Abstract
The key components of the intracellular molecular network required for the expression of a specific function of dendritic cells (DCs) are as yet undefined. Using an in vitro model of human monocyte-derived DC differentiation, this study investigates the role of glycogen synthase kinase 3 (GSK-3), a multifunctional enzyme critical for cellular differentiation, apoptosis, self-renewal, and motility, in this context. We demonstrate that GSK-3 (1) inhibits macrophage development during differentiation of DCs, (2) is constitutively active in immature DCs and suppresses spontaneous maturation, and (3) acquires a proinflammatory functional status mediating high levels of IL-12, IL-6, and TNF-α secretion, and partially inhibits IL-10 in the context of DC activation. In particular, GSK-3 enhances IL-12p35 mRNA expression and thus the production of the proinflammatory cytokine IL-12p70 by integrating the activities of other kinases priming GSK-3 targets and the inhibitory effects of Akt-1. GSK-3 may therefore act as a key integrator of activating and inhibitory pathways involved in proinflammatory DC differentiation and activation.
Introduction
Dendritic cells (DCs) are antigen-presenting cells, which in response to activation stimuli acquire the capacity of transporting antigen from peripheral sites of infection into the draining lymph nodes.1 This coincides with a change in their expression profile of surface markers, resulting in an augmentation of their capacity to interact with and activate naive T cells.2,3 Furthermore, DCs may also secrete high levels of proinflammatory cytokines if appropriately stimulated and interact with cells of the innate immune system, such as natural killer (NK) and NKT cells.4 These characteristics enable DCs to act in a position linking innate and adaptive immune responses. Understanding how the different functional aspects of DCs are regulated may help to design better strategies for using them as adjuvants in vaccination trials or for manipulating these cells in vivo.
Human monocyte-derived dendritic cells (MoDCs) respond to activation stimuli with an adaptive differentiation program that allows the development of diverse functional DC phenotypes. For instance, migratory DCs activated in the presence of prostaglandin E2 (PGE2) or cAMP are unable to secrete inflammatory cytokines, whereas CD40L-activated proinflammatory DCs, which secrete cytokines, are less efficient of migrating toward CCR7 ligands.5-7 These functional characteristics are independently regulated by a network of extra- and intracellular factors and pathways, and influenced by the strength and persistence of the activation stimulus.5,7 We suggested that the intracellular players that compose the molecular network required for the expression of a specific function are organized as a functional unit or “module” (signal response module, SRM).5 Despite an increasing knowledge of factors influencing any given functional module, however, it remains to be defined how the information about this network is integrated.
Due to its involvement in numerous signaling pathways, some of which are known to affect DC differentiation, one candidate regulator of DC function is glycogen synthase kinase 3 (GSK-3). GSK-3 is a multifunctional enzyme now recognized as a key regulator of numerous signaling pathways. More than 40 proteins including 18 transcription factors have been reported to be substrates of GSK-3 (reviewed in Jope and Johnson8 ). Two isoforms of GSK-3 (GSK-3α and GSK-3β) have been described that are highly homologous in their kinase domain, but little is known about isoform-specific functions. GSK-3 is regulated by multiple mechanisms including phosphorylation of GSK-3 itself and its target proteins, intracellular localization, and formation of protein complexes, such as β-catenin/GSK-3 in the canonical wnt pathway.8-10
GSK-3 is linked to a diverse array of neurologic diseases, such as bipolar mood disease,11,12 schizophrenia,13 and Alzheimer disease,14 but also to nonneurologic diseases, such as diabetes mellitus15,16 and cancer.17-19 It is intriguing that most of these diseases are associated with immune deviations. Particularly, patients suffering from bipolar mood disorders and schizophrenia, who were reported to have an increased baseline GSK-3 activity,13 also have increased serum levels of proinflammatory cytokines, such as IL-1,20 IL-6/IL-6R,21-23 IL-12p70,24 and IL-18.25 Chronic treatment with the GSK-3 inhibitor lithium chloride (LiCl) normalizes these deviations together with its therapeutic psychotropic effect. Furthermore, LiCl has successfully been used as a treatment in animal models of autoimmune diseases.26 In vitro, human blood cultures produced significantly less IL-2 and IFN-γ, with an increase in IL-4 and IL-10 levels in the presence of LiCl.27 Recently, Martin et al28 have demonstrated that GSK-3 inhibitors enhance IL-10 secretion and inhibit IL-12p40, IL-6, and TNF-α secretion of human monocytes. Furthermore, mice challenged with endotoxin could be rescued from death by septic shock using GSK-3 inhibitors.28
Based on these observations, this study investigated whether GSK-3 plays a role in regulating DC function. Altogether, GSK-3 emerges as a key checkpoint for the proinflammatory SRM of DCs, thereby being a possible target for therapeutic interventions in autoimmune diseases and transplantation settings.
Materials and methods
Media
Monocytes and MoDCs were cultured in RPMI 1640 (Sigma-Aldrich Chemie, Taufkirchen, Germany) supplemented with 60 mg/L penicillin G, 12.6 mg/L streptomycin, 2 mM l-glutamine, 1% nonessential amino acids, and 10% heat-inactivated fetal calf serum (FCS; Sigma-Aldrich Chemie) in a 5% CO2 incubator.
Monoclonal antibodies, enzyme-linked immunosorbent assay (ELISA) kits, cytokines, and chemokines
Flow cytometric analyses of MoDCs were performed using the following monoclonal antibodies (mAbs): fluorescein isothiocyanate (FITC)–conjugated IgG1 isotype control; phycoerythrin (PE)–conjugated IgG1 isotype control; PE-conjugated IgG2a isotype control; anti–CD86(B70/B7-2)–PE; anti–CD83-PE; anti–CD80(B7-1)–FITC; anti–HLA-A, -B, -C–FITC; and anti–HLA-DR–PE (all from BD Biosciences, Heidelberg, Germany); and anti–CCR7-FITC (R&D Systems, Wiesbaden, Germany). Cytokine ELISA-kits (OptEIA brand) for IL-6, IL-12p40, IL-12p70, TNF-α, and IL-10 were purchased from BD Biosciences. The following cytokines were added to MoDC cultures: recombinant human (rh) GM-CSF (40 ng/mL; Berlex, Seattle, WA), and rhTNF-α (10 ng/mL), rhIL-4 (50 U/mL), and IFN-α2a (2000 IU/mL) (all from PromoCell, Heidelberg, Germany). The BHK cell line expressing CD40L was a gift of Dr E. Leo, University of Heidelberg. Expression of CD40L was confirmed by flow cytometry using an anti-CD40L mAb (BD Biosciences). CCL21 (6Ckine) was purchased from PromoCell and used in migration assays at 40 ng/mL. The following GSK inhibitors were used: LiCl (Merck Biosciences, Darmstadt, Germany), SB216763 and SB415286 (both from Sigma-Aldrich Chemie), and 1-azakenpaullone (Merck Biosciences). The membrane-permeable synthetic cAMP-analog and activator of protein kinase A (PKA) (Sp-5,6-DCl-cBIMPS) was purchased from Biolog (Bremen, Germany). The PI3K inhibitor wortmannin was purchased from Sigma-Aldrich Chemie.
Escherichia coli (XL-1) were cultured in LB-Broth until an OD600 of 0.7, then washed 3 times, resuspended in 60 mM CaCl2, 10 mM PIPES (pH 7.0), 15% glycerol (1/40 of the original volume), and frozen in 50-μL aliquots. Directly prior to activation, one E coli aliquot was thawed, washed in dd H2O, and resuspended in 1 mL H2O. This solution (20 μL) was added to 1 mL MoDC cultures; this concentration is referred to as 1:1.
MoDCs
Peripheral blood mononuclear cells (PBMCs) were obtained from buffy coat preparations from healthy donors from the Red Cross Blood Bank (Heidelberg, Germany) and used to produce MoDCs. CD14+ monocytes were affinity purified using the magnetic-activated cell sorter (MACS) CD14 isolation kit (Miltenyi Biotech, Bergisch-Gladbach, Germany) and either activated immediately or cultured at 0.5 to 1 × 106 cells/mL in culture medium supplemented with GM-CSF (40 ng/mL) and IL-4 (50 U/mL) in 24-well plates. By days 5 to 7, MoDCs represented more than 95% of cultured cells. On days 5 to 7, cells were washed and readjusted to 2 to 3 × 105 MoDCs/mL. Maturation-inducing factors were added, and cells and supernatants were harvested 24 to 36 hours later. All cytokines and DC stimuli used in the present study have previously been tested in dose titration analyses, and the concentrations used in the figures represent those found to be optimal.
Phagocytosis assay
Effector cells were washed twice with PBS and incubated with Saccharomyces cerevisiae at a concentration of 106/mL at 37°C in culture medium. After 1 hour, cells were washed and stained with hematoxylin and eosin. Yeast particles phagocytosed to more than 50% were counted in a total of 100 cells. Percent phagocytosis ([number of cells containing yeast]/[100 cells]) and phagocytic index ([mean number of yeast particles]/[number of phagocytic cells]) were calculated.
Cytokine ELISA
Cytokine secretion by stimulated MoDCs was measured by ELISA. IL-6, IL-12p40, IL-12p70, TNF-α, and IL-10 ELISAs were performed on supernatants of monocyte and MoDC cultures according to the manufacturer's instructions using Maxisorp plates (Nunc, Wiesbaden, Germany). The HRP substrate was tetramethylbenzidine (TMB) peroxidase (BD Biosciences); the color reaction was terminated by adding 100 μL sulfuric acid (2 N). Plates were read in a Sunrise microplate reader (Tecan, Salzburg, Austria).
Migration assays
MoDCs matured with the indicated stimuli for 36 to 48 hours were harvested, washed, and tested for migration toward the chemokine CCL21 (6Ckine, a ligand for CCR7) in a standard transwell assay.1 Briefly, lower chambers of transwell plates (5.0-μm pore size; Costar, Corning, NY) were filled with 350 μL culture medium with or without CCL21 (40 ng/mL). MoDCs (1-2 × 104) were added in 50 μL RPMI/10% FCS into the upper chamber, and cells were incubated at 37°C for 3 to 4 hours. Cells in the lower chambers were harvested, and counted with a hemocytometer. Each condition was performed in duplicate wells.
Western blot analysis
MoDCs activated for 2 to 24 hours with the indicated stimuli were harvested, washed, resuspended at a density of 5 × 106 cells/mL in Western sample buffer (100 mM Tris/HCL [pH 6.8], 4% SDS, 0.2% bromophenol-blue, 20% glycerol, 200 mM DTT), and snap frozen. Cell lysates were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blots as described previously2 using antibodies specific for phospho-Akt (Ser473), phospho–Ser21/9-GSK3α/β (Cell Signaling, New England Biolabs, Frankfurt AM Main, Germany), phospho–(Tyr279/216)-GSK3α/β (BioSource Deutschland, Solingen, Germany), β-catenin (Dianova, Hamburg, Germany), p38 and ERK-1/2 (Santa Cruz, Heidelberg, Germany), β-actin (MP Biomedicals, Illkirch, France), and HRP-conjugated antibodies to rabbit IgG (Santa Cruz). Western blots were developed with enhanced chemiluminescence (ECL) Western blot system (Santa Cruz). For quantitative Western blot analysis, the ECL-Signal was quantified using the Lumi-Imager F1 and the LumiAnalyst Version 3.1 for Windows Software (Roche Diagnostics, Mannheim, Germany).
Real-time reverse-transcription–polymerase chain reaction (RT-PCR) quantification
Cells (2 × 106) were collected in 400 μL lysis buffer from the MagnaPure mRNA Isolation Kit I (RAS, Mannheim, Germany) supplemented with 1% (wt/vol) DTT, and mRNA was isolated with the MagnaPure-LC device using the mRNA-I standard protocol. The elution volume was set to 50 μL. An aliquot of 8.2 μL RNA was reverse transcribed using AMV-RT and oligo-(dT) as primer (First Strand cDNA synthesis kit; RAS) according to the manufacturer's protocol in a thermocycler. After termination of the cDNA synthesis, the reaction mix was diluted to a final volume of 500 μL and stored at −20°C until PCR analysis. Primer sets specific for the sequences of IL-12p35, IL-12p40, IL-6, IL-10, and TNF-α optimized for the LightCycler (RAS) were developed and provided by SEARCH-LC (Heidelberg, Germany). The PCR was performed with the LightCycler FastStart DNA Sybr GreenI kit (RAS) according to the protocol provided in the parameter-specific kits. To control for specificity of the amplification products, a melting curve analysis was performed. No amplification of unspecific products was observed. The copy number was calculated from a standard curve, obtained by plotting known input concentrations of 4 different plasmids at log dilutions to the PCR cycle number (CP) at which the detected fluorescence intensity reaches a fixed value. This approach dramatically reduced variations due to handling errors over several logarithmic dilution steps. To correct for differences in the content of mRNA, the calculated copy numbers were normalized according to the average expression of 2 housekeeping genes, cyclophilin B and β-actin. Values were thus given as input adjusted copy number per microliter of cDNA.
Acquisition of microscope images
Phase-contrast light micrographs of cell cultures were acquired using a Leica DMIL inverted microscope (Leica Microsystems, Wetzlar, Germany) equipped with a Leica 20×/0.4 NA PH1 N PLAN objective lens. Nikon View v4.0 software (Nikon, Düsseldorf, Germany) was used to import images from a Nikon Coolpix 995 photo camera mounted on the microscope. Images were processed with Adobe Photoshop software v7.0 (Adobe Systems, München, Germany).
Results
GSK-3 promotes MoDC differentiation of human monocytes
Differentiation of human MoDCs from CD14+ monocytes requires 4 to 7 days of culture in GM-CSF and IL-4. Addition of the GSK-3 inhibitors LiCl, SB415286, or SB216763 on day 0 inhibited the development of MoDCs resulting in monocytes differentiating toward macrophage-like cells (Figure 1). Cell yields in day-5 cultures were lower in the presence of SB415286 (62% ± 9%, compared with cultures in the absence of the inhibitor [100%]; n = 6). In contrast to MoDCs generated without GSK-3 inhibitors, these cells displayed an elongated morphology, were strongly adherent in culture (Figure 1A), and expressed strong phagocytic activity of Saccharomyces cerevisiae (Figure 1B). Following activation with E coli, they up-regulated MHC classes I and II similarly to MoDCs. However, in contrast to MoDCs, they strongly expressed CD14, lacked expression of CD83, and expressed low levels of CD80 and CD86 (Figure 1C). Furthermore, upon E coli activation, these GSK-3–inhibited cells secreted similar levels of TNF-α, and higher levels of IL-6 and IL-10, but significantly lower levels of IL-12p40 and no IL-12p70 compared with conventional MoDCs (Figure 1D). These data suggest that GSK-3 activity is essential for differentiation of monocytes into MoDCs, and in its absence monocytes will instead differentiate into macrophage-like cells.
GSK-3 inhibits macrophage differentiation of human monocytes. CD14+ monocytes were cultured for 5 days in the presence of GM-CSF and IL-4. The GSK-3 inhibitors LiCl (10 mM), SB415286 (10 μM), and SB216763 (10 μM) were added on day 0. After 5 days of culture, cells were either tested in phagocytosis assay or were activated with intact E coli (1:1000; “Materials and methods”) for an additional 36 hours and examined for phenotypic maturation and cytokine secretion. (A) Photomicrographs of DC culture on day 5 in the absence (top panel) or presence (bottom panel) of the GSK-3 inhibitor (SB415286). One representative of 6 cultures is shown. Similar results were observed in the presence of LiCl and SB216763. (B) Phagocytosis of day-5 cultures with and without GSK-3 inhibitors. (Left panel) Phagocytic index (number of yeast particles per cell). (Right panel) Proportion of cells containing yeast particles. Shown are the means ± SD; n = 4. *P < .05; **P < .01. (C) Flow cytometric analysis of DC surface maturation marker expression following activation with E coli for 36 hours. One representative of 4 experiments is shown. (D) Cytokine secretion of E coli–activated cells. Supernatants were harvested 36 hours following activation. Absolute cytokine levels of E coli–activated MoDCs (set 1): IL-12p70: 5.4 ± 2.5 ng/mL; IL-12p40: 22.2 ± 7.9 ng/mL; IL-6: 28.1 ± 20 ng/mL; IL-10: 2.3 ± 2.3 ng/mL; and TNF-α: 10.6 ± 9.5 ng/mL. Shown are the means ± SD; n = 7-10. *P < .05; **P < .01.
GSK-3 inhibits macrophage differentiation of human monocytes. CD14+ monocytes were cultured for 5 days in the presence of GM-CSF and IL-4. The GSK-3 inhibitors LiCl (10 mM), SB415286 (10 μM), and SB216763 (10 μM) were added on day 0. After 5 days of culture, cells were either tested in phagocytosis assay or were activated with intact E coli (1:1000; “Materials and methods”) for an additional 36 hours and examined for phenotypic maturation and cytokine secretion. (A) Photomicrographs of DC culture on day 5 in the absence (top panel) or presence (bottom panel) of the GSK-3 inhibitor (SB415286). One representative of 6 cultures is shown. Similar results were observed in the presence of LiCl and SB216763. (B) Phagocytosis of day-5 cultures with and without GSK-3 inhibitors. (Left panel) Phagocytic index (number of yeast particles per cell). (Right panel) Proportion of cells containing yeast particles. Shown are the means ± SD; n = 4. *P < .05; **P < .01. (C) Flow cytometric analysis of DC surface maturation marker expression following activation with E coli for 36 hours. One representative of 4 experiments is shown. (D) Cytokine secretion of E coli–activated cells. Supernatants were harvested 36 hours following activation. Absolute cytokine levels of E coli–activated MoDCs (set 1): IL-12p70: 5.4 ± 2.5 ng/mL; IL-12p40: 22.2 ± 7.9 ng/mL; IL-6: 28.1 ± 20 ng/mL; IL-10: 2.3 ± 2.3 ng/mL; and TNF-α: 10.6 ± 9.5 ng/mL. Shown are the means ± SD; n = 7-10. *P < .05; **P < .01.
GSK-3 is constitutively active and inhibits spontaneous maturation of immature MoDCs
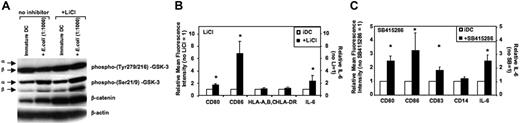
The effects of GSK-3 inhibitors on immature DCs and during activation of DCs were investigated next. Generally, GSK-3 inhibitors did not alter viability or cell yields of DCs during the 36- to 48-hour activation period (n = 5). GSK-3 is negatively regulated by phosphorylation of an N-terminal serine (Ser21 for GSK-3α and Ser9 for GSK-3β). In contrast, GSK-3 activity is facilitated upon phosphorylation of specific tyrosines (Tyr279 in GSK-3α and Tyr216 in GSK-3β).29,30 GSK-3 is constitutively active in immature MoDCs as shown by high Tyr279/216 phosphorylation and low Ser21/9 phosphorylation of GSK-3 (Figure 2A). LiCl inhibits GSK-3 activity in immature MoDCs as demonstrated by increased Ser21/9 phosphorylation; however, tyrosine phosphorylation was not altered (Figure 2A; see also Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). The reduction of GSK-3 activity by LiCl is reflected by increased levels of the GSK-3 substrate β-catenin (Figure 2A). Inhibition of GSK-3 activity in immature MoDCs resulted in increased expression of CD80, CD83, and CD86, and higher levels of IL-6 secretion within 24 to 36 hours (Figure 2B-C). Absolute levels of IL-6 secretion without LiCl were 177 ± 77 pg/mL; in the presence of LiCl (10 mM), these were increased to 298 ± 131 pg/mL and were also elevated to 384 ± 131 pg/mL in the presence of the GSK-3 inhibitor SB415286 (10 μM). These results demonstrate that GSK-3 is constitutively active in immature MoDCs and inhibits their spontaneous maturation.
GSK-3 is constitutively active in and inhibits spontaneous maturation of immature MoDCs. Immature MoDCs were washed on days 5 to 7 of culture and resuspended in culture medium at a concentration of 2 to 3 × 105 cells/mL. Cells were either continued in GM-CSF and IL-4 (iDC) or activated with E coli (1:1000). LiCl (10 mM) or SB415286 (10 μM) was added 20 minutes prior to activation or together with GM-CSF and IL-4. (A) Western blot analysis of DC lysates harvested 2 hours following activation and/or addition of LiCl. β-Actin was used to control for protein loading. One representative of 4 experiments is shown. (B) Flow cytometry analysis and IL-6 ELISAs of the supernatants were performed 36 hours after exposure to LiCl. Mean fluorescence values of CD80, CD86, HLA-A, -B, and -C, and HLA-DR and IL-6 levels in the supernatants are shown for MoDCs relative to MoDCs cultured in the absence of LiCl. Shown are the means ± SD; n = 4 (FACS) and n = 14 (for IL-6). *P < .05 compared with MoDCs not exposed to LiCl. (C) Mean fluorescence values of CD80, CD83, CD86, CD14, and IL-6 levels in the supernatants are shown for MoDCs relative to MoDCs cultured in the absence of SB415286. Shown are the means ± SD; n = 3-6 (FACS) and n = 14 (for IL-6). *P < .05 compared with MoDCs not exposed to LiCl.
GSK-3 is constitutively active in and inhibits spontaneous maturation of immature MoDCs. Immature MoDCs were washed on days 5 to 7 of culture and resuspended in culture medium at a concentration of 2 to 3 × 105 cells/mL. Cells were either continued in GM-CSF and IL-4 (iDC) or activated with E coli (1:1000). LiCl (10 mM) or SB415286 (10 μM) was added 20 minutes prior to activation or together with GM-CSF and IL-4. (A) Western blot analysis of DC lysates harvested 2 hours following activation and/or addition of LiCl. β-Actin was used to control for protein loading. One representative of 4 experiments is shown. (B) Flow cytometry analysis and IL-6 ELISAs of the supernatants were performed 36 hours after exposure to LiCl. Mean fluorescence values of CD80, CD86, HLA-A, -B, and -C, and HLA-DR and IL-6 levels in the supernatants are shown for MoDCs relative to MoDCs cultured in the absence of LiCl. Shown are the means ± SD; n = 4 (FACS) and n = 14 (for IL-6). *P < .05 compared with MoDCs not exposed to LiCl. (C) Mean fluorescence values of CD80, CD83, CD86, CD14, and IL-6 levels in the supernatants are shown for MoDCs relative to MoDCs cultured in the absence of SB415286. Shown are the means ± SD; n = 3-6 (FACS) and n = 14 (for IL-6). *P < .05 compared with MoDCs not exposed to LiCl.
GSK-3 is inhibited during MoDC activation
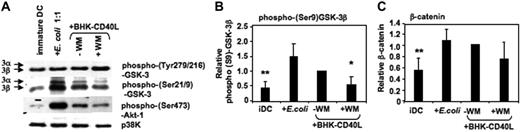
Akt-1 has been shown to phosphorylate GSK-3 at the inhibitory Ser21/9 moiety.31,32 We have recently reported that Akt-1 is activated (ie, Ser473 phosphorylated) during proinflammatory stimulation of DCs.5 Here, we demonstrate that the expression of phospho–Akt-1 correlates with phosphorylation of GSK-3 at Ser21/9. MoDCs with the highest levels of phospho–Akt-1 (following activation with E coli) also displayed the highest levels of phospho–Ser21/9-GSK-3 (Figure 3A-B). As expected, wortmannin (WM) inhibited Akt-1 phosphorylation, and this resulted in reduced Ser21/9 phosphorylation of GSK-3 (Figure 3A-B). Consistent with the observed higher levels of Ser21/9 phosphorylation of GSK-3, the level of β-catenin also increased during DC activation (Figure 3C). These data suggest a net inhibition of GSK-3 activity following the activation of proinflammatory-type MoDCs.
GSK-3 is inhibited during DC activation. MoDCs were prepared as in Figure 2. Cells were either continued in GM-CSF and IL-4 (iDC), or activated with E coli (1:1) or a baby hamster kidney cell line transfected with CD40L (BHK-CD40L, in a 20:1 ratio of DCs to BHK cells) in presence or absence of wortmannin (WM; 100 ng/mL). Cells were harvested and resuspended in lysis buffer 2 hours following activation. (A) Ser21/9 and Tyr279/216 phosphorylation of GSK-3 and Ser473 phosphorylation of Akt-1 were analyzed by Western blot. p38K analysis acts as protein loading control. One representative of 4 experiments is shown. (B) Strength of Ser9 phosphorylation of GSK-3β and (C) intracellular β-catenin expression were assessed by quantitative immunoblotting. Shown are the means ± SD; n = 4.
GSK-3 is inhibited during DC activation. MoDCs were prepared as in Figure 2. Cells were either continued in GM-CSF and IL-4 (iDC), or activated with E coli (1:1) or a baby hamster kidney cell line transfected with CD40L (BHK-CD40L, in a 20:1 ratio of DCs to BHK cells) in presence or absence of wortmannin (WM; 100 ng/mL). Cells were harvested and resuspended in lysis buffer 2 hours following activation. (A) Ser21/9 and Tyr279/216 phosphorylation of GSK-3 and Ser473 phosphorylation of Akt-1 were analyzed by Western blot. p38K analysis acts as protein loading control. One representative of 4 experiments is shown. (B) Strength of Ser9 phosphorylation of GSK-3β and (C) intracellular β-catenin expression were assessed by quantitative immunoblotting. Shown are the means ± SD; n = 4.
GSK-3 inhibitors block E coli–induced IL-12p70 secretion in MoDCs and reduce IL-12p40, IL-6, and TNF-α, but not IL-10, secretion by monocytes or MoDCs
Monocytes stimulated with intact E coli (Figure S2) or LPS (n = 6, data not shown) did not secrete IL-12p70. In contrast, human MoDCs produced high levels of IL-12p70 following E coli stimulation, and this was substantially blocked by either LiCl or SB415286 (Figure 4). IL-12p40, IL-6, and TNF-α secretion were partially inhibited by LiCl or SB415286 in both monocytes and MoDCs (Figure 4; Figure S2). In contrast, IL-10 secretion by either monocytes or MoDCs was not significantly affected by the addition of LiCl, while addition of SB41586 only weakly inhibited IL-10 secretion by MoDCs. As GSK-3 inhibitors have been reported to enhance IL-10 secretion,28 we confirmed our observations using positively and negatively selected CD14+ monocytes activated with LPS (1 μg/mL) and intact E coli at high (1:1) and low (1:1000) concentrations (“Materials and methods”). However, IL-10 secretion was not enhanced in the presence of either LiCl or SB415286 under any of these experimental conditions (n = 4-16, data not shown). Furthermore, using a third GSK-3 inhibitor (1-azakenpaullone, 1 μM), we confirmed the enhancing effects of GSK-3 on proinflammatory cytokines, particularly IL-12p70 (Figure S3). Fluorescence-activated cell sorter (FACS) analysis of DCs activated with E coli in the presence or absence of LiCl or SB415286 showed that the inhibitors reduced, but did not prevent, the expression of the antigen-presenting phenotype (Figures S4–S5).
GSK-3 is necessary for IL-12p70 secretion and enhances IL-12p40, IL-6, and TNF-α, but not IL-10, secretion by DCs activated with E coli. MoDCs were prepared as in Figure 2–3. Cells were either continued in GM-CSF and IL-4 (no activation) or activated with E coli in the presence or absence of LiCl (10 mM) and SB415286 (10 μM) on days 5 to 7 of culture. Supernatants were harvested after 36 hours. Absolute cytokine levels in the absence of inhibitors: IL-12p70: 2.1 ± 1 ng/mL; IL-12p40: 54.2 ± 10.5 ng/mL; IL-6: 109.7 ± 25 ng/mL; IL-10: 7.2 ± 0.8 ng/mL; and TNF-α: 16.1 ± 3.8 ng/mL. Shown are the means ± SEM; n = 6. *P < .05, **P < .01 compared with activation without a GSK-3 inhibitor.
GSK-3 is necessary for IL-12p70 secretion and enhances IL-12p40, IL-6, and TNF-α, but not IL-10, secretion by DCs activated with E coli. MoDCs were prepared as in Figure 2–3. Cells were either continued in GM-CSF and IL-4 (no activation) or activated with E coli in the presence or absence of LiCl (10 mM) and SB415286 (10 μM) on days 5 to 7 of culture. Supernatants were harvested after 36 hours. Absolute cytokine levels in the absence of inhibitors: IL-12p70: 2.1 ± 1 ng/mL; IL-12p40: 54.2 ± 10.5 ng/mL; IL-6: 109.7 ± 25 ng/mL; IL-10: 7.2 ± 0.8 ng/mL; and TNF-α: 16.1 ± 3.8 ng/mL. Shown are the means ± SEM; n = 6. *P < .05, **P < .01 compared with activation without a GSK-3 inhibitor.
The capacity of LiCl and SB415286 to inhibit GSK-3 in activated DCs was confirmed by Western blot analysis. Addition of LiCl enhanced both β-catenin expression and GSK-3–Ser21/9 phosphorylation within 2 hours following activation of MoDCs with low concentrations of E coli (Figure 2A and Figure S1). In contrast, SB415286 did not increase serine phosphorylation of GSK-3 but enhanced β-catenin expression (Figure S1).
GSK-3 enhances proinflammatory cytokine secretion of CD40L-activated MoDCs
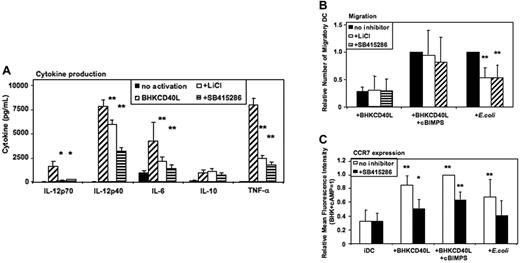
Activation of MoDCs using a baby hamster kidney (BHK) cell line transfected with CD40L mediates a strong proinflammatory signal similar to the effects of soluble CD40L trimers used in previous studies,6,7 resulting in the secretion of high levels of cytokines. As in E coli–activated MoDCs, LiCl, SB415286, and 1-azakenpaullone blocked IL-12p70 secretion and reduced IL-12p40, IL-6, and TNF-α secretion of CD40L-activated MoDCs (Figure 5A and Figure S3). Similarly, IL-10 was not significantly influenced (Figure 5A). FACS analyses of MoDCs activated with BHK-CD40L in the presence of LiCl or SB415286 showed a reduced up-regulation of CD83, but no effect on induction of CD80, CD86, and HLA-I and -II expression (Figures S4–S5).
GSK-3 enhances proinflammatory cytokine secretion by CD40L-activated DCs and reduces migration of E coli–activated DCs. Immature MoDCs were prepared as in Figure 2–3. Cells were either continued in GM-CSF + IL-4 (no activation), or activated with a baby hamster kidney cell line transfected with CD40L (BHK-CD40L) at a 20:1 ratio of MoDCs to BHK-CD40L cells with or without LiCl (10 mM) or with or without SB415286 (10 μM). (A) Cytokine levels in MoDC culture supernatants 36 hours after activation. Shown are means ± SEM; n = 6. *P < .05, **P < .01 compared with activation with the BHK-CD40L cell line. (B) Migration toward CCL21 (40 nM) of MoDCs activated with BHK-CD40L cells and the cAMP-analog Sp-5,6-DCl-cBIMPS (cBIMPS, 50 μM) or with E coli as in Figure 3. Migration was tested in transwell assays with a pore size of 5 μm. Number of separate donors: BHK-CD40L + cBIMPS, n = 6; E coli, n = 15. **P < .01 compared with activation without inhibitor. (C) Expression of CCR7 36 to 38 hours after activation of MoDCs with or without SB415286. Mean fluorescence values relative to DCs activated with BHK-CD40L + cBIMPS (highest levels). Shown are means ± SD; n = 8. *P < .05, **P < .01 compared with immature DCs without the inhibitor.
GSK-3 enhances proinflammatory cytokine secretion by CD40L-activated DCs and reduces migration of E coli–activated DCs. Immature MoDCs were prepared as in Figure 2–3. Cells were either continued in GM-CSF + IL-4 (no activation), or activated with a baby hamster kidney cell line transfected with CD40L (BHK-CD40L) at a 20:1 ratio of MoDCs to BHK-CD40L cells with or without LiCl (10 mM) or with or without SB415286 (10 μM). (A) Cytokine levels in MoDC culture supernatants 36 hours after activation. Shown are means ± SEM; n = 6. *P < .05, **P < .01 compared with activation with the BHK-CD40L cell line. (B) Migration toward CCL21 (40 nM) of MoDCs activated with BHK-CD40L cells and the cAMP-analog Sp-5,6-DCl-cBIMPS (cBIMPS, 50 μM) or with E coli as in Figure 3. Migration was tested in transwell assays with a pore size of 5 μm. Number of separate donors: BHK-CD40L + cBIMPS, n = 6; E coli, n = 15. **P < .01 compared with activation without inhibitor. (C) Expression of CCR7 36 to 38 hours after activation of MoDCs with or without SB415286. Mean fluorescence values relative to DCs activated with BHK-CD40L + cBIMPS (highest levels). Shown are means ± SD; n = 8. *P < .05, **P < .01 compared with immature DCs without the inhibitor.
GSK-3 inhibitors specifically inhibit E coli–induced migration, but have no influence on CD40L/cAMP-induced migration
MoDCs activated with BHK-CD40L migrate poorly toward CCR7 ligands,5-7 whereas E coli–activated MoDCs express significant migratory capacity. Addition of CD40L together with cBIMPS induced migratory capacity but selectively blocked secretion of IL-12p70, TNF-α, and IL-10. IL-6 secretion and IL-12p40 secretion were not influenced by cAMP5 (Figure S6). We next investigated the influence of GSK-3 inhibitors on CD40L- and E coli–induced migration. LiCl and SB415286 did not influence migration of MoDCs induced by CD40L and cAMP (Figure 5B) or CD40L without cAMP (Figure 5B). However, migration induced by E coli was reduced to about 60% by LiCl and SB415286 (Figure 5B) as well as 1-azakenpaullone (Figure S3). This difference between CD40L-activated and E coli–activated DCs is supported by FACS analysis of CC chemokine receptor 7 (CCR7) surface expression. SB415286 significantly reduced CCR7 expression in all protocols of DC activation (Figure 5C). However, this reduction was most relevant for E coli–activated DCs. Here, surface CCR7 expression in the presence of the inhibitor did not differ from expression by immature DCs. This suggests that depending on the activation context, GSK-3 influences migration of MoDCs.
Reverse effects of PI3K and GSK-3 on IL-12 and IL-10 secretion
As mentioned previously, the PI3K/Akt pathway is activated during proinflammatory MoDC stimulation, thus inhibiting GSK-3. It was shown that inhibition of PI3K by wortmannin increases IL-12p70 secretion by activated MoDCs.5 Therefore, we investigated whether inhibiting GSK-3 activity would reverse the effects of wortmannin on MoDC cytokine secretion. LiCl is a complex GSK-3 inhibitor that not only inhibits GSK-3 by replacing Mg2+ at a site distinct from the ATP-binding site,33 but also may directly antagonize GSK-3 by enhancing PI3K/Akt-1 activity and resulting in increased Ser21/9 phosphorylation levels of GSK-3 (Chalecka-Franaszek and Chuang34 and Figure 2A). Thus, some effects of LiCl may also be explained by a modulation of PI3K activity. Therefore, we investigated the interactions between wortmannin and SB415286, a PI3K-independent GSK-3 inhibitor that does not increase GSK-3–Ser21/9 phosphorylation.
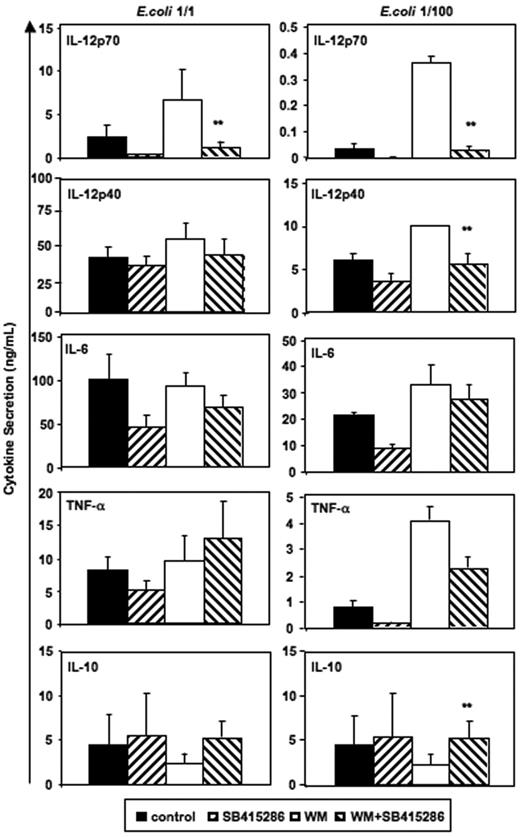
Figure 6 demonstrates that the opposing effects of wortmannin and SB415286 were significant for IL-12p70. SB415286 significantly decreased wortmannin-mediated IL-12p40 secretion only at low concentrations of intact E coli and did not inhibit IL-6 and TNF-α secretion. Similarly, GSK-3 inhibitors (SB415286 [10 μM], LiCl [10 mM], and 1-azakenpaullone [1 μM]) inhibited the wortmannin-enhanced IL-12p70 secretion of MoDCs activated with BHK-CD40L, but did not inhibit the wortmannin-enhanced secretion of IL-12p40, IL-6, or TNF-α (n = 4, data not shown). This suggests that other, indirect effects may explain the activity of GSK-3 inhibitors or wortmannin on the secretion of these cytokines. Alternatively, higher levels of GSK-3 inhibitors may be required to block the secretion of IL-6 and TNF-α, if GSK-3 activity is enhanced by wortmannin. Indeed, SB415286 at a concentration of 25 μM and azakenpaullone at a concentration of 10 μM significantly reduced secretion of all cytokines (induced by BHK-CD40L); however, viability was also reduced by about 30% (n = 4, data not shown), suggesting that this concentration was approaching cell toxic levels.
Reverse effects of PI3K and GSK-3 on IL-12 and IL-10 secretion. Immature MoDCs were prepared and activated as in Figure 2. SB415286 (10 μM) and wortmannin (WM, 100 ng/mL) were added 20 minutes prior to activation; E coli were used in 2 concentrations (1:1 and 1:100; “Materials and methods”). Supernatants were harvested 36 hours after activation. Shown are means ± SEM; n = 8 (E coli, 1:1) and n = 4 experiments (E coli, 1:100). **P < .01 (comparison of WM vs WM + SB415286 only).
Reverse effects of PI3K and GSK-3 on IL-12 and IL-10 secretion. Immature MoDCs were prepared and activated as in Figure 2. SB415286 (10 μM) and wortmannin (WM, 100 ng/mL) were added 20 minutes prior to activation; E coli were used in 2 concentrations (1:1 and 1:100; “Materials and methods”). Supernatants were harvested 36 hours after activation. Shown are means ± SEM; n = 8 (E coli, 1:1) and n = 4 experiments (E coli, 1:100). **P < .01 (comparison of WM vs WM + SB415286 only).
Interestingly, significant effects of wortmannin and SB415286 were observed for IL-10 secretion induced by low concentrations of intact E coli (Figure 6). Here, however, wortmannin inhibited and SB415286 enhanced IL-10 secretion, suggesting that E coli–induced IL-10 secretion is partially inhibited by GSK-3. Similarly, BHK-CD40L–induced IL-10 secretion was enhanced by the GSK-3 inhibitors in the presence of wortmannin (n = 4, data not shown). Our results substantiate that the enhancing effects of wortmannin on IL-12p70 secretion depend on GSK-3, and that the phosphorylation of the Ser21/9 moiety of GSK-3 by Akt-1 inhibits IL-12p70 secretion. Furthermore, our data support observations of Martin et al28 showing that GSK-3 is a partial inhibitor of IL-10 secretion.
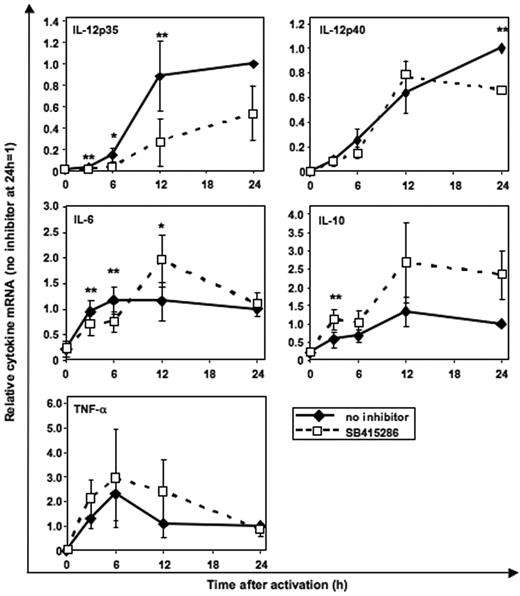
GSK-3 specifically enhances IL-12p35 mRNA expression and inhibits IL-10 mRNA expression
Using real-time PCR quantification, we investigated whether GSK-3 regulates cytokine secretion at the mRNA level. CD40L-induced IL-12p35 mRNA was significantly inhibited by SB415286 (Figure 7) as early as 3 hours following activation. In contrast, IL-12p40 mRNA was reduced in MoDCs only after 24 hours of activation. IL-10 mRNA was enhanced by SB415286 in all 3 MoDC cultures after 12 and 24 hours; however, due to the donor-to-donor variability (1.6- to 3.8-fold enhancement), statistical significance was not reached with 3 experiments. IL-6 mRNA was inhibited after 3 and 6 hours but enhanced after 12 hours following activation. TNF-α mRNA was not significantly changed by the GSK-3 inhibitor (Figure 7).
GSK-3 specifically enhances IL-12p35 mRNA expression and inhibits IL-10 mRNA expression. Real-time PCR quantification of mRNA in MoDCs stimulated with BHK-CD40L cell line as in Figure 3 with or without the GSK-3 inhibitor SB415286. Specificity of the mRNA signal was controlled by adding BHK-CD40L cells to the unstimulated MoDCs (0 h) and by analyzing the influence of mock-transfected BHK cells (no CD40L) (n = 1, not shown) on cytokine mRNA expression levels. Shown are the means ± SD; n = 3, normalized to the 24-hour mRNA levels without inhibitor. *P < .05, **P < .01 (comparison with and without SB415286).
GSK-3 specifically enhances IL-12p35 mRNA expression and inhibits IL-10 mRNA expression. Real-time PCR quantification of mRNA in MoDCs stimulated with BHK-CD40L cell line as in Figure 3 with or without the GSK-3 inhibitor SB415286. Specificity of the mRNA signal was controlled by adding BHK-CD40L cells to the unstimulated MoDCs (0 h) and by analyzing the influence of mock-transfected BHK cells (no CD40L) (n = 1, not shown) on cytokine mRNA expression levels. Shown are the means ± SD; n = 3, normalized to the 24-hour mRNA levels without inhibitor. *P < .05, **P < .01 (comparison with and without SB415286).
These results suggest that GSK-3 regulates IL-12p70 secretion by up-regulating the transcription of IL-12p35 and inhibits IL-10 mRNA expression. In contrast, less direct and less consistent effects were observed for the other 3 cytokines, supporting the hypothesis that indirect effects of GSK-3 may modulate the secretion of IL-6, IL-12p40, and TNF-α.
Discussion
Using our in vitro model of DC differentiation from human monocytes, we demonstrate that GSK-3 is crucially involved in the differentiation and activation of proinflammatory DCs. The present study demonstrates that during the early stages of cell culture in GM-CSF and IL-4, GSK-3 inhibitors can deviate the differentiation of monocytes from developing into MoDCs toward the macrophage lineage. In immature MoDCs, GSK-3 is constitutively active and inhibits their spontaneous maturation in culture over time (ie, increased CD80, CD83, and CD86 expression, and IL-6 secretion). Furthermore, following activation of MoDCs, GSK-3 activity contributed to enhanced IL-6, IL-12p40, and TNF-α secretion, similar to the effects of GSK-3 reported for monocytes by others.28 In contrast, IL-10 secretion was inhibited by GSK-3.28 This was also reflected by increased levels of IL-10 mRNA in the presence of the GSK-3 inhibitor SB415286. However, the strongest and most specific effects of GSK-3 were observed on secretion of IL-12p70, a cytokine that is not produced by monocytes under the activation conditions tested (ie, LPS or E coli). We could demonstrate that SB415286 inhibited CD40L-induced IL-12p35 mRNA expression in MoDCs, suggesting that GSK-3 regulates IL-12p70 production via activating IL-12p35 transcription.
Despite the proinflammatory effects of GSK-3, activation of MoDCs resulted in a net inhibition of GSK-3 activity. This was shown by the increased phosphorylation of GSK-Ser21/9 and the increased expression of β-catenin, a transcription factor that is phosphorylated by GSK-3 and thus targeted for proteasomal degradation.35 GSK-Ser21/9 phosphorylation was partly mediated by Akt-1, as phospho–Akt-1 levels correlated with phospho–Ser21/9-GSK-3 levels following activation, and wortmannin inhibited both Akt-1 and GSK-3–Ser phosphorylation.
Most GSK-3 substrates require “priming” (ie, phosphorylation by other kinases) before they will be further phosphorylated by GSK-3.10 A positively charged pocket of GSK-3, comprising R96, R180, and K205 residues, optimizes binding and orientation of primed targets increasing the efficiency of substrate phosphorylation by 100- to 1000-fold.10,36 The inhibitory Ser21/9 phosphorylation creates a pseudosubstrate that binds intramolecularly to the positively charged pocket. This mechanism of inhibition is competitive so that primed targets, at high enough concentrations, outcompete the pseudosubstrate and become phosphorylated.9,37
Induction of IL-12p70 secretion was strongly influenced by GSK-3 activity. We demonstrate that IL-12p70 secretion was enhanced by the PI3K inhibitor wortmannin, coinciding with reduced phosphorylation of the GSK-3–Ser21/9 moiety. As this enhancing effect of wortmannin could be opposed with the GSK-3 inhibitors LiCl, SB415286, and 1-azakenpaullone, we suggest that the wortmannin effect indeed targeted GSK-3, and competition between primed targets and the phospho-Ser21/9 moiety of GSK-3 may modulate levels of IL-12p70 secretion. Kinases priming GSK-3 targets may include the MAPK p38K and ERK1/2, as they are both activated following DC maturation, and a combination of inhibitors of either MAPK also blocks IL-12p70 secretion.7 In vivo evidence for opposing effects of the PI3K/Akt-1 pathway and GSK-3 during proinflammatory immune responses has recently been demonstrated in rats. Both insulin (activating PI3K) and the GSK-3 inhibitor TDZD-8 attenuated the organ injury/dysfunction associated with sepsis caused by lipopolysaccharide and peptidoglycan.38,39
Our data suggest a scenario where the constitutively active, inhibitory function of GSK-3 in immature DCs changes into a proinflammatory function in response to a changing intracellular context (Figure S7). This new context is characterized by the emergence of primed GSK-3 targets (ie, proteins phosphorylated by other kinases, such as p38K and ERK1/2). Proinflammatory cytokines such as IL-12p70, however, are cachectins and have to be tightly regulated. This regulation is partially achieved by Akt-1, an enzyme activated by all maturation-inducing stimuli used in our system. Akt-1 phosphorylates the serine residues 21/9 of GSK-3, thereby inhibiting the phosphorylation of the GSK-3–primed targets. This results in a down-regulation of IL-12p70 secretion, an effect counteracted by wortmannin. However, the GSK-3 phospho-Ser21/9 moiety acts as a competitive inhibitor of the phospho-binding pocket. High concentrations of primed GSK-3 targets may therefore successfully compete for access to GSK-3, resulting in a controlled proinflammatory activity of this enzyme.
Thus, secretion of IL-12p70 appears to be an integral part of (1) the activity of other kinases priming GSK-3 targets, (2) the activity of Akt-1 that inhibits GSK-3, and (3) the outcome of competition of primed GSK-3 targets and the phospho-Ser21/9 moiety for access to the phospho-binding pocket of GSK-3 (Figure S7). However, this picture is less clear for the other cytokines investigated. Compared with IL-12p70, cytokines such as IL-12p40, IL-6, and TNF-α were only partially inhibited by lithium, SB415286, or 1-azakenpaullone, suggesting GSK-3–dependent and –independent mechanisms. This is further underlined by the lack of efficacy of GSK-3 inhibitors to block WM-enhanced IL-6 and TNF-α secretion. Furthermore, RT-PCR quantification demonstrated that IL-12p40 mRNA levels responded to GSK-3 inhibitors only after 24 hours following activation. IL-6 mRNA was differentially influenced in the early and late periods following activation, whereas TNF-α mRNA was not significantly influenced by GSK-3. On the other side, wortmannin opposed the effects of SB415286 on IL-6 and TNF-α. Although more than one interpretation is possible, this observation may suggest additional inhibitory effects of the PI3K/Akt-1 pathway independent of GSK-3. These effects may also involve posttranslational processing and secretion of these cytokines. IL-10, in contrast, was partially inhibited by GSK-3 and enhanced by PI3K/Akt-1. This effect has been shown by other groups28 and suggests that GSK-3 specifically enhances a proinflammatory function of MoDCs.
Our results demonstrate that GSK-3 is a crucial enzyme involved in differentiation and maintenance of an immature phenotype of DCs. Despite partial functional inhibition, GSK-3 acquires a novel, proinflammatory task in the context of DC activation, probably due to a changing intracellular context. Further studies will have to confirm whether GSK-3 might become a target for therapeutic interventions in clinical immune imbalances, such as autoimmune diseases and transplantation settings. Modulation of this pathway during the course of vaccine-based immunotherapy may enhance DC activation in vivo and thus vaccine-mediated immune responses.
Authorship
Contribution: E.R. designed research, performed research, and collected and analyzed data; M.C. performed research and collected and analyzed data; E.M. analyzed the data and wrote the paper; M.H., M.K., and T.G. performed research and collected data; A.D.H. wrote the paper; M.Z. contributed vital reagents; P.D. wrote the paper; T.L. designed research, performed research, collected and analyzed data, and wrote the paper.
Conflict-of-interest disclosure: One of the authors (E.M.) is employed by a company (CSL Limited), but no reagent or potential product was studied in this paper, nor has this author any financial interest in this work.
Correspondence: Thomas Luft, University of Heidelberg, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; e-mail: thomas_luft@med.uni-heidelberg.de.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (LU830/1-3), and the Tumorzentrum Heidelberg-Mannheim.
We thank Dr U. Klingmüller for support with the use of the Lumi-Imager.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal