Abstract
Recent evidence suggests that mutations in the Gata1 gene may alter the proliferation/differentiation potential of hemopoietic progenitors. By single-cell cloning and sequential replating experiments of prospectively isolated progenitor cells, we demonstrate here that the hypomorphic Gata1low mutation increases the proliferation potential of a unique class of progenitor cells, similar in phenotype to adult common erythroid/megakaryocytic progenitors (MEPs), but with the “unique” capacity to generate erythroblasts, megakaryocytes, and mast cells in vitro. Conversely, progenitor cells phenotypically similar to mast cell progenitors (MCPs) are not detectable in the marrow from these mutants. At the single-cell level, about 11% of Gata1low progenitor cells, including MEPs, generate cells that will continue to proliferate in cultures for up to 4 months. In agreement with these results, trilineage (erythroid, megakaryocytic, and mastocytic) cell lines are consistently isolated from bone marrow and spleen cells of Gata1low mice. These results confirm the crucial role played by Gata1 in hematopoietic commitment and identify, as a new target for the Gata1 action, the restriction point at which common myeloid progenitors become either MEPs or MCPs.
Introduction
Among the GATA family of transcription factors,1 Gata1 exerts a specific role in the control of erythroid,2 megakaryocytic,3,4 eosinophil,5 and mast6 cell differentiation. Genetic alterations of this gene, however, are not only associated with X-linked inherited erythroid or megakaryocytic disorders, but are also found in acquired myeloproliferative disorders. Each mutation is associated with a specific abnormality: point mutations that abrogate the ability of the amino-terminal zinc finger domain of the protein to bind either DNA or Fog1, a partner of Gata1, are found in inherited disorders.7-10 On the other hand, frame shift and splice mutations encoding GATA1s, a protein lacking the amino-terminal domain, are associated not only with impaired inherited erythropoiesis,11,12 but are also found in patients with megakaryocytic leukemia in Down syndrome,13,14 in newborns with transient myeloproliferative syndromes,15 and in one adult patient with megakaryocytic leukemia.16 These observations suggest that, in addition to its effect on terminal differentiation, Gata1 might control the biologic properties of hematopoietic progenitor cells, predisposing them to accumulate secondary mutations in a multistep leukemogenic process. However, direct proof for a possible function of Gata1 in progenitor cells has not been provided as yet.
We had previously described that hematopoietic tissues from mice carrying the hypomorphic Gata1low mutation contain high numbers (∼10%) of “unique” progenitor cells that generate colonies composed of erythroblasts, megakaryocytes, and mast cells.6 Predicted by the stochastic model of hematopoietic commitment,17 such a trilineage progenitor has not been isolated prospectively as yet from the marrow of normal mice. In fact, antigenic profiling has prospectively divided normal murine progenitors into myeloid- and mast cell-restricted. The myeloid-restricted ones are further divided into granulomonocytic progenitors (GMPs), megakaryocytic-erythroid progenitors (MEPs), and common myeloid progenitors (CMPs).18 GMPs correspond to cells previously defined, by functional clonogenesis, as colony-forming cells that generate in 7 days granulocytic, monomacrophagic and granulomonocytic colonies (CFU-Gs, CFU-Ms, and CFU-GMs). MEPs, on the other hand, include cells once functionally defined as those that generate megakaryocytic or erythroid colonies either in 3 days (CFU-MKsday3 and CFU-Es) or 7 days (CFU-MKsday7 and BFU-Es). CMPs were functionally defined as multilineage progenitor cells, that is, those that generate colonies of multiple lineages after 12 to 15 days either in vitro (CFU-mix) or in vivo (spleen colony-forming cells, CFU-Ssday12). Mast cells are localized in extramedullary sites where they engage themselves in the process of allergic response and in the immune reaction against parasites.19,20 As all the other hematopoietic cells, they derive from progenitor cells present in the marrow (and in the spleen) of the mouse. The marrow mast cell-restricted progenitor cells (MCPs) are characterized by the phenotype Lin−c-Kit+Sca-1−Ly6c−FcϵRIα−CD27−β7+ T1/ST2+.21 MCPs normally complete their maturation in extramedullary sites22 through a pathway that involves first up-regulation of c-Kit and T1/ST2 expression (c-Kithigh/T1/ST2high) and, then, induction of the expression of tissue-restricted mast cell proteases (MMCPs)23,24 and of the receptor that binds with high affinity the Fc portion of IgE (FcϵRI). In contrast with most hemopoietic lineages, mastocytic cells retain extensive proliferative activity until completely mature. MCPs give rise in vitro to mast cell colonies within 7 days of culture. On the other hand, CMPs generate MCPs, in addition to GMPs and MEPs, both in vivo and in vitro.21
The aim of this study was to clarify the role of Gata1 in hematopoietic commitment by identifying the antigenic profile and proliferation potential of the Gata1low progenitors giving rise to trilineage colonies. First, we compared the number and function of mast cells generated in bone marrow-derived mast cell cultures (BMMCs) seeded with marrow from Gata1low and wild-type (positive controls) mice. Heterozygous W/Wv mice (ie, expressing the W, truncated, and Wv, kinase defective, form of c-Kit25 ), that do not express mast cells in vivo but whose marrow generates defective mast cells in vitro, were used as negative controls. Next, we examined the frequency of CMPs, MEPs, and MCPs in marrow and spleen from wild-type and Gata1low littermates. The different populations were also isolated and their differentiation and proliferation potential characterized under conditions of limiting dilution followed by single cell recloning. Our results confirm that mast cell differentiation is defective in Gata1low mice (decreased differentiation and increased proliferation). The defect includes the ability to generate, with high frequency, factor-dependent trilineage cell lines. In Gata1low mice, the frequency of cells with the antigenic profile of CMPs, MEPs, and GMPs was normal in marrow and markedly increased in spleen, whereas those with the MCP profile were not detectable. However, mutant cells isolated according to the MEP phenotype, had the “unique” property to generate mast cells and their precursors in 7 days of culture, in addition to erythroblasts and megakaryocytes. Furthermore, the progeny of about 10% of mutant MEPs could be propagated in culture, as single cells, with 95% efficiency, for up to 4 months. In comparison, the progeny of wild-type MEPs became extinguished in 7 to 14 days. These results indicate that cells with the phenotype corresponding to myeloid, but not those corresponding to mastocytic, progenitors are detectable in tissues from Gata1low mice. However, in these mice, the mast cell-generating activity is abnormally acquired by MEPs, which, therefore are antigenically, but not functionally, equivalent to wild-type MEPs. These results indicate that the Gata1low mutation also targets cells at the restriction point between CMPs and either MCPs or MEPs.
Materials and methods
Mice
Mice carrying the hypomorphic Gata1low mutations (ie, deletion of the first enhancer and of the distal promoter of the gene)26 were bred at the Istituto Superiore di Sanità. Transgenic μLCR-hGATA1 males27,28 (carrying the hGATA1 cDNA under the control of a μLCR linked to the β-globin promoter), were crossed with homozygous Gata1low/low females. Because Gata1 is on the X chromosome whereas μLCR-hGATA1 is autosomal, 50% of the male Gata1low/0 offspring will carry the transgene. Offspring were genotyped by polymerase chain reaction (PCR) at birth, as described,29 and μLCR-hGATA1−Gata1low/0 males used as negative controls. WBB6FAW/Wv mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed under good animal care practice conditions and all the experiments were performed with 3- to 4-month old males, under protocols approved by the institutional animal care committee.
Prospective isolation of hematopoietic progenitors
Myeloid (CMP and MEP) and mast cell (MCP) progenitors were purified according to modifications30 of procedures described by Akashi et al18 and Chen et al.21 Briefly, mononuclear cell suspensions were labeled with the biotinylated rat antimouse antibodies CD3, CD45R/B220, CD19, CD127 (IL-7Rαchain), TER-119, IgM, Thy 1.1, Thy 1.2, Sca-1, and Gr-1 (PharMingen, San Diego, CA). Mature (Lin+) cells were removed by binding to sheep anti–rat IgG–conjugated magnetic beads (Dynabeads M-450; Dynal Biotech, Oslo, Norway). The cells eluted from the beads (Lin−) were divided in 2 aliquots. One aliquot was processed for purification of myeloid progenitors by staining with fluorescein isothiocyanate (FITC)–CD34, allophycocyanin (APC)–CD117 (c-kit), phycoerythrin (PE)–CD16/CD32 (FcRγIII/II; PharMingen), and streptavidin-Cy7-PE (Caltag Laboratories, Burlingame, CA). The second aliquot was used for purification of MCPs by staining with FITC-T1/ST2 (BD Biosciences, St. Paul, MN), PE-CD117, and streptavidin-Cy7-PE. Cells labeled with fluorochrome-conjugated isotype antibodies (PharMingen) were used to gate nonspecific signals; dead cells were excluded by propidium iodide staining (5 μg/mL, Sigma, St Louis, MO). Cell analysis and sorting were performed with the FACSAria (Becton Dickinson, San Jose, CA) equipped with 3 air-cooled and solid state lasers (488 nm, 633 nm, and 407 nm).
Flow cytometry
Cells were suspended in Ca++Mg++-free phosphate-buffered saline (PBS) containing 0.5% (vol/vol) bovine serum albumin (BSA; Sigma) and 2 mM EDTA. Mast cells were recognized by labeling with PE-CD117, FITC-CD34, FITC-T1/ST2, and FITC–anti-FcϵRIα (MAR-1; eBioscience, San Diego, CA). Other mature phenotypes analyzed were represented by PE-TER119/FITC-CD71 (erythroblasts), PE-CD61/FITC-CD41 (megakaryocytes), PE-Gr1/FITC-Mac3 (monocytes and granulocytes), and PE-CD11c/FITC-B220 (neutrophils and B cells). Purified anti-CD16/CD32 (FcγRIII/II) was included, as blocker, in all the analyses. Unless stated otherwise, antibodies were from PharMingen and incubated at a concentration of 1 μg/106 cells for 30 minutes on ice. Nonspecific signals and dead cells were excluded, respectively, by appropriate fluorochrome-conjugated isotype and propidium iodide staining. Cell fluorescence was analyzed with the FACSAria (Becton Dickinson).
Liquid cultures of prospectively isolated progenitor cells
MEPs, CMPs, and MCPs (1 × 103 cells/mL) were cultured for 6 to 21 days in Iscove modified Dulbecco medium (IMDM; Gibco, Invitrogen, Carlsband, CA) supplemented with fetal calf serum (FCS, 10% vol/vol; Sigma), 7.5 × 10−5 M β-mercaptoethanol (Sigma), penicillin, and streptomycin sulfate (50 U/mL, Gibco), and glutamine (2 mM, Gibco). Single-cell cloning experiments were performed by limiting dilution, that is, by resuspending the cells as 3 cells/mL and culturing 100 μL of this solution in each of a 96-well plate.31 Cultures were stimulated either with rat SCF (100 ng/mL; Amgen, Thousand Oaks, CA) and murine IL-3 (10 ng/mL, PeproTech, London, United Kingdom)32,33 or with a cocktail containing rat SCF (10 ng/mL), murine IL-3 (10 ng/mL), and GM-CSF (10 ng/mL; PeproTech) and human FLT3-ligand (10 ng/mL; PeproTech), IL-11 (10 ng/mL; PeproTech), thrombopoietin (TPO, 50 ng/mL; PeproTech), and erythropoietin (EPO, 3 U/mL; Hoffman-La Roche, Basel, Switzerland).34 The cultures were incubated at 37°C in a humidified incubator containing 5% CO2 in air and cell growth and phenotype analyzed 6 to 7, 14, and 21 days later. The proliferation potential of the progenitor cell progeny was evaluated by randomly selecting 10 wells and by culturing their content under conditions of limiting dilution. The wells were scored 7 days later for sign of cell proliferation and subcloned again as long as growth was detected.
BMMCs
Marrow and spleen cells (1 × 105 cells/mL) from wild-type, Gata1low, and W/Wv mice were cultured in IMDM supplemented with FBS (30% vol/vol), BSA (1.5% wt/vol), glutamine (2 mM), penicillin and streptomycin sulfate (50 U/mL), SCF (100 ng/mL), and IL-3 (10 ng/mL), as described.6,32,33 Every 3 to 4 days, cells were counted and the cultures replenished with fresh medium to maintain cell density at 2.5 × 105/mL.
Culture of the SN cell lines
The SN cell lines were obtained from Gata1low BMMCs and passed twice a week in IMDM supplemented with FBS (20% vol/vol), BSA (1.5% wt/vol), glutamine (2 mM), penicillin and streptomycin sulfate (50 U/mL), SCF (100 ng/mL), and IL-3 (10 ng/mL).
Immunohistochemistry
Cytocentrifuged cell preparations (3 × 103-104 cells/smear) were stained either with May-Grünwald Giemsa (Sigma), benzidine, acetyl cholinesterase, or Alcian blue, according to standard protocols.6
RNA isolation and RT-PCR analysis
Total RNA was prepared with TRIzol (Gibco), using glycogen (20 μg/L; Hoffman-LaRoche), as carrier. For semiquantitative analysis, RNA (1 μg) was reverse transcribed with random hexamers and Moloney murine leukemia virus reverse transcriptase (RT; Perkin-Elmer, Downers Grove, IL) and cDNA was amplified with the specific sense and antisense primers described in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). The reaction was performed in 100 μL 10 mM Tris-HCl, pH 8.3, containing MgCl2 (2 mM), dNTP (200 μM each), 0.1 μCi (0.037 MBq) [α32 P]dCTP (specific activity 3000 Ci/mmol, Amersham Italia, Cologno Monzese, Italy) and 2 U AmpliTaq DNA polymerase. Primers specific for β2-microglobulin (50 nM each) were added to each amplification after the first 10 cycles as control for the amount of cDNA.6 The reactions were done with the GeneAmp 2400 thermocycler (Perkin-Elmer) and analyzed in the linear amplification range defined by preliminary experiments. Positive (RNA from 32D35 and 32D Epo36 cells) and negative (mock cDNA) controls were included in each experiment. Aliquots (20 μL) were removed from the PCR mixture after selected cycles and the amplified bands separated by electrophoresis on 4% polyacrylamide gel. Gels were dried using the Bio-Rad apparatus (Hercules, CA) and exposed to Hyperfilm-MP (Amersham, Munich, Germany) for 2 hours at −70 °C. All procedures were done according to standard protocols.37 For quantitative analysis, cDNA was synthesized using random primers and SuperScript III (Invitrogen Life Technologies, Bethesda, MD) and amplified with specific TaqMan PCR kits (PE Applied Biosystems, Foster City, CA), as recommended by the manufacture, with the ABI PRISM 7700 Sequence Detection System (PE Applied Biosystems). Quantitative values were obtained from the threshold cycle number (Ct), by subtracting the average Ct of the target gene from that of Gapdh and expressed as 2−ΔCt.
Serotonin-release assay
Cells (1 × 106/mL) were incubated with 5-hydroxy[3H] tryptamine trifluoroacetate (3H-serotonin, 2 μCi/mL [0.074 MBq]); 84.0 Ci/mmol, Amersham) for 6 hours at 37°C, washed twice, and incubated again, at a concentration of 20 × 106/mL, for 1 hour on ice either with 10 μg/mL monoclonal mouse anti–DNP-IgE (clone SPE-7, Sigma) or medium alone. After washing, cells were divided into aliquots (0.4 × 106/50 μL) and stimulated, for 15 minutes at 37°C, with either medium alone, 2 μg/mL monoclonal rat anti–mouse IgE (R35-72, PharMingen), 1 μg/mL DNP-human serum albumin (Sigma), or 1 μg/mL ionomycin (Sigma). Reactions were terminated with 50 μL cold Hanks balanced salt solution (Sigma), cells removed by centrifugation and levels of [3H]serotonin in supernatants measured with the Packard 1600TR liquid scintillator counter (Perkin Elmer). Total 3H-serotonin incorporation was determined by lysing unstimulated cells with 1% Triton X-100 (Sigma). The amount of 3H-serotonin released by the cells was calculated as counts per minute in the supernatant ÷ counts per minute of total incorporation × 100 (see also Rodewald et al22 ).
Statistical analysis
Statistical analysis was performed by analysis of variance (ANOVA test) using Origin 6.1 software for Windows (Microcal Software, Northampton, MA).
Results
Hematopoietic tissues from Gata1low mice contain cells with the antigenic profile of myeloid progenitors but lack those with the mastocytic progenitor profile
Normal progenitor cell subclasses were defined according to the antigenic profiles summarized in Figure 1A. Cells with MCP, MEP and CMP phenotype were detected, at the expected frequencies, in marrow from wild-type animals (Figure 1B; Table 1). The fact that these phenotypes discriminate different populations was confirmed by the observation that the sorted cells retained their phenotype on reanalyses (> 80% purity in all cases; Figure 1B). Lin−c-Kit+ cells from marrow (and spleen) of Gata1low mice were prospectively divided by CD34-CD16/CD32 staining into populations similar to those observed in wild-type tissues (Figure 1B; Table 1) that, once sorted, retained their phenotype on reanalysis (> 88% purity). In contrast, T1/ST2 staining failed to label Lin−c-Kit+ marrow (and spleen) cells from Gata1low mice (Figure 1B; Table 1). To prove that T1/ST2+ cells were indeed absent in Gata1low tissues, Lin−c-Kit+ cells (myeloid progenitors [MPs]) were artificially divided into those in the third lower (MPlow) and third higher (MPhigh) portion of the T1/ST2 profile (Figure 1B). The profile of the 2 populations overlapped on reanalysis (< 60% pure), confirming that T1/ST2+ cells are rare among Gata1low Lin−c-Kit+ cells.
The marrow of Gata1low mice contains progenitor cells with the myeloid, but not with the mastocytic, antigenic profile. (A) Diagrammatic scheme of the antigenic profiling used to define different progenitor cell types in this study. All myeloid and mast cell generating activity is present among the cKit+ cells of the Lin−Sca1−Ly6c−FcϵRIα[−] cells of normal marrow.18,21 The myeloid generating cells are divided by CD34 and CD16/CD32 staining into GMPs (CD34+CD16/CD32+, not shown), MEPs (CD34lowCD16/CD32low), and CMPs (CD34+CD16/CD32low).18 GMPs correspond to progenitor cells functionally defined as CFU-Gs, CFU-Ms, and CFU-GMs, and are not investigated in detail in this study. MEPs are cells restricted toward megakaryocyte and erythroid differentiation and include those cells once functionally defined as CFU-MKday3 and CFU-MKday7, CFU-E, and BFU-E. CMPs give rise to GMPs, MEPs, and MCPs, and correspond to cells functionally defined as capable to generate in vitro CFU-mix and in vivo CFU-Sday 12. The c-Kit+ bone marrow cells can be also divided by T1/ST2 staining into T1/ST2− cells that contain all of the myeloid cells described (myeloid progenitors, MPs) and T1/ST2+ cells, defined as MCPs, that generate c-Kit+/T1/ST2+ cells and mast cells within 7 days both in vitro (in semisolid and liquid cultures) and in vivo (in extramedullary sites).21 (B) Prospective identification and isolation of MEPs, CMPs, and MCPs from the marrow of Gata1low and wild-type littermates. Lin−c-Kit+ cells from normal marrow (the c-Kit staining of the CD34-CD16/CD32 analysis is not shown, for clarity) were further divided by CD34-CD16/CD32 (on the left) and T1/ST2 (on the right) staining according to prospective gates (indicated by rectangles) corresponding to MEPs or CMPs and MPs and MCPs, respectively. Wild-type MPs are defined as cells with the c-Kit+/T1/ST2− phenotype and contain, by definition, a mixture of CMPs, MEPs, and GMPs. In the case of wild-type cells, all of the sorted fractions were 80% to 95% pure, as shown by the presented reanalysis. The Lin−c-Kit+ cells from Gata1low bone marrow were also divided by CD34-CD16/CD32 staining according to the prospective gates corresponding to MEPs or CMPs (∼90% pure, by reanalysis). In contrast, T1/ST2+ cells were not detectable among Gata1low Lin−c-Kit+ cells. The absence of MCPs among the marrow cells from Gata1low mice was confirmed by arbitrarily sorting T1/ST2− cells into the one third-lower (MPlow) and one third-higher (MPhigh) portion of the staining, as indicated, and by showing that the profile of the 2 sorted populations was largely overlapping (< 60% pure). Results from 2 representative experiments are shown. Similar results were observed in at least 3 independent experiments per group. The mean (± SD) frequency of each population observed in multiple experiments is summarized in Table 1. (C) Gata1 expression in different classes of progenitor cells prospectively isolated from the marrow of wild-type and Gata1low mice, as indicated. Results are presented as mean (± SD) of at least 3 independent determinations for progenitor cell type. Values of Gata1 expression statistically different (P < .05) from those expressed by the corresponding CMPs are indicated by a single asterisk, whereas differences statistically significant between values observed in populations with the same profile purified from Gata1low and wild-type littermates are indicated by double asterisks.
The marrow of Gata1low mice contains progenitor cells with the myeloid, but not with the mastocytic, antigenic profile. (A) Diagrammatic scheme of the antigenic profiling used to define different progenitor cell types in this study. All myeloid and mast cell generating activity is present among the cKit+ cells of the Lin−Sca1−Ly6c−FcϵRIα[−] cells of normal marrow.18,21 The myeloid generating cells are divided by CD34 and CD16/CD32 staining into GMPs (CD34+CD16/CD32+, not shown), MEPs (CD34lowCD16/CD32low), and CMPs (CD34+CD16/CD32low).18 GMPs correspond to progenitor cells functionally defined as CFU-Gs, CFU-Ms, and CFU-GMs, and are not investigated in detail in this study. MEPs are cells restricted toward megakaryocyte and erythroid differentiation and include those cells once functionally defined as CFU-MKday3 and CFU-MKday7, CFU-E, and BFU-E. CMPs give rise to GMPs, MEPs, and MCPs, and correspond to cells functionally defined as capable to generate in vitro CFU-mix and in vivo CFU-Sday 12. The c-Kit+ bone marrow cells can be also divided by T1/ST2 staining into T1/ST2− cells that contain all of the myeloid cells described (myeloid progenitors, MPs) and T1/ST2+ cells, defined as MCPs, that generate c-Kit+/T1/ST2+ cells and mast cells within 7 days both in vitro (in semisolid and liquid cultures) and in vivo (in extramedullary sites).21 (B) Prospective identification and isolation of MEPs, CMPs, and MCPs from the marrow of Gata1low and wild-type littermates. Lin−c-Kit+ cells from normal marrow (the c-Kit staining of the CD34-CD16/CD32 analysis is not shown, for clarity) were further divided by CD34-CD16/CD32 (on the left) and T1/ST2 (on the right) staining according to prospective gates (indicated by rectangles) corresponding to MEPs or CMPs and MPs and MCPs, respectively. Wild-type MPs are defined as cells with the c-Kit+/T1/ST2− phenotype and contain, by definition, a mixture of CMPs, MEPs, and GMPs. In the case of wild-type cells, all of the sorted fractions were 80% to 95% pure, as shown by the presented reanalysis. The Lin−c-Kit+ cells from Gata1low bone marrow were also divided by CD34-CD16/CD32 staining according to the prospective gates corresponding to MEPs or CMPs (∼90% pure, by reanalysis). In contrast, T1/ST2+ cells were not detectable among Gata1low Lin−c-Kit+ cells. The absence of MCPs among the marrow cells from Gata1low mice was confirmed by arbitrarily sorting T1/ST2− cells into the one third-lower (MPlow) and one third-higher (MPhigh) portion of the staining, as indicated, and by showing that the profile of the 2 sorted populations was largely overlapping (< 60% pure). Results from 2 representative experiments are shown. Similar results were observed in at least 3 independent experiments per group. The mean (± SD) frequency of each population observed in multiple experiments is summarized in Table 1. (C) Gata1 expression in different classes of progenitor cells prospectively isolated from the marrow of wild-type and Gata1low mice, as indicated. Results are presented as mean (± SD) of at least 3 independent determinations for progenitor cell type. Values of Gata1 expression statistically different (P < .05) from those expressed by the corresponding CMPs are indicated by a single asterisk, whereas differences statistically significant between values observed in populations with the same profile purified from Gata1low and wild-type littermates are indicated by double asterisks.
Frequency of progenitor cells prospectively isolated from marrow and spleen of Gata1low and wild-type littermates
| . | GMPs, % . | CMPs, % . | MEPs, % . | MCPs, % . |
|---|---|---|---|---|
| Wild type | ||||
| BM | 36.7 ± 3.4 | 34.1 ± 0.7 | 20.8 ± 0.7 | 4.0 ± 0.2 |
| Spleen | BD | BD | BD | BD |
| GATA-1low | ||||
| BM | 32.8 ± 3.9 | 35.5 ± 3.7 | 15.5 ± 1.5* | < 0.1* |
| Spleen | 18.3 ± 6.3 | 26.6 ± 3.8 | 34.1 ± 6.0 | < 0.1* |
| . | GMPs, % . | CMPs, % . | MEPs, % . | MCPs, % . |
|---|---|---|---|---|
| Wild type | ||||
| BM | 36.7 ± 3.4 | 34.1 ± 0.7 | 20.8 ± 0.7 | 4.0 ± 0.2 |
| Spleen | BD | BD | BD | BD |
| GATA-1low | ||||
| BM | 32.8 ± 3.9 | 35.5 ± 3.7 | 15.5 ± 1.5* | < 0.1* |
| Spleen | 18.3 ± 6.3 | 26.6 ± 3.8 | 34.1 ± 6.0 | < 0.1* |
GMPs are defined as CD16/CD32+/CD34+; CMPs, as CD16/CD32−/CD34+; MEPs, as CD16/CD32−/CD34−; and MCPs, as T1/ST2+. All the cells are c-Kit+. Results are presented as the mean ± SD of at least 3 experiments per group of mice.
BM indicates bone marrow; BD, below detectable levels.
Values statistically different (P < .05) from those observed in wild-type littermates.
The levels of Gata1 expressed by progenitor cells prospectively isolated from Gata1low and wild-type marrow are compared in Figure 1C. The wild-type cells expressing the highest and lowest Gata1 levels were MEPs (2−ΔCt = 0.1 ± 0.05) and CMPs (2−ΔCt = 0.014 ± 0.006, 1 log lower), respectively. CMPs and MEPs isolated from Gata1low mutants expressed levels of Gata1 lower than those expressed by the corresponding normal cells. Surprisingly, the Gata1low cells in which Gata1 expression was reduced the most were represented by CMPs (2−ΔCt = 0.10 ± 0.01 × 10−2, ∼1-log reduction) and not MEPs (2−ΔCt = 0.023 ± 0.001, ∼4-fold reduction; Figure 1C). In addition, the expression of Fog1 was similar in wild-type CMPs and MEPs (2−ΔCt = 0.10-0.11, in both) and decreased by 50% in wild-type MCPs (2−ΔCt = 0.06 ± 0.01, P < .05). Also Gata1low CMPs and MEPs expressed similar Fog1 levels, but the values were significantly higher than those expressed by wild-type cells (2−ΔCt = 0.13-0.14, P < .05).
In conclusion, whereas MCPs were barely detectable, CMPs and MEPs were identified in hematopoietic tissues from Gata1low mice and expressed levels of Gata1 about 0.5 to 1-log lower and of Fog1 about 50% higher than the corresponding wild-type cells.
The altered proliferation and differentiation observed in BMMCs from Gata1low mice is permissive to the establishment of factor-dependent cell lines
In BMMCs seeded with either wild-type or W/Wv cells, the cell number increased regularly (by 1 log) during the first 3 weeks to remain constant at later time points (Figure 2A). In contrast, in BMMCs from Gata1low mice, the cell number increased regularly up to the fourth to fifth week and was significantly higher than in wild-type and W/Wv BMMCs after 25 to 30 days (Figure 2A).
Gata1low BMMCs are characterized by high proliferation rates and generate mast cell precursors as early as day 7, but the cells remain partially immature up to day 26. (A) BMMCs from Gata1low mice generate more cells and for longer time than BMMCs from wild-type littermates and W/Wv mice. Time-course analysis of BMMCs seeded with marrow cells from wild-type, Gata1low, and W/Wv mice are presented in the top, middle, and bottom panel, as indicated. The dotted lines indicate the regular demipopulation necessary to feed the cultures. Results are presented as mean (± SD) of at least 6 independent cultures per experimental animal. Asterisk indicates values statistically different (P < .001) between BMMCs seeded with Gata1low and wild-type, or W/Wv cells. (B) Representative flow cytometric analysis for c-Kit and FcϵRI expression of cells obtained in BMMCs seeded with marrow from wild-type, Gata1low, and W/Wv mice, as indicated. Cells were analyzed either at day 7, 21, or 26 of culture. Negative controls were represented by cells labeled with irrelevant antibodies and are not presented for convenience. Similar results were obtained in at least 3 independent experiments per group of mice (see panel C). (C) Frequency of c-Kithigh (on the left) and FcϵRI+ (as percent of c-Kithigh, on the right) cells in BMMCs seeded with bone marrow from wild-type (large hatched bars), Gata1low (⊡), and W/Wv (tight hatched bars) mice. Cells were analyzed either at day 7, 21, or 26 of culture, as indicated on the x-axes. Results are presented as the mean (± SD) of at least 3 independent experiments per group of mice. Values observed in Gata1low BMMCs and statistically different from those obtained in the corresponding cultures with wild-type and W/Wv cells are indicated with a single asterisk (P < .05) and double asterisks (P < .01), respectively. (D). Semiquantitative RT-PCR analysis for the expression of mast cell specific genes (MMCP-6, MC-CPA, and MITF) as well as of Gata1, Gata2, and NFE2 in cells obtained after 26 days in BMMCs seeded with marrow from wild-type, Gata1low, or W/Wv mice, as indicated. The Gata1low c-Kithigh cells were divided into immature (FcϵRI−) and mature (FcϵRI+) cells by sorting (> 95% purity on reanalysis, not shown). c-KithighFcϵRI− cells from wild-type and W/Wv BMMCs could not be isolated because of their infrequency. The triangle on top of the panels indicates increasing numbers of cycles. Similar results were observed in 2 additional experiments per experimental group; n.d. indicates not done. (E) Time-course analysis of serotonin uptake (on the top) and release (on the bottom) by cells obtained in BMMCs from wild-type (large hatched bars), Gata1low (⊡), and W/Wv (tight hatched bars) mice. Cells were analyzed either at 7, 21, or 26 days of culture, as indicated on the x-axes. Levels of serotonin are expressed as cpm/105 cells. The total serotonin incorporated was measured by lysing BMMCs in Triton. Serotonin release was induced by IgE-αIgE stimulation. Positive and negative controls were represented by cells stimulated with IgE alone, medium + αIgE; medium + HSA + DNP and ionomycin and are not shown for clarity. Results are presented as the mean (± SD) of at least 3 separate experiments, per group of mice, performed in duplicate. Values observed in Gata1low BMMCs and statistically different from those obtained in the corresponding cultures with wild-type and W/Wv cells are indicated with a single asterisk (P < .05) and double asterisks (P < .01), respectively.
Gata1low BMMCs are characterized by high proliferation rates and generate mast cell precursors as early as day 7, but the cells remain partially immature up to day 26. (A) BMMCs from Gata1low mice generate more cells and for longer time than BMMCs from wild-type littermates and W/Wv mice. Time-course analysis of BMMCs seeded with marrow cells from wild-type, Gata1low, and W/Wv mice are presented in the top, middle, and bottom panel, as indicated. The dotted lines indicate the regular demipopulation necessary to feed the cultures. Results are presented as mean (± SD) of at least 6 independent cultures per experimental animal. Asterisk indicates values statistically different (P < .001) between BMMCs seeded with Gata1low and wild-type, or W/Wv cells. (B) Representative flow cytometric analysis for c-Kit and FcϵRI expression of cells obtained in BMMCs seeded with marrow from wild-type, Gata1low, and W/Wv mice, as indicated. Cells were analyzed either at day 7, 21, or 26 of culture. Negative controls were represented by cells labeled with irrelevant antibodies and are not presented for convenience. Similar results were obtained in at least 3 independent experiments per group of mice (see panel C). (C) Frequency of c-Kithigh (on the left) and FcϵRI+ (as percent of c-Kithigh, on the right) cells in BMMCs seeded with bone marrow from wild-type (large hatched bars), Gata1low (⊡), and W/Wv (tight hatched bars) mice. Cells were analyzed either at day 7, 21, or 26 of culture, as indicated on the x-axes. Results are presented as the mean (± SD) of at least 3 independent experiments per group of mice. Values observed in Gata1low BMMCs and statistically different from those obtained in the corresponding cultures with wild-type and W/Wv cells are indicated with a single asterisk (P < .05) and double asterisks (P < .01), respectively. (D). Semiquantitative RT-PCR analysis for the expression of mast cell specific genes (MMCP-6, MC-CPA, and MITF) as well as of Gata1, Gata2, and NFE2 in cells obtained after 26 days in BMMCs seeded with marrow from wild-type, Gata1low, or W/Wv mice, as indicated. The Gata1low c-Kithigh cells were divided into immature (FcϵRI−) and mature (FcϵRI+) cells by sorting (> 95% purity on reanalysis, not shown). c-KithighFcϵRI− cells from wild-type and W/Wv BMMCs could not be isolated because of their infrequency. The triangle on top of the panels indicates increasing numbers of cycles. Similar results were observed in 2 additional experiments per experimental group; n.d. indicates not done. (E) Time-course analysis of serotonin uptake (on the top) and release (on the bottom) by cells obtained in BMMCs from wild-type (large hatched bars), Gata1low (⊡), and W/Wv (tight hatched bars) mice. Cells were analyzed either at 7, 21, or 26 days of culture, as indicated on the x-axes. Levels of serotonin are expressed as cpm/105 cells. The total serotonin incorporated was measured by lysing BMMCs in Triton. Serotonin release was induced by IgE-αIgE stimulation. Positive and negative controls were represented by cells stimulated with IgE alone, medium + αIgE; medium + HSA + DNP and ionomycin and are not shown for clarity. Results are presented as the mean (± SD) of at least 3 separate experiments, per group of mice, performed in duplicate. Values observed in Gata1low BMMCs and statistically different from those obtained in the corresponding cultures with wild-type and W/Wv cells are indicated with a single asterisk (P < .05) and double asterisks (P < .01), respectively.
In wild-type and W/Wv BMMCs, few (5%-10%) cells expressed the mature c-KithighFcϵRI+ mast cell phenotype by day 7 (Figure 2B-C) but almost all (90%-100%) of them were c-KithighFcϵRI+ by day 21 to 26. In contrast, in BMMCs from Gata1low mice, although many (35%) of the cells were c-Kithigh already by day 7, many (20%) of the c-Kit+ cells remained FcϵRI− until day 21 to 26 (Figure 2B-C). Differences were observed in level of antigen expression between cells obtained after 26 days in BMMCs from different mouse groups. As expected,32 W/Wv BMMCs expressed less c-Kit and FcϵRI than the corresponding wild-type cells (mean fluorescence intensity/cell: c-Kit = 31 ± 1 versus 192 ± 22, P < .01, and FcϵRI = 7 ± 1 versus 18 ± 1, P < .05, respectively). On the other hand, Gata1low BMMCs expressed levels of c-Kit similar to those expressed by normal cells (mean fluorescence intensity/cell = 265 ± 17) but levels of FcϵRI receptor similar to those expressed by W/Wv cells (mean fluorescence intensity/cell = 8 ± 1). Expression profiling confirmed the immature nature of day 26 Gata-1low BMMCs. In fact, they expressed consistently more Gata1, Gata2, MITF, and MMCP-6 (early marker38 ) but less MC-CPA (a serosal maturation marker39 ) than the corresponding wild-type cells (Figure 2D). Interestingly, these cells expressed NFE2, a gene involved in the control of megakaryocytic-erythroid, rather than mastocytic, differentiation40 (Figure 2D). BMMCs from all the mice groups investigated were capable of incorporating and releasing serotonin after IgE-αIgE stimulation already by day 7 (Figure 2E). In all the cases, however, maximal levels of serotonin were released by cells obtained by day 21. Those released by Gata1low cells remained significantly lower than those released by the other 2 cell types at all time points (Figure 2E).
The proliferation observed after the first 3 weeks in Gata1low BMMCs prompted us to ask whether these cells had become cell lines. To test this hypothesis, Gata1low, and wild-type as control, BMMCs were regularly replenished with fresh medium and growth factors for 2 additional months. Whereas wild-type cultures became extinguished within 1 month, Gata1low cells were maintained and, by the end of the 2 months, cultured as single cells in 96-well plates. One week later, cell proliferation was observed in about 30% of the wells and the cells in 10, randomly selected, wells transferred into flasks. These cultures, once replenished with fresh medium and growth factors, could be regularly passed for 6 additional months. At this time point (9 months from the beginning of the Gata1low BMMCs), the cells in each culture were considered as clonal cell lines. Because similar results were obtained in 2 additional experiments, a total of 30 individual lines were established over the course of the study.
Ten of 30 Gata1low BMMC lines were characterized in terms of morphology, growth factor requirement, and antigenic profile, and 3 of them were also analyzed by expression profiling and serotonin-releasing activity. Because results obtained with different clones were similar, only those obtained with the representative SN1 cell line are presented (Figure 3). SN1 cells were large with few cytoplasmic granules reactive with Alcian blue (5%-10%), acetyl cholinesterase A (5%; Figure 3A), and benzidine (0.1%; not shown).
Gata1low BMMCs generate with high efficiency trilineage growth factor-dependent cell lines. (A) SN1, a representative clonal cell line obtained in BMMCs seeded with marrow cells from Gata1low mice, expresses morphologic markers of the mastocytic (Alcian blue) and megakaryocytic (acetylcholine esterase A) lineage. The procedure to isolate the SN1 cell line is described in “Results.” By May-Grünwald-Giemsa (B1) staining, SN1 cells were large and contained several cytoplasmic granules, that reacted with acetylcholine esterase A (B2) or Alcian blue (B3). Original magnification, × 64. Images were obtained with a Leica DMLS2 microscope equipped with an N PLAN 10×/2.1 objective lens at 10×/0.05 magnification (Leica, Solms, Germany). Photographs were taken with a Leica DFC 280 digital camera, and were acquired with Leica IM50 4.0 software. Subsequent image processing was performed with Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA). (B) The SN1 cells are growth factor dependent and require, for optimal growth, SCF and IL-3. Growth factor requirement was measured by counting the number of cells present after 5 days in cultures supplemented with the growth factors indicated on the abscises. The detailed growth curve of cells in cultures stimulated with SCF and IL-3 is presented in the insert. Results are presented as mean (± SD) cell numbers observed in at least 3 independent experiments. (C) Antigenic profiling of SN1 cells. The different panels present, in the order, dot plot analysis for side-scatter, c-Kit expression and gate used to analyze, in the following histograms, the expression of CD34, SCA-1, and FcϵRI, as indicated. In these histograms, the gray area corresponds to SN1 cells labeled with the antibody indicated on the abscissa, the dotted lines present the profile of serosal Gata1low mast cells labeled with the same antibody (positive control), and the straight lines correspond to SN1 cells labeled with an irrelevant antibody (negative control). (D) Levels of serotonin (as percent of total serotonin uptake) released on IgE + αIgE stimulation by SN1 cells. Positive and negative controls were represented by cells stimulated with IgE alone, medium + αIgE; medium + HSA + DNP and ionomycin, as indicated. The levels of total serotonin that had been incorporated by the cells was measured by Triton lysis. Although low, the levels of serotonin released on αIgE-IgE stimulation are statistically (P < .05) different from those released on stimulation with IgE alone. Results are presented as the mean (± SD) of at least 3 separate experiments performed in triplicate. (E) Expression profiling of SN1 cells by semiquantitative RT-PCR. The gene analyzed in each reaction is indicated on the right. The triangle on the top of the panels indicates increasing numbers of cycles. The levels of expression are compared to those measured in parallel experiments using, as template, cDNA from a mastocytic (32D)35 and an erythroid (32D EPO) cell line.36 Similar results were observed with 3 additional clonal cell lines obtained from Gata1low BMMCs (not shown).
Gata1low BMMCs generate with high efficiency trilineage growth factor-dependent cell lines. (A) SN1, a representative clonal cell line obtained in BMMCs seeded with marrow cells from Gata1low mice, expresses morphologic markers of the mastocytic (Alcian blue) and megakaryocytic (acetylcholine esterase A) lineage. The procedure to isolate the SN1 cell line is described in “Results.” By May-Grünwald-Giemsa (B1) staining, SN1 cells were large and contained several cytoplasmic granules, that reacted with acetylcholine esterase A (B2) or Alcian blue (B3). Original magnification, × 64. Images were obtained with a Leica DMLS2 microscope equipped with an N PLAN 10×/2.1 objective lens at 10×/0.05 magnification (Leica, Solms, Germany). Photographs were taken with a Leica DFC 280 digital camera, and were acquired with Leica IM50 4.0 software. Subsequent image processing was performed with Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA). (B) The SN1 cells are growth factor dependent and require, for optimal growth, SCF and IL-3. Growth factor requirement was measured by counting the number of cells present after 5 days in cultures supplemented with the growth factors indicated on the abscises. The detailed growth curve of cells in cultures stimulated with SCF and IL-3 is presented in the insert. Results are presented as mean (± SD) cell numbers observed in at least 3 independent experiments. (C) Antigenic profiling of SN1 cells. The different panels present, in the order, dot plot analysis for side-scatter, c-Kit expression and gate used to analyze, in the following histograms, the expression of CD34, SCA-1, and FcϵRI, as indicated. In these histograms, the gray area corresponds to SN1 cells labeled with the antibody indicated on the abscissa, the dotted lines present the profile of serosal Gata1low mast cells labeled with the same antibody (positive control), and the straight lines correspond to SN1 cells labeled with an irrelevant antibody (negative control). (D) Levels of serotonin (as percent of total serotonin uptake) released on IgE + αIgE stimulation by SN1 cells. Positive and negative controls were represented by cells stimulated with IgE alone, medium + αIgE; medium + HSA + DNP and ionomycin, as indicated. The levels of total serotonin that had been incorporated by the cells was measured by Triton lysis. Although low, the levels of serotonin released on αIgE-IgE stimulation are statistically (P < .05) different from those released on stimulation with IgE alone. Results are presented as the mean (± SD) of at least 3 separate experiments performed in triplicate. (E) Expression profiling of SN1 cells by semiquantitative RT-PCR. The gene analyzed in each reaction is indicated on the right. The triangle on the top of the panels indicates increasing numbers of cycles. The levels of expression are compared to those measured in parallel experiments using, as template, cDNA from a mastocytic (32D)35 and an erythroid (32D EPO) cell line.36 Similar results were observed with 3 additional clonal cell lines obtained from Gata1low BMMCs (not shown).
These cells failed to survive in the absence of growth factors (Figure 3B). Few of them (20%-30%) survived in the presence of either SCF, IL-3, or TPO alone. For optimal proliferation, they required the presence of both SCF and IL-3. In their presence, a doubling time of about 24 hours was observed (Figure 3B).
SN1 cells expressed 10 times more surface c-Kit, similar levels of CD34 and Sca1, and lower levels of FcϵRI than the original Gata1low mast cells from which they had derived (Figure 3C). Consequently, although capable of some (∼8%) serotonin release after IgE/αIgE stimulation, their serotonin-releasing activity remained modest (Figure 3D). Some SN1 cells expressed the erythroid TER119 (21% ± 4%) or megakaryocytic 2D5 (61% ± 3%) markers, with 15% of them expressing both (not shown). Because almost all SN1 cells expressed FcϵRI (Figure 3C), erythroid, megakaryocytic, and mastocytic surface markers were coexpressed in about 15% of the cells. By molecular profiling, SN1 cells expressed mast cell specific genes as well as erythroid (β-globin) and megakaryocytic (GpIa and GpIIb)–specific genes (Figure 3E). Interestingly, they expressed high levels of NFE2, Gata2, and Gata1. This last result indicates a different Gata1 regulation in these cells as compared with that of erythroid Gata1low progenitors. The levels of Gata1 expressed by SN1 cells are higher than those expressed by normal hematopoietic cells and resemble those found in MEL cells.41 As in the case of these erythroleukemic cells, such a high level of Gata1 expression was not sufficient per se to restore proper terminal differentiation of SN1 cells.
In conclusion, BMMCs seeded with marrow (and spleen, not shown) cells from Gata1low mice are characterized by increased proliferation and decreased cell differentiation and consistently generate 3-lineage growth factor-dependent cell lines.
Gata1low MEPs generate mast cells, and their precursors, in BMMC cultures
The fact that mast cells, although defective, were present in skin and peritoneum of Gata1low mice, as well as in BMMCs seeded with their marrow (Migliaccio et al6 and this report), indicated that the mastocytic activity of Gata1low stem cells was not impaired. The fact that MCPs were not detectable in Gata1low tissues implies, then, that a cell different from an MCP acquired MCP function in these mutants. To identify which of the Gata1low progenitor cells acquired mast cell-generating activity, cells prospectively isolated from tissues of wild-type and Gata1low littermates were cultured in BMMCs and the cultures analyzed 7 days later for presence of mast cells (c-KithighFcϵRI+) and of their precursors (c-Kithigh/T1/ST2high; Figure 4; Table S2).
MEPs prospectively isolated from Gata1low marrow generate many mast cells when cultured under BMMC conditions. (A) Fold increase observed after 7 days in cultures seeded with either MEPs (CD34lowCD16/CD32low), CMPs (CD34+CD16/CD32low), MPs (T1/ST2−), or MCPs (T1/ST2+) isolated from the marrow of wild-type and Gata1low littermates, as indicated. By definition, wild-type MPs are a mixture of CMPs, MEPs, and GMPs. In the case of the Gata1low mice, MPlow and MPhigh cells (Figure 2B) were independently cultured. All of the cultures were seeded with 103 cells/mL and stimulated with SCF plus IL-3. Results are presented as mean (± SD) of at least 3 separate experiments per experimental group. (B) Flow cytometry analysis for the presence of cells with the mastocytic phenotype in day 7 cultures of MEPs, CMPs, MPs, and MCPs, prospectively isolated from the marrow of wild-type and Gata1low mice, as indicated. The c-KithighT1/ST2high antigenic profile corresponds to that of mast cell precursors and the c-KithighFcϵRI+ one to that of mature mast cells.6,21 Results from a representative experiment are shown. The mean (± SD) frequency obtained in at least 3 separate cultures per experimental point is reported in Table S2. (C) Quantitative RT-PCR analysis for the expression of Gata1 and of mast cell-specific genes by c-KithighFc_RI+ cells obtained after 7 days in cultures of the different progenitor cells prospectively isolated from the marrow of either wild-type or Gata1low mice, as indicated. The mast cell-specific genes analyzed were represented by MMCP-6, a marker for early stages of mast cell maturation,38 and MC-CPA and MMCP-7, markers for late differentiation stages of serosal24 and dermal39 mast cells, respectively. The expression profile of mast cells obtained from wild-type MCPs, for its low MMCP-7 level, is similar to that reported to be expressed by murine mast cells obtained in BMMCs.24 On the other hand, the high level of expression of MMCP-7 in mast cells obtained from wild-type MCPs suggests that this population might be preferentially enriched for precursors of serosal mast cells. The mast cells obtained from all of the Gata1low progenitors expressed a similar profile, resembling that of mast cells derived from wild-type CMPs. All of the sorted populations analyzed were more than 95% pure, on reanalyses (not shown). Results are expressed and mean (± SD) 2−ΔCt obtained in at least 3 experiments per group. Single and double asterisks indicate expression levels statistically different (P < .01) from that of the progeny of CMPs purified from the same animal and between the progeny of Gata1low cells and those of the corresponding wild-type progenitor cells.
MEPs prospectively isolated from Gata1low marrow generate many mast cells when cultured under BMMC conditions. (A) Fold increase observed after 7 days in cultures seeded with either MEPs (CD34lowCD16/CD32low), CMPs (CD34+CD16/CD32low), MPs (T1/ST2−), or MCPs (T1/ST2+) isolated from the marrow of wild-type and Gata1low littermates, as indicated. By definition, wild-type MPs are a mixture of CMPs, MEPs, and GMPs. In the case of the Gata1low mice, MPlow and MPhigh cells (Figure 2B) were independently cultured. All of the cultures were seeded with 103 cells/mL and stimulated with SCF plus IL-3. Results are presented as mean (± SD) of at least 3 separate experiments per experimental group. (B) Flow cytometry analysis for the presence of cells with the mastocytic phenotype in day 7 cultures of MEPs, CMPs, MPs, and MCPs, prospectively isolated from the marrow of wild-type and Gata1low mice, as indicated. The c-KithighT1/ST2high antigenic profile corresponds to that of mast cell precursors and the c-KithighFcϵRI+ one to that of mature mast cells.6,21 Results from a representative experiment are shown. The mean (± SD) frequency obtained in at least 3 separate cultures per experimental point is reported in Table S2. (C) Quantitative RT-PCR analysis for the expression of Gata1 and of mast cell-specific genes by c-KithighFc_RI+ cells obtained after 7 days in cultures of the different progenitor cells prospectively isolated from the marrow of either wild-type or Gata1low mice, as indicated. The mast cell-specific genes analyzed were represented by MMCP-6, a marker for early stages of mast cell maturation,38 and MC-CPA and MMCP-7, markers for late differentiation stages of serosal24 and dermal39 mast cells, respectively. The expression profile of mast cells obtained from wild-type MCPs, for its low MMCP-7 level, is similar to that reported to be expressed by murine mast cells obtained in BMMCs.24 On the other hand, the high level of expression of MMCP-7 in mast cells obtained from wild-type MCPs suggests that this population might be preferentially enriched for precursors of serosal mast cells. The mast cells obtained from all of the Gata1low progenitors expressed a similar profile, resembling that of mast cells derived from wild-type CMPs. All of the sorted populations analyzed were more than 95% pure, on reanalyses (not shown). Results are expressed and mean (± SD) 2−ΔCt obtained in at least 3 experiments per group. Single and double asterisks indicate expression levels statistically different (P < .01) from that of the progeny of CMPs purified from the same animal and between the progeny of Gata1low cells and those of the corresponding wild-type progenitor cells.
Numerous cells (∼300-500-fold increase with respect to day 0) were generated in 7 days of culture by all of the prospectively isolated progenitor cells (wild-type and Gata1low alike), with the exception of wild-type MPs that grew poorly (Figure 4A). As expected, wild-type MEPs and CMPs generated mostly erythroid and megakaryocytic cells (Table S2 and Suda et al,17 Akashi et al,18 and Nakorn et al34 ), and few (1%-2%) mast cells and their precursors. These cells represented, instead, more than 7% to 8% of the progeny of wild-type MCPs (Figure 4B; Table S2; Akashi et al18 ). In contrast, MEPs and CMPs isolated from Gata1low animals consistently generated many (> 20%) c-KithighFcϵRI+ and c-Kithigh/T1/ST2high cells, in addition to erythroblasts and megakaryocytes (Figure 4B; Table S2).
The mastocytic nature of cells present among the progeny of Gata1low MEPs was confirmed by functional assay (c-KithighT1/ST2high cells; Table S3) and expression profiling (c-KithighFcϵRI+ cells; Figure 4C). c-KithighT1/ST2high cells, derived from Gata1low MEPs, generated, after 14 days of BMMCs, more c-KithighFcϵRI+ cells that the corresponding progeny of wild-type MCPs (fold increase = 97- and 600-fold, P < .01, respectively; Table S3).
On the other hand, c-KithighFcϵRI+ cells generated by the various wild-type progenitor cells had different expression profiling (Figure 4C). Those generated by MCPs and CMPs expressed robust levels of the late MC-CPA marker and low level of expression of the early MMCP-6 marker.24,39 Wild-type CMP-derived c-KithighFcϵRI+ cells expressed also high levels of MMCP-7, a protease usually not expressed by BMMC-derived mast cells.24 In contrast, mast cell-specific genes were poorly expressed by c-KithighFcϵRI+ cells derived from wild-type MEPs. In comparison, c-KithighFcϵRI+ cells generated by all of the Gata1low progenitors, including MEPs, expressed a homogenous profile, characterized, as that of wild-type CMP-derived cells, by robust expression of all the mast cell-specific genes analyzed, including MMCP-7.
To further confirm that Gata1low MEPs, but not wild-type MEPs, expressed mast cell-generating activity, the progeny of both cells was cultured for additional 14 to 21 days (Figure S1). High numbers of mast cells were consistently detected in cultures seeded with Gata1low MEPs but not in those seeded with the corresponding wild-type cells.
In conclusion, Gata1low MEPs generate numerous mature and precursor mast cells in BMMC cultures.
Altered differentiation/proliferation potential of single Gata1low MEPs
To confirm that Gata1low MEPs generate mast cells and to determine the extent of the abnormal proliferation of their progeny, prospectively isolated Gata1low, and wild-type as controls, MEPs were cultured as single cells, using the limiting dilution protocol. The morphology and recloning potential of their progeny was then evaluated at weekly intervals (Figure 5A).
Single MEPs isolated from Gata1low mice generate with high frequency cells with extensive proliferation capacity under BMMC conditions. (A) Experimental design used to measure the differentiation and proliferation potential of single Gata1low MEPs. MEPs isolated from the marrow of Gata1low mice were diluted at a concentration of 3 cells/mL, and 100 μL of this solution cultured in each well of a 96-multiwell plate (Table S4). At this cell concentration, 30% of the wells will contain, on average, one cell and the probability that one of them will contain 2 cells is less than 9%. Seven days later, the wells were scored for sign of proliferation. Number and morphology of the cells present in 10 randomly selected wells were analyzed. Fourteen days later, the cells contained in 10 additional randomly selected wells, were harvested, counted, and cloned under limiting conditions in secondary single-cell cultures. This procedure was repeated again after 21 (tertiary cultures) and 28 (quaternary cultures) days, as indicated. MCPs purified from wild-type mice were processed in parallel, as control. (B) Cloning efficiency over time (ie, number of wells in which cell growth was detected) observed in primary cultures seeded with single wild-type MCPs (▪) and Gata1low MEP (•). Results are presented as mean (± SD) of those obtained in 4 independent experiments per animal group (see also Table S4). (C) May-Grünwald staining of the progeny observed over time in cultures of a representative wild-type MCP and a representative Gata1low MEP, as indicated. At day 7, the progeny of wild-type MCP have an homogeneous mastocytic morphology, whereas megakaryocytes (Mk), erythroblasts (Ery), and mast cells (MC) are present among the progeny of Gata1low MEPs. From 14 days on, only cells with mast cell morphology were detectable in the cultures. The results are representative of those obtained with at least 10 individual wells per experimental point; b.d. = below detectable levels. Original magnification, × 40. Images were obtained using a Zeiss Axiostat Plus microscope equipped with a Zeiss CP-ACHORMAT 40×/0.65 objective lens (Zeiss, Arese, Italy). Photographs were taken with an Olympus Camedia c-5060 digital camera and were acquired with Camedia Master 4.1 software (Opera Zerbo, Milan, Italy). Subsequent image processing was performed with Adobe Photoshop 6.0 software. (D) Total cell number per well and replating efficiency (ie, number of single cells that proliferated in following passages) in single-cell cultures of the progeny of wild-type MCPs (▪) and Gata1low MEPs (•). Results are presented as mean (± SD) of those obtained in 3 independent experiments per animal group. Values statistically different (*P < .01, **P < .001) between cultures of wild-type and Gata1low cells (see also Table S4).
Single MEPs isolated from Gata1low mice generate with high frequency cells with extensive proliferation capacity under BMMC conditions. (A) Experimental design used to measure the differentiation and proliferation potential of single Gata1low MEPs. MEPs isolated from the marrow of Gata1low mice were diluted at a concentration of 3 cells/mL, and 100 μL of this solution cultured in each well of a 96-multiwell plate (Table S4). At this cell concentration, 30% of the wells will contain, on average, one cell and the probability that one of them will contain 2 cells is less than 9%. Seven days later, the wells were scored for sign of proliferation. Number and morphology of the cells present in 10 randomly selected wells were analyzed. Fourteen days later, the cells contained in 10 additional randomly selected wells, were harvested, counted, and cloned under limiting conditions in secondary single-cell cultures. This procedure was repeated again after 21 (tertiary cultures) and 28 (quaternary cultures) days, as indicated. MCPs purified from wild-type mice were processed in parallel, as control. (B) Cloning efficiency over time (ie, number of wells in which cell growth was detected) observed in primary cultures seeded with single wild-type MCPs (▪) and Gata1low MEP (•). Results are presented as mean (± SD) of those obtained in 4 independent experiments per animal group (see also Table S4). (C) May-Grünwald staining of the progeny observed over time in cultures of a representative wild-type MCP and a representative Gata1low MEP, as indicated. At day 7, the progeny of wild-type MCP have an homogeneous mastocytic morphology, whereas megakaryocytes (Mk), erythroblasts (Ery), and mast cells (MC) are present among the progeny of Gata1low MEPs. From 14 days on, only cells with mast cell morphology were detectable in the cultures. The results are representative of those obtained with at least 10 individual wells per experimental point; b.d. = below detectable levels. Original magnification, × 40. Images were obtained using a Zeiss Axiostat Plus microscope equipped with a Zeiss CP-ACHORMAT 40×/0.65 objective lens (Zeiss, Arese, Italy). Photographs were taken with an Olympus Camedia c-5060 digital camera and were acquired with Camedia Master 4.1 software (Opera Zerbo, Milan, Italy). Subsequent image processing was performed with Adobe Photoshop 6.0 software. (D) Total cell number per well and replating efficiency (ie, number of single cells that proliferated in following passages) in single-cell cultures of the progeny of wild-type MCPs (▪) and Gata1low MEPs (•). Results are presented as mean (± SD) of those obtained in 3 independent experiments per animal group. Values statistically different (*P < .01, **P < .001) between cultures of wild-type and Gata1low cells (see also Table S4).
Approximately 15% of single wild-type MCPs proliferated by day 7, giving rise to about 6 × 103 cells by day 14 of culture (Figure 5B). In all the cases, the progeny had mast cell morphology (Figure 5C). Also 15% of single Gata1low MEPs proliferated under these conditions, generating about 6 × 103 to 105 cells by day 7 to 14 (Figure 5B). The morphology of the Gata1low MEP-derived progeny included mast cells, in addition to megakaryocytes and erythroblasts, by day 7 (Figure 5C). Starting from day 14, the progeny of selected wells was harvested, and recultured again under conditions of limiting dilution at weekly intervals. The replating efficiency of wild-type MCP-derived cells declined over time from 5% at day 14 to undetectable levels at day 21. These cultures were discontinued. In contrast, the number and replating efficiency of the Gata1low MEP-derived progeny increased over time. By the third to fourth passage, each Gata1low cell generated about 8 to 10 × 105 cells/wk, with a cloning efficiency of about 30%. When corrected for the limiting dilution factor (0.3 cells/well), a cloning efficiency of 30% indicates that all the cells had proliferated (Figure 5D; Table S4). The progeny of Gata1low MEPs could be propagated as single cells for at least 4 additional months. The design of the replating experiments allows tracking of the proliferation potential of the progeny of individual MEPs. The tracking indicates that about 11% of single Gata1low MEPs have the potential to generate cells capable of growth with 100% efficiency after 4 passages (Table S4).
Ectopic expression of hGATA1 restores the functions of Gata1low MEPs
To prove that the differentiation/proliferation alterations expressed by Gata1low MEPs are a direct consequence of reduced Gata1 expression, we analyzed the biologic properties of MEPs purified from marrow of μLCRhGATA1 Gata1low/0 males (Figure 6). MEPs purified from μLCRhGATA1/Gata1low/0 mice expressed, as those purified from their Gata1low/0 littermates, low levels of mGata1 (Figure 6). These cells, however, also expressed hGATA1 (Figure 6). Once again, MEPs from Gata1low littermates generated many (≈13%) mast cells (c-KithighFcϵRI+). In contrast, those isolated from μLCRhGATA1/Gata1low mice, as those isolated from wild-type mice, generated few (< 2%) mast cells. Furthermore, these cells expressed low levels of mast cell-specific genes (Figure 6). Last, but not least, the progeny of μLCRhGATA1/Gata1low MEPs, as that derived from wild-type MEPs, did not proliferate beyond 15 to 21 days of culture.
MEPs isolated from Gata1low/hGATA1 mice generate few mast cells in BMMC cultures. The gate used to isolate cells with the MEP phenotype (c-Kit+CD34−) from the marrow of Gata1low/hGATA1 mice and Gata1low littermates, as well as from wild-type controls, is presented in the panels on the left, which also include reanalysis for purity (> 95% in all cases) and the levels of mGata1 and hGATA1 gene expressed in the sorted cells (as measured by a specific real-time RT-PCR). The frequency of c-KithighFcϵRI+ cells in the progeny of MEP purified from the different animals after 6 days of BMMC cultures is presented in the middle panels. The c-KithighFcϵRI+ cells obtained in the different cultures were purified by sorting (> 95% pure by reanalysis) and analyzed, by real-time RT-PCR, for mGata1 and hGATA1 expression, as well as for expression of mast cell markers, as shown in the right panels. Expression levels are presented as mean ± SD of 2−ΔCt obtained in three separate experiments per group of mice. Values statistically (P < .01) different from those expressed by wild-type and Gata1low cells are indicated by single and double asterisks, respectively.
MEPs isolated from Gata1low/hGATA1 mice generate few mast cells in BMMC cultures. The gate used to isolate cells with the MEP phenotype (c-Kit+CD34−) from the marrow of Gata1low/hGATA1 mice and Gata1low littermates, as well as from wild-type controls, is presented in the panels on the left, which also include reanalysis for purity (> 95% in all cases) and the levels of mGata1 and hGATA1 gene expressed in the sorted cells (as measured by a specific real-time RT-PCR). The frequency of c-KithighFcϵRI+ cells in the progeny of MEP purified from the different animals after 6 days of BMMC cultures is presented in the middle panels. The c-KithighFcϵRI+ cells obtained in the different cultures were purified by sorting (> 95% pure by reanalysis) and analyzed, by real-time RT-PCR, for mGata1 and hGATA1 expression, as well as for expression of mast cell markers, as shown in the right panels. Expression levels are presented as mean ± SD of 2−ΔCt obtained in three separate experiments per group of mice. Values statistically (P < .01) different from those expressed by wild-type and Gata1low cells are indicated by single and double asterisks, respectively.
Discussion
It is shown here that the marrow and spleen from Gata1low mice contain cells expressing the antigenic profile of wild-type GMPs CMPs, and MEPs but not those resembling wild-type MCPs (Figure 1B). The frequency of CMPs and MEPs is normal in marrow and increases, by several fold, in spleen of mutant mice (Table 1). The hypomorphic mutation reduces by 0.5 to 1-log Gata1 expression in CMPs and MEPs (Figure 1C). Reduced levels of Gata1 expression are, then, associated with increased proliferation and altered differentiation of prospectively isolated CMPs and MEPs that generate many mast cells, and their precursors, in addition to erythroblasts and megakaryocytes, both in mass and in single-cell cultures (Figures 4–5; Tables S1–S4). Furthermore, the progeny of about 11% of MEPs express unlimited proliferation potential in vitro, as reflected by the frequent generation of trilineage factor-dependent cell lines from Gata1low BMMCs (Figure 3). These results identify the MEP as the “unique” trilineage progenitor, the CFU-EMKMc, previously identified in hematopoietic tissues from Gata1low mice.
The observation that Fog1 represses FcϵRI expression38 suggests that Fog1 might represent a repressor of mastocytopoiesis. To test this hypothesis, we compared the level of expression of Fog1 in wild-type and Gata1low MEPs. However, the mutant cells expressed values of Fog1 significantly higher, and not lower than, those expressed by wild-type MEPs. Such a higher Fog1 expression in Gata1low MEPs, though not providing any clue for their high mastocytopoietic activity, is consistent with their robust capacity to generate megakaryocytes in cultures (Table S2).
Physiologic manipulations that would alter commitment by targeting a transcription factor have not been reported so far. The most promising of those manipulations would be represented by those that, by inducing stress, would stimulate animals to quickly amplify cells for a specific lineage. However, the hematopoietic system does not respond to stress by amplifying specific progenitor cell compartments but rather by activating alternative differentiation routes. These routes, first identified for the crossing-point between the lymphoid and myeloid lineage,43 are also undertaken for quick recovery from chemically induced hemolytic anemia.44 In fact, the spleen from mice recovering from this anemia, contains, in addition to numerous MEPs, bipotent erythroid-megakaryocytic precursors (PEMs).44 PEMs are clearly distinct from MEPs because they do not express c-Kit, do not respond to SCF, and do not form erythroid/megakaryocytic colonies in vitro or spleen colonies in vivo.30,44 That PEMs are generated through an “alternative spleen-specific route” is suggested by the observation that MEPs do not generate PEMs.30 On the other hand, the reported observation that Gata1null TER119+ proerythroblasts exposed to soluble growth factors and the OP9 stromal cell line revert to a mast cell phenotype45 addresses more properly cell plasticity rather than commitment.
Recently, it has been proposed a transcription factor–based model for lineage commitment according to which lineage-specification is determined by the balance between the relative concentrations of transcription factors for alternative lineages.46,47 This model is based on the observation that single hematopoietic progenitors activate transcription factor gene expression in random combinations and before the stage when their fate is fixed.48 This model is also supported by results on forced transcription factor expression on cell fate. In fact, forced Gata1 expression favors generation of erythroid cells in multiple models of stem cell commitment (in vivo, in mouse transplantation models49,50 and zebrafish development51 and, in vitro, avian myeloid cells52 ). The observation, however, that Gata1null embryonic stem cells do generate proerythroblasts,53,54 although apoptotic,55 in vitro, has been considered for a long time as a proof that Gata1, although required for erythroid maturation, is dispensable for commitment. By showing that Gata1low MEPs (and CMPs) are capable of differentiating into mast cells, in addition to erythroblasts and megakaryocytes, we demonstrate for the first time that the level of Gata1 expression in the progenitor cell compartments determine the lineage toward which their progeny will be committed. As counter-proof, forced expression of μLCRhGATA1 restored the function of Gata1low MEPs (Figure 6). These results reinforce the concept that Gata1 is a key element, not only for maturation, but also for commitment toward erythroid, megakaryocytic, and mast cell lineage.
The results presented here, and an overview of published results that did not formally involve analysis of prospectively isolated cells, not only confirm the transcription factor-based model for lineage specification46,47 but, as suggested in Figure 7, indicate that the extent of Gata1 reduction might determine the level, in the hematopoietic hierarchy, of the cell targeted by the mutation. In fact, the Gata1null mutation affects bipotent megakaryocytic/erythroid cells (the PEM?) that, although maturation defective, have such high proliferation activity to generate, both in culture and in the liver of chimeric mice, TPO-dependent megakaryocytic cell lines.59 As shown here, the hypomorphic Gata1low mutation affects cells at the restriction point between CMPs and MEPs or MCPs. On the other hand, the hypomorphic Gata10.5 mutation,60 which induces in chimeric mice either myeloid (at 2 months) or B-cell (at 1 year) leukemia,58 might affect cells at the restriction point between myeloid and B-cell development. Because the levels of Gata1 expression in Gata10.5 progenitor cells are unknown, the model cannot be completed with the hypothesis that the extent of Gata1 expression impairment is inversely correlated with the spectrum of progenitor cells affected.
A unifying model for the effects of different Gata1 mutations in hematopoiesis. All of the different Gata1 mutations described up to now affect the end stage of erythroid, megakaryocytic, and mast cell differentiation.1,56 Several Gata1 mutations also affect the biology of stem/progenitor cells. The Gata1s mutation increases the proliferation potential of fetal progenitor cells.57 We propose that the Gata10.5 mutation, being associated with myeloid and B-lymphoid leukemia,58 may act at the restriction point between hematopoietic stem cells (HSCs) and CMPs and common lymphoid progenitors (CLPs). The Gata1low mutation affects cells at the restriction point between CMPs and MEPs and MCPs (this report). On the other hand, the Gata1null mutation is associated with development of thrombopoietin-dependent cell lines and has been suggested to affect the restriction point between MEPs and CFU-MKday3 and CFU-E.59 Modified from Stachura et al59 with permission.
A unifying model for the effects of different Gata1 mutations in hematopoiesis. All of the different Gata1 mutations described up to now affect the end stage of erythroid, megakaryocytic, and mast cell differentiation.1,56 Several Gata1 mutations also affect the biology of stem/progenitor cells. The Gata1s mutation increases the proliferation potential of fetal progenitor cells.57 We propose that the Gata10.5 mutation, being associated with myeloid and B-lymphoid leukemia,58 may act at the restriction point between hematopoietic stem cells (HSCs) and CMPs and common lymphoid progenitors (CLPs). The Gata1low mutation affects cells at the restriction point between CMPs and MEPs and MCPs (this report). On the other hand, the Gata1null mutation is associated with development of thrombopoietin-dependent cell lines and has been suggested to affect the restriction point between MEPs and CFU-MKday3 and CFU-E.59 Modified from Stachura et al59 with permission.
The increased proliferation potential of mice carrying the Gata10.5 mutation is associated with development of leukemia.58 Furthermore, mice carrying the Gata1s mutation, which is associated with transient myeloproliferative disorders of newborns,13-15 have increased proliferation capacity of a “unique” fetal stem/progenitor cell extinguished in adult life.57 These results suggest that altered Gata1 expression, by increasing progenitor cell proliferation, may predispose to leukemia by favoring accumulation of secondary mutations. Although Gata1low mice do not develop leukemia, they develop a syndrome similar to idiopathic myelofibrosis (CD1) or essential thrombocytopenia (DBA/2), depending on background,29,61 2 diseases that may evolve to leukemia in humans.62 The high number (∼11%) of Gata1low MEPs that generate factor-dependent cell lines suggest that these mice, if exposed to stimuli such as SCF or IL-3 (or both), that increase MEP proliferation, might develop leukemia as well. Further studies will be necessary to clarify this point.
In conclusion, these results confirm the crucial role played by Gata1 in hematopoietic commitment and identify as new target for Gata1 activity the restriction point between CMPs and MEPs or MCPs.
Authorship
Contribution: B.G. designed the experiments, performed the cell purifications, the cultures, the serotonin release assays, prepared the data for publication, and wrote the manuscript; M.S. designed the experiments and supervised cell purification; F.M. performed cytofluorimetric analysis and cell sorting; G.A. performed quantitative RT-PCR analysis; A.M.V. established and characterized the SN cell lines and discussed the results; G.M. analyzed the data and discussed the results; S.H.O. analyzed the data and wrote the manuscript; and A.R.M. designed research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
All of the authors have read the manuscript, concur with its content, and state that the data have not been submitted anywhere else for publication.
Correspondence: Anna Rita Migliaccio, Hematology, Oncology and Molecular Medicine, Istituto Superiore Sanità, Viale Regina Elena 299, 00161 Rome, Italy; e-mail: migliar@iss.it.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Recombinant rat SCF was provided by Amgen (Thousand Oaks, CA; MTA no. 19982634-005). George Stamatoyannopoulos and Qiliang Li are gratefully acknowledged for support and encouragement.
This work was supported by Ministero per l'Università e la Ricerca Scientifica e Tecnologica (grants RBNE0189JJ 006, RBNE01sp72 003, and MM06103241); institutional funds from Istituto Superiore Sanità, Rome, Italy, and the University of Illinois at Chicago; National Institutes of Health (S.H.O); and National Cancer Institute (PO1-ca108671-01a2; mpd-rc) grants.

![Figure 1. The marrow of Gata1low mice contains progenitor cells with the myeloid, but not with the mastocytic, antigenic profile. (A) Diagrammatic scheme of the antigenic profiling used to define different progenitor cell types in this study. All myeloid and mast cell generating activity is present among the cKit+ cells of the Lin−Sca1−Ly6c−FcϵRIα[−] cells of normal marrow.18,21 The myeloid generating cells are divided by CD34 and CD16/CD32 staining into GMPs (CD34+CD16/CD32+, not shown), MEPs (CD34lowCD16/CD32low), and CMPs (CD34+CD16/CD32low).18 GMPs correspond to progenitor cells functionally defined as CFU-Gs, CFU-Ms, and CFU-GMs, and are not investigated in detail in this study. MEPs are cells restricted toward megakaryocyte and erythroid differentiation and include those cells once functionally defined as CFU-MKday3 and CFU-MKday7, CFU-E, and BFU-E. CMPs give rise to GMPs, MEPs, and MCPs, and correspond to cells functionally defined as capable to generate in vitro CFU-mix and in vivo CFU-Sday 12. The c-Kit+ bone marrow cells can be also divided by T1/ST2 staining into T1/ST2− cells that contain all of the myeloid cells described (myeloid progenitors, MPs) and T1/ST2+ cells, defined as MCPs, that generate c-Kit+/T1/ST2+ cells and mast cells within 7 days both in vitro (in semisolid and liquid cultures) and in vivo (in extramedullary sites).21 (B) Prospective identification and isolation of MEPs, CMPs, and MCPs from the marrow of Gata1low and wild-type littermates. Lin−c-Kit+ cells from normal marrow (the c-Kit staining of the CD34-CD16/CD32 analysis is not shown, for clarity) were further divided by CD34-CD16/CD32 (on the left) and T1/ST2 (on the right) staining according to prospective gates (indicated by rectangles) corresponding to MEPs or CMPs and MPs and MCPs, respectively. Wild-type MPs are defined as cells with the c-Kit+/T1/ST2− phenotype and contain, by definition, a mixture of CMPs, MEPs, and GMPs. In the case of wild-type cells, all of the sorted fractions were 80% to 95% pure, as shown by the presented reanalysis. The Lin−c-Kit+ cells from Gata1low bone marrow were also divided by CD34-CD16/CD32 staining according to the prospective gates corresponding to MEPs or CMPs (∼90% pure, by reanalysis). In contrast, T1/ST2+ cells were not detectable among Gata1low Lin−c-Kit+ cells. The absence of MCPs among the marrow cells from Gata1low mice was confirmed by arbitrarily sorting T1/ST2− cells into the one third-lower (MPlow) and one third-higher (MPhigh) portion of the staining, as indicated, and by showing that the profile of the 2 sorted populations was largely overlapping (< 60% pure). Results from 2 representative experiments are shown. Similar results were observed in at least 3 independent experiments per group. The mean (± SD) frequency of each population observed in multiple experiments is summarized in Table 1. (C) Gata1 expression in different classes of progenitor cells prospectively isolated from the marrow of wild-type and Gata1low mice, as indicated. Results are presented as mean (± SD) of at least 3 independent determinations for progenitor cell type. Values of Gata1 expression statistically different (P < .05) from those expressed by the corresponding CMPs are indicated by a single asterisk, whereas differences statistically significant between values observed in populations with the same profile purified from Gata1low and wild-type littermates are indicated by double asterisks.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/4/10.1182_blood-2006-07-030726/4/m_zh80040708390001.jpeg?Expires=1765970704&Signature=BYix5UB7IBsy1cwOYC~SvdtYXHrVKlwursfL6sbUa1BfIaCGeT61DkcBATVjwSU87Qy4iOZbuqnB0poRf-pAQFNhpMK2OYwX-6t-8DzokNHTPjFsYcOIhALt14hyCezvK14hRwz9KxaskBGjV4d7aBuFoBOinRuDRYz1sCHOQQ8QFCk0qS1lYzsip4qUwYQzHoEyHARM749lI~Z1tqcbTIwk0ZlQgSa0tz0FtZvgiFZUo~MyHXFG8YdZ8IFEl5xsDvz-QjydPktd3bc-6TzlOxhQ56whGyzQWuRrINKvMOJxO6LZ~lR1~egXItZS3h2hC9CCbJG7nTKXCFQfZT7mGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
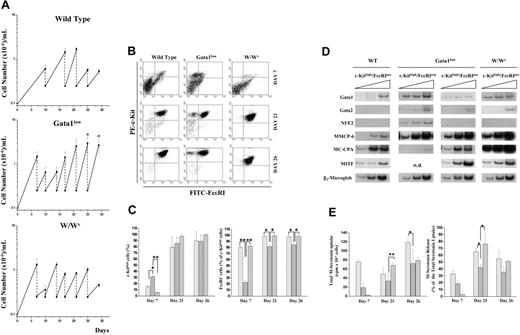
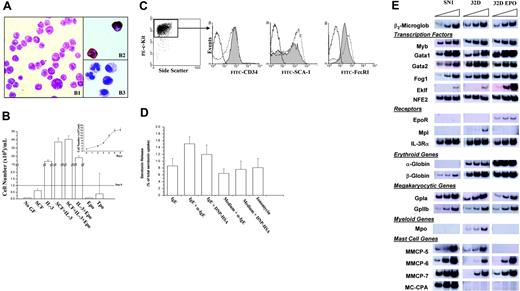
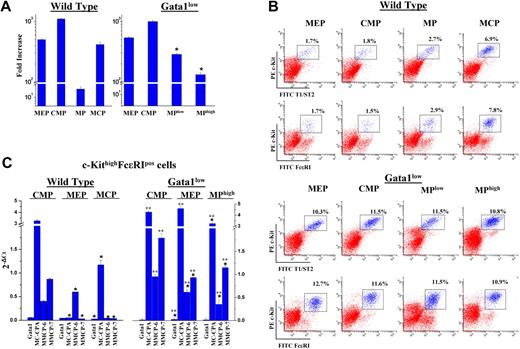


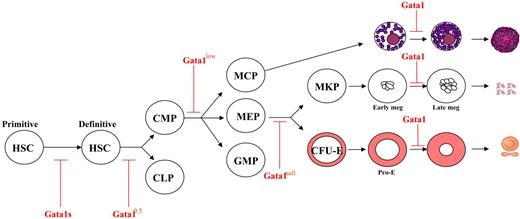
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal