Abstract
We previously developed a multivariate model based on the RNA expression of 6 genes (LMO2, BCL6, FN1, CCND2, SCYA3, and BCL2) that predicts survival in diffuse large B-cell lymphoma (DLBCL) patients. Since LMO2 emerged as the strongest predictor of superior outcome, we generated a monoclonal anti-LMO2 antibody in order to study its tissue expression pattern. Immunohistologic analysis of over 1200 normal and neoplastic tissue and cell lines showed that LMO2 protein is expressed as a nuclear marker in normal germinal-center (GC) B cells and GC-derived B-cell lines and in a subset of GC-derived B-cell lymphomas. LMO2 was also expressed in erythroid and myeloid precursors and in megakaryocytes and also in lymphoblastic and acute myeloid leukemias. It was rarely expressed in mature T, natural killer (NK), and plasma cell neoplasms and was absent from nonhematolymphoid tissues except for endothelial cells. Hierarchical cluster analysis of immunohistologic data in DLBCL demonstrated that the expression profile of the LMO2 protein was similar to that of other GC-associated proteins (HGAL, BCL6, and CD10) but different from that of non-GC proteins (MUM1/IRF4 and BCL2). Our results warrant inclusion of LMO2 in multivariate analyses to construct a clinically applicable immunohistologic algorithm for predicting survival in patients with DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL), the most common adult non-Hodgkin lymphoma, is well recognized as a heterogeneous entity in which less than half of all affected patients are cured by currently available therapies.1,2 Although clinical indicators such as the international prognostic index (IPI) are used to define prognostic subgroups of DLBCL,3 these surrogates fail to fully reflect the underlying heterogeneity of the disease, since patients with identical IPIs can have strikingly different outcomes. A pivotal study in 2000, employing gene-expression profiling, reported that DLBCL could be classified into 2 molecularly distinct subtypes: germinal-center B-cell (GCB)–like DLBCL, exhibiting a gene-expression signature similar to normal germinal-center B cells; and activated B-cell (ABC)–like DLBCL, typified by the gene-expression signature of stimulated peripheral-blood B cells.4 The overall survival in patients treated with anthracycline-containing chemotherapy was significantly longer for GCB-like DLBCL compared with ABC-like DLBCL.4 That study led to the construction of several models predicting survival of DLBCL patients based on RNA and protein expression5 ; however, a consensus approach for predicting DLBCL prognosis and risk-adapted management of this lymphoma has not been achieved.
To this end, we previously evaluated 36 genes that were reported to predict survival in DLBCL, correlating their expression levels (as measured by quantitative reverse transcriptase–polymerase chain reaction [RT-PCR]) with their ability to predict survival in univariate analyses.6 The 6 genes with the strongest predictive value, LMO2, BCL6, FN1, CCND2, SCYA3, and BCL2, were then selected to construct a multivariate model that predicted survival in a second independent cohort of DLBCL patients.6
The gene with the strongest predictive power for long survival, LMO2 (also known as RBTN2 and TTG2), is a cysteine-rich LIM domain-containing transcription factor that plays an important role in angiogenesis and erythropoiesis and is required for definitive hematopoiesis during mouse embryogenesis.7,8 In humans, it is activated by chromosomal translocations at t(11;14)(p13;q11) or t(7;11)(q35;p13) in T-cell acute lymphoblastic lymphoma/leukemia (T-ALL).9 LMO2 is not expressed in mature T cells10 but its mRNA is widely detected in fetal tissues, particularly in the liver.11 Its expression has also been reported in adult spleen and mouse B-cell lines11 and, more recently, in germinal-center B cells.4 However, despite these extensive studies of LMO2 mRNA expression and its role in mouse hematopoiesis, thymic development, and leukemogenesis, the distribution of the protein in tissue has not been studied. We have therefore generated a monoclonal anti-LMO2 antibody and characterized LMO2 protein expression in normal and neoplastic hematopoietic and nonhematopoietic tissues. A comparison of the LMO2 protein-expression profile with that of other well-characterized GC markers, such as HGAL, BCL6, and CD10, and non-GC markers, such as BCL2 and MUM1/IRF4, was undertaken to determine whether LMO2 expression correlates with prognostic subclasses of DLBCL.
Materials and methods
Generation of monoclonal anti-LMO2 antibody
We generated a GST-LMO2 construct in pGEX-2T vector (Pharmacia Biotech, Uppsala, Sweden). The GST-LMO2 fusion protein, expressed in BL21 cells (Novagene, Madison, WI), was purified on a solid-phase glutathione column. The resulting protein was approximately 40% pure by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). This protein was used for immunization of mice: 25 to 30 mg of total protein was mixed with Freund complete or incomplete adjuvant for the first and 2 subsequent injections, respectively. Injections were given into the footpads of mice at 2-week intervals, followed by 3 injections every 3 days prior to undertaking fusion of draining lymph node or spleen cells to K6H6B5 fusion partner hybridoma cells, as reported previously.12 Enzyme-linked immunosorbent assay (ELISA) using GST-LMO2 fusion protein or an unrelated GST fusion protein was used for initial screening of hybridoma supernatants. The secreting hybridoma cells were subcloned by serial dilution and then further screened for specific antibody production by immunoblotting cellular lysates from LMO2-expressing cells (Daudi cells and HeLa cells stably transfected with pIRES-LMO2 construct) and cellular lysates from cells not expressing LMO2 (pIRES-transfected HeLa cells). The antibody chosen for the current study, LMO2 subclone 1A9-1, is an IgG1 monoclonal containing a kappa light chain.
Tissue samples and cell lines
Paraffin-embedded tissue.
Formalin-fixed, paraffin-embedded tissue samples of normal and neoplastic hematolymphoid cases were obtained from the archives of the Departments of Pathology, Stanford University Medical Center, Stanford, CA, and Aarhus University Hospital, Denmark. Institutional Review Board (IRB) approval was obtained from all participating institutions. The cases were studied by immunohistochemistry on tissue microarrays and on whole sections wherever detailed morphologic analysis was deemed necessary. For expression in normal hematopoietic tissues, 3 examples each of tonsil, lymph node, thymus, spleen, and bone marrow core biopsies were used. Hematolymphoid neoplasia was classified according to the current World Health Organization (WHO) scheme.1 Tissue microarray (TMA) construction has been described elsewhere.13,14
To screen for the expression of LMO2 protein in nonhematopoietic tissue, a TMA containing 110 samples was used. This TMA contained 2 normal examples from each of the following organs or tissue type: adrenal, bladder, brain, breast, colon, kidney, liver, lung, muscle (heart and skeletal), ovary, pancreas, prostate, stomach, testis, thyroid, and uterus. Neoplastic tissue samples included carcinomas of the adrenal cortex (2), bladder (2), breast (8), colon (6), head and neck squamous cell (2), liver (hepatocellular, 4; and cholangiocarcinoma, 4), lung (adenocarcinoma, 4; and squamous cell carcinoma, 4), ovary (6), parathyroid (4), prostate (3), stomach (2), thyroid (2), and uterus (4) as well as glioblastoma multiforme (2), seminoma (2), and soft-tissue sarcomas (13).
Cell lines.
The cell lines used in this study include 2 cell lines classified as GCB-like (SU-DHL4, OCI-LY19), 2 classified as non–GCB-like (OCI-LY3, OCI-LY10) by gene-expression analysis,4 2 Burkitt lymphoma cell lines (Raji and Ramos), and 1 nonhematolymphoid cell line (HeLa). All cell lines, except OCI-LY10 and OCI-LY3, were grown in RPMI 1640 medium (Fisher Scientific, Santa Clara, CA), supplemented with 10% fetal calf serum, 2 mM/L glutamine (GIBCO BRL, Grand Island, NY), and penicillin/streptomycin (GIBCO BRL). The OCI-LY10 and OCI-LY3 cell lines were grown in IMDM essential medium (Fisher Scientific), supplemented with 20% fresh human plasma and 50 mM 2-beta mercaptoethanol.
Immunoblot analysis
Whole-cell extracts for immunoblot analysis were prepared by lysing cells (5 × 106) with RIPA buffer (1 × phosphate-buffered saline [PBS], 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 mM phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin, and 100 mM sodium orthovanadate) on ice for 30 minutes. After centrifugation at 3000g, the supernatant was assayed for protein concentration by bicinchoninic acid (BCA) assay (Pierce Biotechnology, Rockford, IL). For immunoblotting, 20 μg of whole-cell lysate was separated on 10% SDS–polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes (BioRad Laboratories, Hercules, CA), and probed with anti-LMO2 at 1:10 000 dilution and anti–β-actin antibodies at 1:10 000 dilution (Sigma, St Louis, MO) overnight at 4°C. These antibodies were detected using goat anti–rabbit or anti–mouse horseradish peroxidase (HRP)–conjugated antibodies at 1:10 000 dilution (Jackson Immuno Research Laboratories, West Grove, PA), respectively, and visualized by the Super Signal West Pico Chemiluminescent Substrate kit (Pierce Biotechnology).
Immunohistochemistry
Serial 4-μM–thick sections from paraffin-embedded conventional tissue and tissue microarray blocks were deparaffinized in xylene and hydrated in a series of graded alcohols. Heat-induced antigen retrieval was carried out by microwave pretreatment in Tris (5 mM, pH 10.0 for 20 minutes) for 15 minutes. Anti-LMO2 antibody was used at a dilution of 1:150. Detection was carried out using the DAKO Envision method (DAKO, Carpinteria, CA). Staining was optimized on normal paraffin-embedded tonsil sections and a cutoff of staining in greater than 30% of lymphoma cells was assigned a positive score. This cutoff was based on the need for using a nonambiguous threshold for scoring TMAs and does not reflect differences in staining intensity between normal and neoplastic tissue or among different diagnoses. The cutoff was chosen before correlation with other immunohistologic markers. The distinction between positive and negative cases was relatively straightforward. Materials and methods for HGAL, BCL6, CD10, BCL2, and MUM1/IRF4 immunostaining have been described previously.13
Double-immunofluorescence labeling was performed as previously described.15 Antibodies to CD20, CD3, myeloperoxidase (MPO), glycophorin A, and CD79a were purchased from DAKO. Anti-LAT antibody was kindly provided by Professor V. Horejsi (University of Prague, Czech Republic).
Images of immunohistologic staining were acquired using a Nikon Eclipse E400 microscope (Nikon, Tokyo, Japan) and Nikon digital camera (DS-L1; Nikon, Tokyo, Japan), using a 10×/0.30 numerical aperture (NA) Plan Fluor (Figure 2A-B,I-J,L) or a 20×/0.50 NA (Figure 2E,H,M-N), 40×/0.75 NA (Figures 2F-G,O, 3), or 60×/0.85 NA (Figure 2C-D,K) Plan Fluor objective lens. Digitized images were processed using Adobe Photoshop 7 image processing and manipulation software (Adobe Systems, San Jose, CA).
Data analysis and visualization
The stained lymphoma TMA slides were scanned and stored as high-resolution images using an automated scanner (Bacus Laboratories Slide Scanner [BLISS]; http://www.bacuslabs.com) and are accessible at http://tma.stanford.edu/tma_portal/LM02. The “Deconvoluter” algorithm (custom WBS macro, Excel; Microsoft, Redmond, WA) with appropriate layout for use in the Cluster software was used for hierarchical clustering to integrate all immunohistologic staining results as previously described (http://genome-www.stanford.edu/TMA/).16 Positive staining is represented as red, lack of staining as green, and noninterpretable staining as white.
Results
Specificity of anti-LMO2 antibody
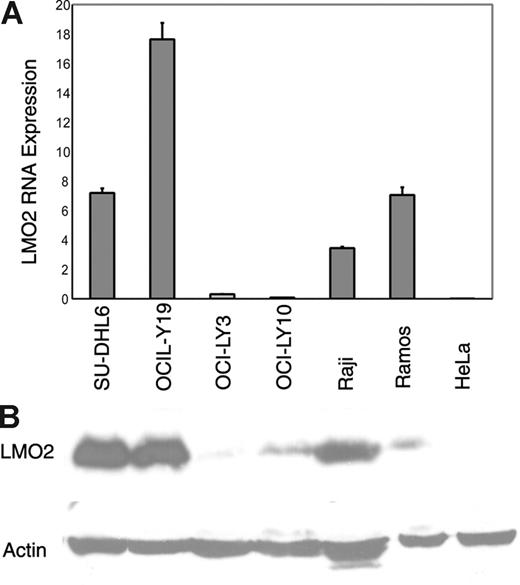
The specificity of the anti-LMO2 monoclonal antibody 1A9-1 was investigated by immunoblotting of cell lysates from HeLa cells transfected with pIRES-LMO2. A 24-kDa band was detected (Figure 1A) of identical size to the protein detected with an anti-Flag antibody (LMO2 fused to the flag epitope in the construct). This band was absent in mock-transfected cells (Figure 1A).
Specificity of anti-LMO2 monoclonal antibody and correlation with LMO2 mRNA expression. (A) Immunoblot analysis using the anti-LMO2 monoclonal antibody shows a specific band corresponding to LMO2 protein expression in HeLa cells stably transfected with LMO2 but not in native or mock-transfected HeLa cells. (B) LMO2 mRNA and protein expression were analyzed by real-time RT-PCR in GC-like (SU-DHL-4, OCI-LY7) and in non–GC-like (OCI-LY3, OCI-LY10) DLBCL cell lines and in Raji, Ramos, and HeLa cell lines. Immunoblot analysis shows that LMO2 protein expression corresponds to LMO2 mRNA expression in the cell lines analyzed. Error bars indicate average of 2 triplicate RT-PCR experiments.
Specificity of anti-LMO2 monoclonal antibody and correlation with LMO2 mRNA expression. (A) Immunoblot analysis using the anti-LMO2 monoclonal antibody shows a specific band corresponding to LMO2 protein expression in HeLa cells stably transfected with LMO2 but not in native or mock-transfected HeLa cells. (B) LMO2 mRNA and protein expression were analyzed by real-time RT-PCR in GC-like (SU-DHL-4, OCI-LY7) and in non–GC-like (OCI-LY3, OCI-LY10) DLBCL cell lines and in Raji, Ramos, and HeLa cell lines. Immunoblot analysis shows that LMO2 protein expression corresponds to LMO2 mRNA expression in the cell lines analyzed. Error bars indicate average of 2 triplicate RT-PCR experiments.
Correlation of LMO2 mRNA and protein expression
To evaluate the relationship between LMO2 mRNA and protein expression, we measured LMO2 mRNA by quantitative real-time RT-PCR and LMO2 protein expression by immunoblot analysis in 6 lymphoma cell lines (SU-DHL4, OCI-LY19, OCI-LY3, OCI-LY10, Ramos, and Raji) and in HeLa cells (LMO2 negative). LMO2 mRNA expression was high in the 2 GC-like DLBCL cell lines (SU-DHL4 and OCI-LY19) and in Burkitt lymphoma cell lines (Raji and Ramos) and low in the remaining cell lines. Similarly, we found high LMO2 protein expression in the GCB-like SU-DHL4 and OCI-LY19 cell lines and in the Raji cell line, whereas it was undetectable or present at low levels in the ABC-like OCI-LY3 and OCI-LY10 DLBCL cell lines and HeLa cells. However, in Ramos cells, LMO2 protein expression as detected by immunoblotting was low, whereas RNA expression was high, suggesting that in these cells there is posttranscriptional regulation of LMO2 protein expression (Figure 1B).
Expression of LMO2 protein in normal hematolymphoid tissue
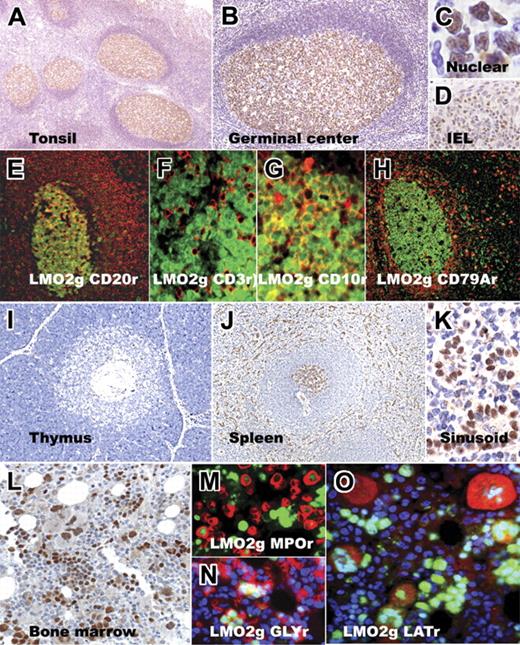
LMO2 protein was highly expressed in lymphocytes within the germinal center in normal tonsils and lymph nodes; the staining was crisply localized to the nucleus. Mantle zones showed only a few scattered positive cells, although at higher concentrations of the antibody weak staining was detected in a small proportion of mantle cells. The interfollicular areas showed LMO2 staining on endothelial cells and scattered histiocytes, and some intraepithelial lymphocytes in the tonsil were LMO2 positive. The interfollicular areas also exhibited scattered LMO2-positive lymphocytes that were partially highlighted by staining for CD30, indicating that these are likely to be activated B or T cells. Double-immunofluorescence microscopy showed that the germinal-center–associated staining for LMO2 was confined to CD20+ GC B cells and absent in CD3+ T cells within and outside germinal centers. CD10 was coexpressed on the majority of LMO2-positive GC B cells. Double-immunofluorescence labeling for LMO2 and CD79a highlighted a pattern similar to that obtained for LMO2 and CD20 staining within the germinal centers and also showed that plasma cells (detected by CD79a reactivity) were LMO2 negative (Figure 2).
Immunohistochemical staining for LMO2 in normal hematopoietic tissue. Low- and high-magnification images of normal tonsil sections show LMO2-specific staining within germinal centers; LMO2 staining is localized to the nucleus and is also found in intraepithelial lymphocytes (IELs) within the tonsil epithelium. Double-immunofluorescence labeling on tonsil tissue shows colocalization of LMO2 staining (green nuclear stain in all panels) with CD20 (red, membrane), CD3 (red, membrane/cytoplasmic), CD10 (red, membrane), and CD79a (red, cytoplasmic) staining in germinal-center cells. CD79a+ plasma cells lack LMO2 staining. Normal thymus shows rare, scattered LMO2-positive cells in the cortex and medulla. Normal spleen shows LMO2-specific staining in germinal centers of secondary lymphoid follicles and sinusoidal lining cells, whereas the mantle and marginal zones lack staining. The bone marrow shows LMO2 staining in subsets of immature myeloid (MPO-positive, red) and erythroid (glycophorin-positive, red) precursors and in megakaryocytes (LAT-positive, red). Red immunofluorescence is indicated by r; green immunofluorescence, by g.
Immunohistochemical staining for LMO2 in normal hematopoietic tissue. Low- and high-magnification images of normal tonsil sections show LMO2-specific staining within germinal centers; LMO2 staining is localized to the nucleus and is also found in intraepithelial lymphocytes (IELs) within the tonsil epithelium. Double-immunofluorescence labeling on tonsil tissue shows colocalization of LMO2 staining (green nuclear stain in all panels) with CD20 (red, membrane), CD3 (red, membrane/cytoplasmic), CD10 (red, membrane), and CD79a (red, cytoplasmic) staining in germinal-center cells. CD79a+ plasma cells lack LMO2 staining. Normal thymus shows rare, scattered LMO2-positive cells in the cortex and medulla. Normal spleen shows LMO2-specific staining in germinal centers of secondary lymphoid follicles and sinusoidal lining cells, whereas the mantle and marginal zones lack staining. The bone marrow shows LMO2 staining in subsets of immature myeloid (MPO-positive, red) and erythroid (glycophorin-positive, red) precursors and in megakaryocytes (LAT-positive, red). Red immunofluorescence is indicated by r; green immunofluorescence, by g.
In normal thymi, LMO2 protein and staining was restricted to rare scattered lymphocytes, the majority of which were found in the medulla. In the spleen, germinal centers within secondary lymphoid follicles of the white pulp showed LMO2 staining, whereas the mantle and marginal zones lacked staining. As shown in Figure 2, splenic sinusoidal lining cells (littoral cells) within the red pulp exhibited strong staining for LMO2 protein.
In normal bone marrow, LMO2 staining was detected in all 3 hematopoietic lineages. A subset of myeloid precursors showed weak to moderate staining for LMO2 as highlighted by double-immunofluorescence labeling of immature myeloid precursors for LMO2 and myeloperoxidase; however, mature myeloid lineage cells, including neutrophils, lacked LMO2 staining. Clusters of erythroid precursors, highlighted by immunofluorescence labeling for glycophorin A (Gly), showed intense staining for LMO2. Similarly, megakaryocytes, detected by immunofluorescence staining for LAT, showed moderate to strong staining for LMO2.
Expression of LMO2 protein in hematolymphoid neoplasia
Results of immunohistologic staining in hematolymphoid neoplasia are summarized in Table 1 and specific examples are illustrated in Figure 3. Immunoreactivity for LMO2 was present in follicular lymphomas of all 3 histologic grades (80/159), Burkitt lymphomas (8/19), lymphocyte-predominant Hodgkin lymphomas (9/15), mediastinal large B-cell lymphomas (8/13), and DLBCLs (92/197). LMO2 staining was absent in marginal-zone lymphomas of all types, with the exception of a single case of nodal marginal-zone lymphoma (of 5 tested). All cases of mantle-cell lymphomas (0/18), small lymphocytic lymphoma/chronic lymphocytic leukemias (0/38), and lymphoplasmacytic lymphomas (0/5) lacked staining. Rare myeloma biopsies (3/174) showed LMO2 staining. Among precursor lymphoid neoplasms, B-cell (5/12) and T-cell (8/14) acute lymphoblastic lymphoma/leukemia showed staining. LMO2 staining was absent in peripheral T-cell lymphomas (0/33) but was present in rare NK-cell lymphomas (5/91). Unlike lymphocyte-predominant Hodgkin lymphomas, none of the 107 cases of classical Hodgkin lymphomas showed staining for LMO2.
Immunohistologic analysis of LMO2 protein expression in hematolymphoid neoplasia
| Lymphoma subtype . | Total positive* . | % positive . |
|---|---|---|
| B-cell lymphoma; n = 487 | ||
| Follicular lymphoma | 80/159 | 50 |
| Grade 1 | 16/41 | 39 |
| Grade 2 | 24/53 | 45 |
| Grade 3 | 40/65 | 62 |
| Diffuse large B-cell lymphoma | 92/197 | 47 |
| Mediastinal large B-cell lymphoma | 8/13 | 62 |
| Burkitt lymphoma | 5/10 | 50 |
| Extranodal marginal-zone lymphoma | 0/27 | 0 |
| Splenic marginal-zone lymphoma | 0/5 | 0 |
| Nodal marginal-zone lymphoma | 1/5 | 20 |
| Mantle-cell lymphoma | 0/18 | 0 |
| Small lymphocytic lymphoma/CLL | 0/36 | 0 |
| Lymphoplasmacytic lymphoma | 0/5 | 0 |
| Precursor B-lymphoblastic lymphoma | 5/12 | 42 |
| T-cell lymphoma; n = 138 | ||
| Precursor T-lymphoblastic lymphoma | 8/14 | 57 |
| Peripheral T-cell lymphoma | 0/25 | 0 |
| Anaplastic large-cell lymphoma | 0/8 | 0 |
| NK lymphoma | 5/91 | 5 |
| Plasma-cell neoplasms; n = 174 | ||
| Multiple myeloma | 3/153 | 2 |
| Plasma-cell leukemia | 0/13 | 0 |
| Monoclonal gammopathy | 0/8 | 0 |
| Hodgkin lymphoma; n = 122 | ||
| Lymphocyte predominant | 9/15 | 60 |
| Classical Hodgkin | 0/107 | 0 |
| Myeloid leukemia; n = 11 | ||
| Acute myeloid leukemia | 8/10 | 80 |
| Chronic myeloid leukemia | 1/1 | 100 |
| Lymphoma subtype . | Total positive* . | % positive . |
|---|---|---|
| B-cell lymphoma; n = 487 | ||
| Follicular lymphoma | 80/159 | 50 |
| Grade 1 | 16/41 | 39 |
| Grade 2 | 24/53 | 45 |
| Grade 3 | 40/65 | 62 |
| Diffuse large B-cell lymphoma | 92/197 | 47 |
| Mediastinal large B-cell lymphoma | 8/13 | 62 |
| Burkitt lymphoma | 5/10 | 50 |
| Extranodal marginal-zone lymphoma | 0/27 | 0 |
| Splenic marginal-zone lymphoma | 0/5 | 0 |
| Nodal marginal-zone lymphoma | 1/5 | 20 |
| Mantle-cell lymphoma | 0/18 | 0 |
| Small lymphocytic lymphoma/CLL | 0/36 | 0 |
| Lymphoplasmacytic lymphoma | 0/5 | 0 |
| Precursor B-lymphoblastic lymphoma | 5/12 | 42 |
| T-cell lymphoma; n = 138 | ||
| Precursor T-lymphoblastic lymphoma | 8/14 | 57 |
| Peripheral T-cell lymphoma | 0/25 | 0 |
| Anaplastic large-cell lymphoma | 0/8 | 0 |
| NK lymphoma | 5/91 | 5 |
| Plasma-cell neoplasms; n = 174 | ||
| Multiple myeloma | 3/153 | 2 |
| Plasma-cell leukemia | 0/13 | 0 |
| Monoclonal gammopathy | 0/8 | 0 |
| Hodgkin lymphoma; n = 122 | ||
| Lymphocyte predominant | 9/15 | 60 |
| Classical Hodgkin | 0/107 | 0 |
| Myeloid leukemia; n = 11 | ||
| Acute myeloid leukemia | 8/10 | 80 |
| Chronic myeloid leukemia | 1/1 | 100 |
LMO2 immunostaining was similar in intensity to normal germinal-center B cells and was localized to the nuclei in all hematopoietic neoplasms tested. Cases were scored positive if more than 30% of lymphoma cells stained for LMO2.
Immunohistologic staining for LMO2 in hematolymphoid neoplasia. Representative examples of LMO2 immunostaining in lymphomas show LMO2 expression in Burkitt, DLBCL, precursor T-acute lymphoblastic lymphoma, and lymphocyte-predominant (LP) Hodgkin lymphoma, whereas it is absent in extranodal marginal-zone lymphoma and classical Hodgkin lymphoma. Among leukemias, immature blasts in an example of acute myeloid leukemia (AML; M4) and acute megakaryoblastic leukemia (AML; M7) show LMO2 staining. A case of chronic myeloid leukemia (CML) shows LMO2 staining in micromegakaryocytes and in a subset of early myeloid precursors but not in mature neutrophils.
Immunohistologic staining for LMO2 in hematolymphoid neoplasia. Representative examples of LMO2 immunostaining in lymphomas show LMO2 expression in Burkitt, DLBCL, precursor T-acute lymphoblastic lymphoma, and lymphocyte-predominant (LP) Hodgkin lymphoma, whereas it is absent in extranodal marginal-zone lymphoma and classical Hodgkin lymphoma. Among leukemias, immature blasts in an example of acute myeloid leukemia (AML; M4) and acute megakaryoblastic leukemia (AML; M7) show LMO2 staining. A case of chronic myeloid leukemia (CML) shows LMO2 staining in micromegakaryocytes and in a subset of early myeloid precursors but not in mature neutrophils.
Among myeloid leukemias, immature blasts in the majority of acute myeloid leukemias (AMLs) expressed LMO2 protein (8/10), including 3 with multilineage dysplasia, 1 with 11q23 abnormality, and 1 with megakaryocytic differentiation (AML, M7). A case of chronic myeloid leukemia (CML; chronic phase) harboring the BCR-ABL translocation showed LMO2 staining in micromegakaryocytes and in a subset of early myeloid precursors but not in mature neutrophils.
Expression of LMO2 protein in nonhematolymphoid tissues
One hundred ten samples of nonhematopoietic tissue from various adult organs and corresponding tumors were stained for LMO2 and no LMO2 staining was detected in any nonhematopoietic cell type, with the exception of vascular endothelial cells.
Expression of LMO2 protein in DLBCL
Staining for LMO2 protein was detected in 92 (47%) of 197 cases of DLBCL; the intensity of staining was similar to that in normal germinal-center B cells. Its expression was further studied in 143 cases of DLBCL compared with that of 5 additional markers (HGAL, BCL6, CD10, BCL2, and MUM1/IRF4) documented in our previous work.13 Hierarchical cluster analysis demonstrated that LMO2 protein correlated with the expression patterns of GC-specific markers HGAL, BCL6, and CD10 but not with non-GC markers MUM1/IRF4 or BCL2 (Figure 4, Table 2). Using the immunohistologic algorithm proposed by Hans et al17 to classify DLBCL into GC and non-GC subtypes, we found that LMO2 staining was present in 45 of 62 cases classified as GC type and in 30 of 66 cases classified as non-GC type (Table 3). We have previously reported that HGAL is also present in a substantial number of non-GC type cases,13 but there was no correlation in this category between expression of HGAL and LMO2. It should be noted that application of a different cutoff for definition of LMO2 positivity (eg, 20%) resulted in identical findings (data not shown), reflecting the robustness of LMO2 staining and ease of interpretation.
Hierarchical cluster analysis of immunohistologic data. The expression patterns of 6 proteins (LMO2, HGAL, CD10, BCL6, MUM1/IRF4 [MUM1], and BCL2) in 143 cases of DLBCL are shown. Positive staining is indicated in red, lack of staining in green, and uninformative data in white. LMO2 protein expression is clustered on the same branch of the dendrogram as germinal-center proteins HGAL, BCL6, and CD10 and away from non–germinal-center proteins MUM1 and BCL2.
Hierarchical cluster analysis of immunohistologic data. The expression patterns of 6 proteins (LMO2, HGAL, CD10, BCL6, MUM1/IRF4 [MUM1], and BCL2) in 143 cases of DLBCL are shown. Positive staining is indicated in red, lack of staining in green, and uninformative data in white. LMO2 protein expression is clustered on the same branch of the dendrogram as germinal-center proteins HGAL, BCL6, and CD10 and away from non–germinal-center proteins MUM1 and BCL2.
Comparative immunohistologic studies in 143 cases of DLBCL
| Score . | LMO2 . | HGAL . | CD10 . | BCL6 . | MUM1 . | BCL2 . |
|---|---|---|---|---|---|---|
| Positive | 78 | 97 | 51 | 78 | 65 | 95 |
| Negative | 63 | 41 | 80 | 54 | 66 | 32 |
| Equivocal | 2 | 5 | 12 | 11 | 12 | 16 |
| Score . | LMO2 . | HGAL . | CD10 . | BCL6 . | MUM1 . | BCL2 . |
|---|---|---|---|---|---|---|
| Positive | 78 | 97 | 51 | 78 | 65 | 95 |
| Negative | 63 | 41 | 80 | 54 | 66 | 32 |
| Equivocal | 2 | 5 | 12 | 11 | 12 | 16 |
Positive indicates staining in greater than 30% lymphoma cells; negative, lack of staining in greater than 30% lymphoma cells; and equivocal, lack of core for scoring.
Comparison with cell-of-origin classification
| Classification* . | Total no. . | LMO2 positive, no. . | LMO2 negative, no. . |
|---|---|---|---|
| GC | 62 | 45 | 18† |
| Non-GC | 66 | 30† | 36 |
| Cannot classify | 15 | 5 | 9 |
| Classification* . | Total no. . | LMO2 positive, no. . | LMO2 negative, no. . |
|---|---|---|---|
| GC | 62 | 45 | 18† |
| Non-GC | 66 | 30† | 36 |
| Cannot classify | 15 | 5 | 9 |
Discussion
The LMO2 gene is of major importance in hematopoiesis: its deletion in mice is lethal because of loss of yolk-sac erythropoiesis, and studies in embryonic stem cells and chimeric mice have shown that LMO2 mRNA contributes to the development of all hematopoietic lineages.18 LMO2 appears to act as an intranuclear bridging molecule in hematopoietic cells, orchestrating protein-protein interactions in the formation of multiprotein complexes that are necessary for specification of cell lineage and differentiation.19 LMO2 can also play a central role in leukemic transformation: chromosomal translocations involving LMO2 occur at a frequency of 8% to 10% in T-cell acute lymphoblastic leukemia,20 and it appears to function synergistically with other transcription factors (HOX11, TAL1/SCL, LYL1, LMO1, LMO2) to induce oncogenic transformation.21,22 Furthermore, independently of chromosomal translocations, LMO2 activation leading to T-ALL–like lymphoproliferative disorders has been observed in 3 of 9 children treated for X-linked severe combine immunodeficiency when an IL2Rγ2-bearing retroviral vector integrated itself in proximity to the LMO2 promoter.23,24 Finally, LMO2 is of clinical relevance in DLBCL, since its expression level in DLBCL is a powerful predictor of patient survival, probably in part because it identifies cases of germinal-center cell origin.6
However, despite these extensive investigations based on the assessment of mRNA levels, there have been no studies of LMO2 at the protein level. In this paper we document for the first time, using a newly generated antibody reactive with routinely processed biopsy tissue, the expression of LMO2 in normal and neoplastic human tissues. Our results not only throw new light on the site of expression of LMO2 but also document a unique antibody that is suitable for use in the routine hematopathology laboratory.
In normal bone marrow we found that all 3 hematopoietic lineages expressed LMO2 protein, although in the myeloid and erythroid series this expression appeared to disappear with differentiation. These results are in keeping with prior mRNA studies25 and they underscore the central role of LMO2 in hematopoiesis. Its physiologic expression in bone marrow may also be relevant to our observation of LMO2 expression in 8 of 10 cases of AML (which is in keeping with published mRNA studies25 ) and in neoplastic megakaryocytes in CML.
We also observed LMO2 expression in nonmyeloid acute leukemia, namely in 8 of 14 cases of T-ALL and 5 of 12 cases of B-ALL. It is well recognized that LMO2 mRNA is overexpressed in many more cases of T-ALL than carry the t(11;14)(p13;q11) and t(7;11)(q35;p13) translocations, and this is comparable to the aberrant overexpression of other transcription factors (eg, HOX11, TAL1) in T-ALL in the absence of cytogenetic abnormalities.25 It is not clear whether this expression in the absence of translocation reflects derivation of the leukemias from a T-cell precursor that normally expresses LMO2 or whether there is a leukemia-specific mechanism of LMO2 activation. In the former context, LMO2 protein in the thymus in the present study was confined to a few scattered cells in the medulla and cortex. This finding corroborates studies in mouse embryogenesis where LMO2 mRNA is absent from fetal and adult thymus (but present in many other fetal tissues).11 However, Asnafi et al26 have shown that LMO2 is most commonly expressed in T-ALLs derived from immature, CD4−, and CD8− (double-negative, DN1-2 stage) thymocytes. These are present in normal thymi as a very minor population, residing near the cortico-medullary junction, and they may not be detectable by conventional immunostaining of the thymus due to their rarity. However, it is also possible that the normal equivalent of T-ALL lacks LMO2 and that a specific genetic alteration mechanism causes LMO2 deregulation leading to oncogenesis. Some support for this hypothesis comes from recent studies that have shown frequent biallelic activation of 1 or more transcription factors in T-ALL, indicating that aberrant cis-acting regulatory elements are not the only pathway to induction of leukemias.27
The observed expression of LMO2 protein in 5 of 12 cases of B-ALL may also reflect their derivation from a B-cell precursor that normally expresses this transcription factor, and more studies to address this possibility are in progress. In this context, it may be relevant that Foroni et al11 previously predicted a role for LMO2 in B-cell development based on the expression of LMO2 mRNA in mouse fetal liver, adult spleen, and B-cell lines.
Our observation that LMO2 is expressed in germinal centers is fully in keeping with gene-expression profiling studies that showed high levels of LMO2 mRNA in GC B cells.4 Our double-immunofluorescence results for CD20, CD10, and CD3 confirm that LMO2 is expressed specifically in GC B cells, in keeping with mRNA studies. Consistent with its expression in GC B cells, our staining results in 1021 lymphomas show that the LMO2 protein is expressed in GC-derived B-cell lymphomas, namely follicular, Burkitt, lymphocyte-predominant Hodgkin, and diffuse large B-cell neoplasms. With the exception of rare cases of myelomas and NK lymphomas, LMO2 staining was essentially lacking in all other mature B- and T-cell lymphomas and in classical Hodgkin lymphomas. Among DLBCLs, LMO2 tended to be expressed in cases assigned by phenotyping to the GC categories and it can therefore be added to the panel of markers that pathologists may use to subcategorize lymphomas.
Gene-expression profiling has been used successfully to predict prognostic subgroups of DLBCL, but the expense and need for fresh tissue for this technology pose major constraints on its use in routine clinical practice. Several studies have therefore focused on the search for immunohistologic markers that can identify risk groups. However, at present the panel as proposed by Hans et al17 is based on only 3 markers, BCL6, CD10 and MUM1, and studies of prognosis based on this and other similar algorithms have yielded conflicting results.28-31 We have previously explored the use of 2 other GC-associated gene products (HGAL and JAW1) whose overexpression at the mRNA has been documented at high levels in GC and GC-derived tumors and have shown them to be preferentially expressed in GC DLBCL at the protein level.13,32 A subset (34%) of our DLBCL cases showed an LMO2 staining pattern that was not consistent with the result that would be expected based on the Hans algorithm. This could be attributed to “promiscuity” of GC markers that result in their expression in a subset of non-GC–derived DLBCLs. In the germinal-center microenvironment where there is a continuum of B-cell differentiation, it is likely that specific GC markers are up- or down-regulated at any one particular stage. However, the expression of any one of the GC markers is independent of its ability, when combined with other markers, to define DLBCL subtypes. Our data raise the important consideration that the “GC profile” likely comprises more than 1 molecular signature of partially overlapping protein expression patterns that underlie the genetic heterogeneity of DLBCL. Alternatively, LMO2 staining in non-GC–derived DLBCL may suggest that the Hans algorithm misclassifies a significant proportion of DLBCL cases. Indeed, in their original paper, approximately 20% of cases were misclassified in comparison to the gold standard of gene-array–based classification of the same cases.17 It is possible that the incorporation of additional newly characterized GC markers such as LMO2, HGAL, and JAW1 may be necessary to improve the robustness of an immunohistologic predictor in the prognostic stratification of patients with DLBCL.
In addition to its interest in the context of DLBCL, immunohistologic detection of LMO2 may be of practical value for the diagnosis of small-cell B-cell neoplasms, particularly for distinguishing low-grade B-cell lymphomas (marginal zone, lymphoplasmacytic, and small lymphocytic neoplasms) from follicular lymphomas. In this regard, LMO2 is similar to HGAL, another GC-specific marker we previously characterized, whose expression is also restricted to GC B cells.13 Both markers are therefore superior to BCL6 in distinguishing follicular colonization by marginal-zone lymphoma or a diffuse infiltrate of small lymphocytic lymphoma from a true follicular lymphoma. In contrast to HGAL, LMO2 is less frequently expressed in follicular lymphomas. Thus, although both LMO2 and HGAL are highly specific to GC B cells, the expression of LMO2 is more restricted than HGAL, and, in the diagnostic setting, HGAL is a more sensitive marker in the discrimination of a GC-derived lymphoma from its morphologic mimics than is LMO2. On the other hand, the crisp nuclear staining associated with LMO2 protein offers easier interpretation in comparison to the diffuse cytoplasmic staining associated with HGAL. Thus, LMO2 could be extremely valuable in the diagnosis of small tissue samples and needle biopsies.
In conclusion, we have raised an anti-LMO2 antibody and shown that the LMO2 protein is expressed in normal human germinal centers and GC-derived lymphomas. In addition, it is also expressed at high levels in endothelial cells, spleen, hematopoietic precursors, and a significant proportion of acute lymphoblastic and myeloid leukemias. In DLBCL, LMO2 protein expression is aligned with GC markers HGAL, CD10, and BCL6, indicating a potential role for LMO2 in the prognostic stratification of DLBCL patients. The anti-LMO2 antibody exhibits robust nuclear labeling in paraffin sections and is suitable for hematopathologic diagnosis as well as for the study of leukemogenesis, angiogenesis, and thymic development.
Authorship
Contribution: Y.N., D.Y.M., and I.S.L. designed research, analyzed data, and wrote the paper; S.Z., J.C., B.T., and M.J. performed research and analyzed data; A.S.H. and S.H.D. contributed vital clinical material; and R.L. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
I.S.L. and R.L. contributed equally to this work.
Correspondence: Y. Natkunam, Department of Pathology, L235, Stanford University School of Medicine, 300 Pasteur Dr, Stanford, CA 94305; e-mail: yaso@stanford.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Teresa Marafioti and Soo-Yong Tan for valuable scientific input and Thelma Santa-Maria for administrative assistance.
This work was supported partly by National Institutes of Health (NIH) grants CA34233, CA33399, and CA109335; a Leukemia and Lymphoma Society Support of Continuous Research Excellence (SCORE) grant; and the Dwoskin Family Foundation. R.L. is an American Cancer Society Clinical Research Professor.


![Figure 4. Hierarchical cluster analysis of immunohistologic data. The expression patterns of 6 proteins (LMO2, HGAL, CD10, BCL6, MUM1/IRF4 [MUM1], and BCL2) in 143 cases of DLBCL are shown. Positive staining is indicated in red, lack of staining in green, and uninformative data in white. LMO2 protein expression is clustered on the same branch of the dendrogram as germinal-center proteins HGAL, BCL6, and CD10 and away from non–germinal-center proteins MUM1 and BCL2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/4/10.1182_blood-2006-08-039024/4/m_zh80040708070004.jpeg?Expires=1765916671&Signature=DJe6sLBIi8tas7SwduLZTKo8d0PjIY-mTUIglfXtD0i-klcfncKaYZH-mxbbnvIUbaKXrhvSYuWw2UdflII4WjVJlgE0j28Xph0IepBmuBlohpdVK7hPkDTogSsSOg7wbJfBETrX0gfbQnYtiAbqUuRWleVN1l6JVZwm21ViK6SZ~sZ9XGrmB9GHPReSyZasBEfAtVmwPgooVjseQLwKHL5C8LgXXRwgn80lkmSjZeZOepwJIviWL2L90MGsaaiZXS70svARxoHEN2gA~rqCI6G1nNdA038dTxeM1CamsnCfqcBS4Rqn8A1Dc1plsoRJmwHvkUVlTmZcs-n-JywUdg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal