Abstract
In this study, we focused primarily on the antileukemic activity of interferon-β (IFN-β) in a murine xenograft model of acute myeloid leukemia (AML). Bolus administration of recombinant IFN-β via the subcutaneous or intravenous route failed to show efficacy in mice injected with AML cells despite achieving peak plasma IFN-β levels of more than 200 IU/mL. In contrast, stable expression of IFN-β following adeno-associated virus (AAV) vector–mediated gene transfer resulted in significant antileukemic activity against primary AML cells derived from patients with poor prognostic markers. An almost linear relationship was observed with stable plasma levels of IFN-β and antileukemic activity in mice. Even levels below 10 IU/mL were able to reduce tumor load by 50-fold when compared with control animals. These levels of IFN-β are likely to be nontoxic in humans. Therefore, approaches capable of maintaining stable plasma levels of IFN-β merit further clinical evaluation in patients with AML.
Introduction
Despite significant advances in the treatment of acute myeloid leukemia (AML), long-term survival is limited to about 30% of patients; hence the urgent need for alternative treatment strategies.1 Type I IFNs (IFN-α and IFN-β) have shown considerable promise in the treatment of many solid tumors2 and have proven to be highly effective in several hematologic malignancies, including chronic myeloid leukemia.3 Although in vitro studies with type I IFNs have shown impressive cytotoxicity against AML cells,4 their antitumor efficacy in vivo remains largely untested in animal models or in patients with AML, except for the United Kingdom Medical Research Council (UK MRC) AML 11 trial, in which maintenance treatment with IFN-α failed to prevent relapse or improve overall survival.5 However, it remains unclear whether this lack of efficacy was related to poor antileukemic effect in vivo or a failure to maintain stable therapeutic IFN-α levels because of its short half-life.6 In this study, we compare the antileukemic effects of IFN-α and β against a variety of AML cell lines in a xenograft model. Two different schedules of administration were used: the traditional high-dose multiple bolus injection approach using recombinant IFN-β, as well as continuous low-dose delivery, achieved here through gene transfer with adeno-associated virus (AAV) vectors.
Materials and methods
Cell culture and cell proliferation assays
The AML cell lines HL-60, KG1α, AML193, and OCI AML5 were cultured in RPMI medium with 10% fetal calf serum. GM-CSF at 100 pg/mL was added to the medium for AML193 and OCI AML5 cells. Frozen AML cells obtained from leukopheresis of patients with poor-risk AML were used for our primary AML experiments. Cell lines were seeded in 96-well plates at 20 000 cells per well to which varying concentrations of recombinant human IFNβ-1a or IFNα-2b were added. Following stimulation for 3 or 5 days, cells were pulsed with 3H-thymidine (0.5 μCi [0.0185 MBq] per well) for 4 hours, harvested, and 3H-thymidine incorporation measured by scintillation counting.
AAV2/8 hIFN-β and AAV2/8 hFIX vector production
As previously described, recombinant AAV vectors pseudotyped with serotype 8 capsid were generated by transient transfection of 293T cells using either the pAV2 CAGG hIFN-β or pAV2 CAGG human factor IX (hFIX) control vector plasmids together with pAAV2/8 packaging and pHTGI adeno helper plasmids.7 The AAV2/8 vectors were purified using an ion exchange chromatography method, and slot blot analysis was used to determine the titer of the vectors.
Human IFNα-2b and IFNβ-1a bioassay
Plasma levels of human IFN-α and IFN-β were determined using a validated antiviral assay with a sensitivity of 0.5 IU/mL as described before.8
AML murine models
Xenograft models of AML were established by injecting 1 × 107 primary AML cells or HL-60 cells (AML-M2 cell line) into the tail veins of sublethally (300 cGy) irradiated β2mnull nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice as described before.9 Infiltration of organs by AML cells was assessed using flow cytometric and immunohistochemical staining for human CD45. Primary AML cells exclusively infiltrated the bone marrow, while HL-60 cells also infiltrated the spleen and liver.
Immunohistochemistry and TUNEL assay
Formalin-fixed paraffin-embedded tissue sections were stained with a primary monoclonal anti–human CD45RB antibody (M0833, 1:100 dilution; Dako, Carpinteria, CA) after blocking with a mouse immunoglobulin–blocking reagent (PK-2200; Vector Laboratories, Burlingame, CA). Slides were then incubated with a biotinylated goat antimouse antibody and stained with streptavidin peroxidase before final addition of a chromogenic substrate (Dako ChemMate Detection kit K5001).
Frozen sections from subcutaneously implanted HL-60 tumors as well as formalin-fixed paraffin-embedded tissue sections from intravenously injected HL-60 leukemic mice were used for TUNEL staining. Frozen sections were fixed in 4% paraformaldehyde, rehydrated in PBS, and permeabilized in 1% Triton X-100, 0.1% sodium citrate. Paraffin sections were dewaxed, rehydrated, and proteinase K treated prior to permeabilization. A fluorescein TUNEL labeling kit (Roche Diagnostics, West Sussex, United Kingdom) was then used to label apoptotic cells followed by counterstaining with DAPI. Slides were analyzed by fluorescence microscopy.
Results and discussion
Effects of IFN-α and β on AML cell lines in vitro and in vivo
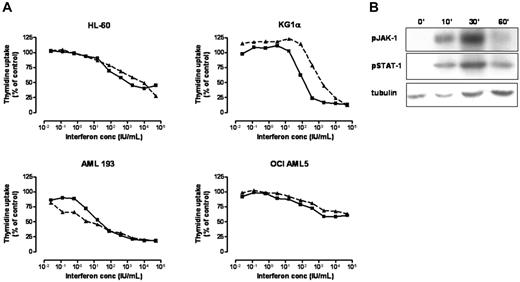
We compared the activity of IFNα-2b and IFNβ-1a against a range of AML cell lines (HL-60, OCI AML5, AML193, KG1α). After 3 days of in vitro culture we observed significant and equivalent antiproliferative activity, as assessed by a thymidine uptake assay, with both hIFN-α and hIFN-β against all AML cell lines. The minimum dose required for both agents was 10 IU/mL with a reduction in proliferation of 50% to 80% occurring at a dose of approximately 1000 IU/mL. Higher IFN concentrations did not result in any further reduction in AML cell proliferation (Figure 1A). In keeping with published results in other tumor types,10 the biologic effects of hIFN-β on AML cells appeared to be mediated largely through activation of the JAK-STAT signaling pathway, as demonstrated by phosphorylation of JAK-1 and STAT-1 in HL-60 cells in response to hIFN-β stimulation (Figure 1B).
Effects of recombinant hIFN-α/β on AML proliferation and cell signaling. (A) 3H-thymidine uptake assay showing a linear reduction in proliferation with an increasing dose of hIFN-α (dashed line) or hIFN-β (solid line) following stimulation of AML cell lines in vitro for 3 days. (B) Western blot of HL-60 cells showing phosphorylation of JAK-1 and STAT-1 in response to hIFN-β stimulation at time points of 0, 10, 30, and 60 minutes.
Effects of recombinant hIFN-α/β on AML proliferation and cell signaling. (A) 3H-thymidine uptake assay showing a linear reduction in proliferation with an increasing dose of hIFN-α (dashed line) or hIFN-β (solid line) following stimulation of AML cell lines in vitro for 3 days. (B) Western blot of HL-60 cells showing phosphorylation of JAK-1 and STAT-1 in response to hIFN-β stimulation at time points of 0, 10, 30, and 60 minutes.
Having demonstrated the antileukemic activity of hIFN-α and hIFN-β in vitro, we next assessed their in vivo efficacy in a xenograft model of AML. We chose to focus on hIFN-β because of its reported stronger antiproliferative activity against other tumor types.11-14 The use of our immunodeficient model offers an opportunity to selectively assess the antiproliferative effects of hIFN-β in the absence of its immunomodulatory15 and direct antiangiogenic properties,16 because hIFN-β does not cross-react with its cognate murine receptor.17 In our initial experiments, recombinant hIFN-β was administered either subcutaneously (2000 IU per mouse thrice weekly) or intravenously (60 000 IU per mouse daily) into cohorts of β2mnull NOD/SCID mice a week after intravenous injection with HL-60 cells with an additional untreated cohort (n = 10) serving as controls. The dose administered weekly was approximately 3-fold or 200-fold higher for the subcutaneous or intravenous cohorts, respectively, than the equivalent standard adult human dose of hIFN-β given clinically.18 Despite administering such high doses, hIFN-β was undetectable in the plasma of animals treated subcutaneously (n = 4) at any time point. In contrast, at 2 hours after intravenous injection (n = 9) the mean plasma level of hIFN-β was 400 IU/mL (range, 200 to 1000 IU/mL), which declined to undetectable levels by 24 hours, consistent with its short half-life. Four weeks after injection of HL-60 cells, heavy but equivalent levels of tumor infiltration were observed in all 3 cohorts (Table 1). These results contrast with the in vitro effects of hIFN-β on AML cells and may be explained either by its rapid clearance or by its poor biologic activity in vivo.
Outcome of recombinant hIFN-β treatment of HL-60 leukemic mice
| Treatment cohort . | Proportion of animals with significant disease* . | Peak plasma hIFN-β levels, IU/mL . | Trough plasma hIFN-β levels, IU/mL . |
|---|---|---|---|
| Controls | 10/10 | < 0.5 | < 0.5 |
| Subcutaneous hIFN-β | 4/4 | < 0.5 | < 0.5 |
| Intravenous hIFN-β | 8/10† | 400 ± 100 | < 0.5 |
| Treatment cohort . | Proportion of animals with significant disease* . | Peak plasma hIFN-β levels, IU/mL . | Trough plasma hIFN-β levels, IU/mL . |
|---|---|---|---|
| Controls | 10/10 | < 0.5 | < 0.5 |
| Subcutaneous hIFN-β | 4/4 | < 0.5 | < 0.5 |
| Intravenous hIFN-β | 8/10† | 400 ± 100 | < 0.5 |
Peak levels were measured at 2 hours after injection; trough at 24 hours.
More than 30% infiltration.
Two early deaths from radiation toxicity.
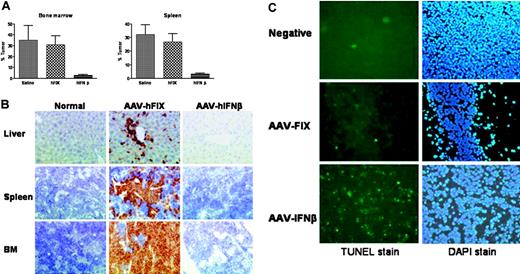
To resolve this issue we established stable long-term expression of hIFN-β in vivo following gene transfer with AAV2/8 vectors encoding hIFN-β. We have previously demonstrated success with this approach in the treatment of solid tumors in murine models.19 Systemic administration of AAV2/8 vectors results in rapid and high-level transgene expression from the liver, which is preferentially transduced.20 β2mnull NOD/SCID mice were injected with HL-60 cells as previously, and a week later AAV2/8 hIFN-β was administered at a dose of 1 × 1011 vector particles. Peak plasma hIFN-β levels measuring 20 000 to 100 000 IU/mL were achieved within a week of vector injection and were stably maintained until the mice were culled after 4 weeks. Six of 8 animals in the hIFN-β–transduced cohort had no evidence of leukemia when analyzed by flow cytometry or immunohistochemistry. In contrast, all control mice (n = 7) expressing hFIX after AAV-mediated gene transfer had widespread evidence of leukemic infiltration at levels observed in saline-treated controls (n = 6) (Figure 2A-B). TUNEL staining confirmed increased apoptosis of HL-60 cells within AAV hIFN-β–transduced animals compared with tumors within control mice (Figure 2C), which is consistent with our in vitro observations (data not shown). The antileukemic effect of hIFN-β seen here is unlikely to be mediated by direct in vivo transduction of AML cells by AAV, because HL-60 cells are refractory to AAV-mediated gene transfer.21,22
Antileukemic efficacy of AAV2/8-mediated hIFN-β expression in a xenograft AML model. (A) HL-60 tumor burden in β2mnull NOD/SCID mice following AAV2/8 hIFN-β gene transfer; quantitative results from fluorescence-activated cell sorter (FACS) analysis of bone marrow and spleen single-cell suspensions. Control animals included a saline-treated cohort and a cohort receiving AAV2/8 hFIX vector. Error bars represent SEM. (B) Human CD45 immunohistochemistry confirming the widespread tissue infiltration of control hFIX-transduced mice with HL-60 cells, in marked contrast to hIFN-β–transduced animals. (C) TUNEL staining of subcutaneous HL-60 tumor implants following systemic transduction with AAV2/8 hFIX or AAV2/8 hIFN-β. The negative tumor control was stained without prior treatment with TdT enzyme. All slides were counterstained with DAPI. Slides were examined using an Olympus BX-51 microscope equipped with a 40×/0.85 numerical aperture oil objective (Olympus, Melville, NY). Images were captured using an Olympus digital camera model U-TVO using the SIS image analysis system (Soft Imaging Systems, Münster, Germany). Adobe Photoshop version 5 (Adobe Systems, San Jose, CA) was used to edit the images.
Antileukemic efficacy of AAV2/8-mediated hIFN-β expression in a xenograft AML model. (A) HL-60 tumor burden in β2mnull NOD/SCID mice following AAV2/8 hIFN-β gene transfer; quantitative results from fluorescence-activated cell sorter (FACS) analysis of bone marrow and spleen single-cell suspensions. Control animals included a saline-treated cohort and a cohort receiving AAV2/8 hFIX vector. Error bars represent SEM. (B) Human CD45 immunohistochemistry confirming the widespread tissue infiltration of control hFIX-transduced mice with HL-60 cells, in marked contrast to hIFN-β–transduced animals. (C) TUNEL staining of subcutaneous HL-60 tumor implants following systemic transduction with AAV2/8 hFIX or AAV2/8 hIFN-β. The negative tumor control was stained without prior treatment with TdT enzyme. All slides were counterstained with DAPI. Slides were examined using an Olympus BX-51 microscope equipped with a 40×/0.85 numerical aperture oil objective (Olympus, Melville, NY). Images were captured using an Olympus digital camera model U-TVO using the SIS image analysis system (Soft Imaging Systems, Münster, Germany). Adobe Photoshop version 5 (Adobe Systems, San Jose, CA) was used to edit the images.
Parallel administration of either AAV2/8 hIFN-α or AAV2/8 hIFN-β vector at a dose of 3 × 1010 vector particles per animal (n = 4 each cohort) resulted in disparate levels of IFN expression (3000 ± 400 and 6000 ± 400 IU/mL, respectively) due to the inherent variability in biologic activity of different vector stocks. The tumor load in hIFN-α–expressing mice was substantially lower than in control hFIX-transduced animals (10.7% ± 5.4% versus 76.3% ± 5%, respectively) but higher than that in the hIFN-β cohort (0.9% ± 0.5%). The higher residual tumor load in the hIFN-α cohort is probably a reflection of lower plasma hIFN-α levels, although differences in antitumor activity in vivo between hIFN-α and hIFN-β, as observed in many solid tumors, cannot be excluded. Nevertheless, we have shown that stable expression of both hIFN-α and β at supraphysiological levels results in significant antileukemic activity against HL-60 cells in vivo, which appears to be mediated (at least in part) through a proapoptotic mechanism.
Effects of IFN-β on primary AML cells in vivo
We next evaluated the effects of stable hIFN-β expression on the proliferation of primary AML cells in vivo. In separate sets of experiments, β2mnull NOD/SCID mice were implanted with 1 × 107 primary leukemic cells taken from 3 different patients with poor-risk AML (high white cell count, Flt3-ITD mutation) followed, 2 weeks later, by transduction with 1 × 1011 vector particles of AAV2/8 hIFN-β or an equivalent dose of AAV2/8 hFIX. After 6 weeks mice were analyzed, and in all 3 experiments there was clear evidence of AML infiltration within the bone marrow of control animals (n = 11) whereas none of the hIFN-β–transduced mice (n = 12) had any evidence of leukemia (Table 2). Plasma levels of hIFN-β in these experiments ranged from 7000 to 90 000 IU/mL (median, 48 000 IU/mL).
Phenotypic characteristics of AML donors used in our experiments and outcome of engraftment studies following AAV2/8-mediated hIFN-β expression in vivo
| Patient no. . | Patient characteristics . | Proportion of mice engrafted with AML . | |||
|---|---|---|---|---|---|
| AML . | Flt3/ITD . | WBC count, × 109/L . | hFIX transduced . | hIFN-β transduced . | |
| 1 | M4 | Flt3/ITD+ | 308 | 3/3 | 0/4 |
| 2 | M1 | Flt3/ITD+ | 150 | 5/5 | 0/4 |
| 3 | M1 | Flt3/ITD+ | — | 3/4* | 0/4 |
| Patient no. . | Patient characteristics . | Proportion of mice engrafted with AML . | |||
|---|---|---|---|---|---|
| AML . | Flt3/ITD . | WBC count, × 109/L . | hFIX transduced . | hIFN-β transduced . | |
| 1 | M4 | Flt3/ITD+ | 308 | 3/3 | 0/4 |
| 2 | M1 | Flt3/ITD+ | 150 | 5/5 | 0/4 |
| 3 | M1 | Flt3/ITD+ | — | 3/4* | 0/4 |
Primary AML engraftment was defined as more than 1% tumor infiltration of bone marrow by CD45 FACS analysis.
WBC indicates white blood cells; —, not available.
One death from radiation toxicity.
In subsequent experiments, 3 doses of AAV2/8 hIFN-β vector (1 × 1011, 1 × 1010, or 1 × 109 particles per animal) were administered into cohorts of mice injected 2 weeks previously with primary AML cells. The degree of AML infiltration within bone marrow was found to correlate directly with mean plasma hIFN-β levels (Figure 3) with a 700-fold and 140-fold reduction in tumor load at plasma hIFN-β levels of 55 000 ± 12 000 and 1500 ± 130 IU/mL, respectively. Significantly, a 50-fold reduction in primary AML infiltration was seen even when plasma levels were less than 10 IU/mL (4 ± 0.8 IU/mL). Pharmacokinetic studies in humans have shown a great deal of variability in plasma hIFN-β levels achieved following recombinant hIFN-β treatment, but levels below 10 IU/mL have been relatively well tolerated.23
Efficacy of hIFN-β expression against primary AML cells in vivo. (A) Plasma hIFN-β levels in β2mnull NOD/SCID mice following AAV2/8-mediated transduction at different vector doses. Measurements were made in duplicates on 2 separate occasions. (B) Effects of plasma hIFN-β levels on primary AML proliferation in vivo; quantitative results from FACS analysis of bone marrow.
Efficacy of hIFN-β expression against primary AML cells in vivo. (A) Plasma hIFN-β levels in β2mnull NOD/SCID mice following AAV2/8-mediated transduction at different vector doses. Measurements were made in duplicates on 2 separate occasions. (B) Effects of plasma hIFN-β levels on primary AML proliferation in vivo; quantitative results from FACS analysis of bone marrow.
Our study demonstrates that type I interferons α and β have significant activity against AML in the setting of a xenograft model. Detailed analysis with hIFN-β showed that sustained expression even at low but potentially clinically tolerable levels, achieved here by gene transfer, resulted in significant inhibition of leukemic cell proliferation in vivo. These results suggest that alternative delivery strategies such as the use of continuous intravenous or subcutaneous administration protocols or the longer-acting pegylated form of IFN-β may prove more effective in patients with AML.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Amit C. Nathwani, Department of Haematology, University College London, 98 Chenies Mews, London WC1E 6HX, United Kingdom; e-mail: a.nathwani@ucl.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by the Kay Kendall Leukaemia Fund, which provided R.B. with a Clinical Research Fellowship.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal