-1/actin ratio. The image is representative of 4 independent experiments. (B) Jurkat cells were transiently transfected with luciferase reporter constructs and plasmids containing vehicle (-), 4.9 kb HO-1 promoter (4.9 kb), MARE mutant M1, mutant M2, both M1+M2 site mutants, and 3.8 kb promoter region (3.8 kb) devoid of MARE sites, together with Bach2 expression vector (+) or vehicle (-). Luciferase activity was examined after 24 hours and measured as luciferase–Renilla luciferase activity ratio. The results are the mean ± standard deviation of 3 independent experiments, each carried out in duplicate. *Differences in activity of the indicated assays in the presence or absence of Bach2 are statistically significant according to the Mann-Whitney U test (P < .05).
Bach2 regulates the HO-1 promoter. (A) 293T cells were transiently transfected with vectors encoding BACH2 or vehicle (control) and harvested after 2 days. Cell lysates were separated on 10% SDS-PAGE and immunoblotted with anti–HO-1, anti-Bach2, and antiactin antibodies. The bar graph shows the densitometry of the bands indicating the HO-1/actin ratio. The image is representative of 4 independent experiments. (B) Jurkat cells were transiently transfected with luciferase reporter constructs and plasmids containing vehicle (-), 4.9 kb HO-1 promoter (4.9 kb), MARE mutant M1, mutant M2, both M1+M2 site mutants, and 3.8 kb promoter region (3.8 kb) devoid of MARE sites, together with Bach2 expression vector (+) or vehicle (-). Luciferase activity was examined after 24 hours and measured as luciferase–Renilla luciferase activity ratio. The results are the mean ± standard deviation of 3 independent experiments, each carried out in duplicate. *Differences in activity of the indicated assays in the presence or absence of Bach2 are statistically significant according to the Mann-Whitney U test (P < .05).
Discussion
Oncoproteins use several molecular mechanisms to render a cell phenotypically abnormal and endowed with a growth advantage. In the case of Bcr-Abl, constitutive activation of the PI-3K pathway is fundamental for its antiapoptotic effect.39,40 This is exerted through up-regulation and/or activation of prosurvival molecules such as Bcl-XL and Bcl-2 and down-regulation/suppression of proapoptotic factors such as Bad and Bim.40-44 In the present study, we show that the transcription factor Bach2 is another important target of the PI-3K pathway in BCR-ABL–positive cells and that its defective function may contribute to their failure in undergoing apoptosis in the presence of oxidative stress.
We have observed that Bach2 is phosphorylated via PI-3K in B-lymphoid cells and that this can be reversed by treatment with LY294002. In Ph-positive lymphoid cells, excessive signaling via PI-3K leads to Bach2 existing predominantly in its phosphorylated form, with dephosphorylation being accomplished by inhibition of either Bcr-Abl itself or PI-3K.
By dissecting the downstream pathways from PI-3K/Akt, we found that ectopic expression of constitutively activated S6Ks led to marked phosphorylation of Bach2. Because S6K1 has been shown to be constitutively activated in BCR-ABL–positive cells,31 our findings suggest that Bcr-Abl phosphorylates Bach2 via PI-3K/S6K but mainly independently of mTOR. S6K1, also known as p70S6 kinase, and S6K2 are important downstream effectors of PI-3K and mTOR in the control of cell growth and are also regulated by MAPK/ERK.45-47 In CML cells, S6K appears to be activated not only via PI-3/Akt but also through other pathways, because Bcr-Abl SH2 domain mutants that fail to activate PI-3/Akt can still stimulate S6K.39 Moreover, the S6 ribosomal protein, an S6K substrate, is phosphorylated both in a Bcr-Abl kinase–, PI-3K–, and mTOR-dependent manner and by Abl-kinase–independent mechanisms in BCR-ABL–positive cells.48 These observations highlight the importance and complexity of the PI-3K/S6K pathway in BCR-ABL–positive cells.
S6K1 has been shown to phosphorylate several proteins such as BAD, CREM, eEF2k, CBP80, SKAR, and IRS-1 (reviewed by Richardson et al49 and Harrington et al50 ). Ribosomal S6 and eIF4B are phosphorylated by both S6K1 and S6K2.51 However, none of these substrates was shown to be regulated by S6Ks at the subcellular localization level. Thus, this is the first report on S6K being able to regulate the subcellular localization of one of its downstream effectors through its kinase activity. Nevertheless, it is important that the subcellular localization of Bach2 is also regulated through a CLS by Crm1/exportin1-dependent nuclear export12 and by sumoylation, which determines its nuclear accumulation into promyelocytic leukemia (PML) bodies.35 Moreover, it has been also shown that overexpression of its small Maf heterodimerization partners is capable of inducing nuclear translocation of Bach2.52 Structurally, Bach2 also possesses a nuclear localization sequence (NLS) in the basic region12 (K.I., unpublished data, July 1999). Altogether, the data suggest that the subcellular localization of Bach2 is regulated by multiple mechanisms. It is thus possible that Bcr-Abl affects its intracellular traffic not only by phosphorylation but also through these alternative pathways, because the nuclear translocation of Bach2 upon treatment with LY294002 is not complete and residual protein remains in the cytoplasm (Figure 4B).
Although we have shown that Bach2 is phosphorylated on S521 by the PI3-K/S6K pathway, it is not clear whether S6Ks phosphorylate Bach2 directly or indirectly. Thus, we cannot exclude the possibility that this serine could be phosphorylated not only by S6Ks but also by Akt, because the consensus sequences for Akt and S6Ks phosphorylation are similar and the phosphorylation of Bach2 is only partially inhibited by rapamycin. It is also possible that Bach2 can be phosphorylated on other aa residues. Nevertheless, S521 is undoubtedly an important target site of the PI-3K/S6K pathway because its phosphorylation clearly regulates the subcellular localization of Bach2 (Figures 3C and 4E), and its mutation affects the proliferation of CML cells (Figure 5). There are several mechanisms by which phosphorylation of S521 may regulate the location of Bach2. It is possible that phosphorylation of S521 interferes with the NLS or CLS functions. Alternatively, S521 could bind to 14-3-3, because the consensus sequence for 14-3-3 binding is similar to the sequence around S521, and binding of 14-3-3 to PI-3K and S6K phosphorylation sites has been reported in many proteins. Of note, PI-3K is known to regulate differentiation, activation, and proliferation of B cells.53 The expression pattern of Bach2 in B-cell lines and the phenotype of knockout mice demonstrate that Bach2 is important for B-cell differentiation.14,18 We have previously shown that PI-3K regulates the expression of Bach216 and show here that this pathway is also crucial for regulating its phosphorylation status. Taken together, the data suggest that the activity of Bach2 may be tightly regulated by the PI-3K pathway during B-cell differentiation. In CML cells, inhibition of Bach2 could lead to a differentiation arrest in transformed B cells, but further studies are needed to clarify the issue.
Under oxidative stress, cells respond by activating signaling pathways that will determine their fate (ie, survival or apoptosis). In B cells, it is known that the Syk phosphotyrosine kinase activates the PI-3K survival pathway and enhances cellular resistance to oxidative stress-induced apoptosis. On the other hand, Syk also accelerates apoptosis through its downstream effector PLC-γ2.54 This observation suggests that cells try to survive in response to relatively low levels of oxidative stress but undergo apoptosis if the stimulus and induced damage are too strong to sustain survival. This is supported by recent observations that low levels of oxidative stress lead to activation of Akt, but higher doses may induce obstruction of the pathway.55 Although it has been shown that ROSs induce phosphorylation of S6Ks,56,57 activation of these kinases may also follow the pattern described for Akt in response to higher levels of oxidative stress, because they are downstream of Akt. Therefore, in conditions of mild oxidative stress, Bach2 is phosphorylated by activated PI-3K/S6K and retained in the cytoplasm, allowing the cells to repair the damage and survive. Conversely, when cells find themselves under intense oxidative stress, Bach2 may be dephosphorylated through inhibition of the Akt/S6K pathway and translocates to the nucleus by disruption of Crm1-dependent nuclear export.12 The ROS-induced relocation of Bach2 to the nucleus may be only partially dependent on the S6K pathway and is likely to be also under concurrent control of the CLS and other mechanisms. In CML cells, constitutive phosphorylation of Bach2 induced by Bcr-Abl via Akt/S6Ks, as well as overall reduced expression of this transcription factor,16 may be major mechanisms by which they are resistant to oxidative stress. However, we cannot exclude at this time the role of mechanisms such as elevated expression of Bcl-XL, enhanced DNA repair, and others, which have also been associated to impaired apoptosis under conditions of mutagenic stimuli.41,58,59
The effector transcriptional targets of Bach2 involved in triggering the apoptotic process under conditions of oxidative stress are unknown. Here we show that HO-1 is one of the genes transcriptionally repressed by Bach2 and is likely to be involved in this process. Expression of HO-1 is regulated by several transcription factors, such as NF-E2–related factor 2 (Nrf2) and Bach1, both binding to the MAREs on its promoter.37,60 Nrf2 activates but Bach1 inhibits its expression, suggesting that MARE is an important binding element that is tightly regulated for transcription of HO-1. This is supported in our study and that of others23 by the fact that disruption of the 2 MAREs led to lower HO-1 promoter activity than that of the wild-type promoter, as shown in Figure 6B. HO-1 was originally identified as an enzyme that catalyzes oxidative degradation of heme to form biliverdin, carbon monoxide, and free ion. Its expression is induced by stress-inducing stimuli such as hypoxia, heavy metals, UV radiation, reactive nitric oxide, and ROSs.61 There is recent evidence that HO-1 also has cytoprotective and antiapoptotic functions against oxidative injury.61 Expression of HO-1 is high in various malignancies, and it has an antiapoptotic role in tumor growth.62-64 In CML, it is up-regulated by Bcr-Abl, and hemin-induced overexpression leads to in vitro resistance to imatinib.36 Because Bach2 is induced by treatment with imatinib in CML cells, this is likely to suppress the expression of HO-1, disabling its antiapoptotic function. Interestingly, in a recent microarray-based gene expression profiling of myeloma cells resistant to arsenic trioxide, a producer of ROSs, HO-1 was found as one of the most up-regulated and Bach2 the most down-regulated gene.65 These observations suggest that Bach2 may be one of the important regulators that serve to bridge the gap between ROSs and HO-1.
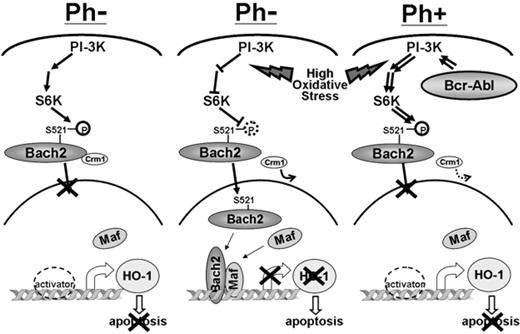
In summary, Bcr-Abl inhibits the nuclear translocation of Bach2 through phosphorylation of S521 via the PI-3K/S6K pathway and therefore suppresses Bach2 proapoptotic function. Altogether, the data presented here combined with work by others18,19 are consistent with a model in which Bach2 is a crucial mediator of the response to mutagenic stimuli (Figure 7). Cells such as CML lymphoid blasts harboring too little and constitutively phosphorylated Bach2 are unable to trigger the necessary apoptotic response under conditions of oxidative stress, and this may allow the accumulation of harmful mutations that contribute to transformation of the leukemia.
A model for the effect of Bach2 subcellular localization inBCR-ABL–positive (Ph+) and –negative (Ph−) cells. In normal conditions, Bach2 is phosphorylated via the PI-3K/S6K pathway and retained in the cytoplasm (left). Under intensive oxidative stress, the signal for Bach2 phosphorylation and the Crm1-dependent nuclear export is abolished; it migrates to the nucleus and inhibits HO-1 transcription, resulting in induction of apoptosis in Ph-negative cells (middle). In contrast, Bcr-Abl constitutively phosphorylates Bach2 via the PI-3K/S6K pathway, preventing its nuclear translocation in response to oxidative stress, allowing Ph-positive cells to survive even if carrying damaged DNA as a consequence of the mutagenic stimulus (right).
A model for the effect of Bach2 subcellular localization inBCR-ABL–positive (Ph+) and –negative (Ph−) cells. In normal conditions, Bach2 is phosphorylated via the PI-3K/S6K pathway and retained in the cytoplasm (left). Under intensive oxidative stress, the signal for Bach2 phosphorylation and the Crm1-dependent nuclear export is abolished; it migrates to the nucleus and inhibits HO-1 transcription, resulting in induction of apoptosis in Ph-negative cells (middle). In contrast, Bcr-Abl constitutively phosphorylates Bach2 via the PI-3K/S6K pathway, preventing its nuclear translocation in response to oxidative stress, allowing Ph-positive cells to survive even if carrying damaged DNA as a consequence of the mutagenic stimulus (right).
Authorship
Contribution: C.Y. performed experiments and wrote the draft manuscript; F.Y., D.E.S., S.M.H., D.I., and A.M. performed experiments; S.B. and K.I. provided advice on the design of the study, provided vital new reagents, and commented on the manuscript; and J.V.M. conceived and designed the study, supervised its execution, helped write the paper, and had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Junia V. Melo, Department of Haematology, Imperial College London, Hammersmith Hospital, Du Cane Road, London W12 0NN, United Kingdom; e-mail: j.melo@imperial.ac.uk.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by the Leukaemia Research Fund (United Kingdom). The authors thank all researchers who kindly donated materials and reagents and were cited in the relevant sections.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal