The article of Pilotti and colleagues aims to elucidate the anti-HIV mechanism(s) related to HTLV-II coinfection in intravenous drug users (IDUs). The authors demonstrate spontaneous production in vitro of an isoform of MIP-1α (CCL3L1) in PBMCs from HTLV-II–infected subjects, and this isoform was the major determinant of anti-HIV activity in coinfected HTLV/HIV subjects, possibly due to interference with chemokine receptor CCR5. Additional studies provide indirect evidence that the mechanism should act at a transcriptional level.
First isolated in association with a rare form of hairy cell leukemia,1 and subsequently classified in 2 different subtypes (a and b), human T-lymphotropic virus type II (HTLV-II) has been associated with several lymphoproliferative manifestations, although its direct role in causing a disease has never been unequivocally demonstrated. Based on evidence in vitro and epidemiologic studies, it has been speculated that type a could exert a more detrimental role interacting with the immune system than type b.
HTLV-II has been shown to be endemic among various American-Indian populations in North America, in Panama, and in South America. HTLV-II can be transmitted in 3 ways: mother-to-child transmission (associated mainly with prolonged breastfeeding), mainly in tropical developing countries; sexual transmission (mainly from men to women); and parenteral transmission (by needle sharing and transfusion).
HTLV-II infection has also been endemic for the past 10 to 20 years among intravenous drug users (IDUs) in Europe, especially in Italy, Spain, and Ireland, as well as in North America. From epidemiologic studies, it was soon clear that HTLV-II did not cause any fast progression to acquired immunodeficiency syndrome (AIDS) in IDUs, but on the contrary it was surprisingly associated with a delayed progression. It was only after the discovery of chemokines as natural inhibitors of human immunodeficiency virus (HIV) infection,2 that the possible mechanism of HTLV-II protective action started to be elucidated. Indeed, an increased C-C chemokine production in certain coinfected patients was noted.3,4
The current article by Pilotti and colleagues provides a better comprehension of this mechanism. In fact, it appears that HTLV-II infection triggers production of CCL3L1, an isoform of MIP-1α that is primarily responsible for protection (see figure). This induction seems to act at a transcriptional level, though the authors do not provide any direct evidence to sustain this hypothesis. In addition, other “protective” cytokines were produced as a result of HTLV-II infection. The effect was noted not only in stimu-lated peripheral blood mononuclear cells (PBMCs), but also in unstimulated cells. The authors thus propose that HTLV-II confers a protective state able to reduce susceptibility to HIV infection.
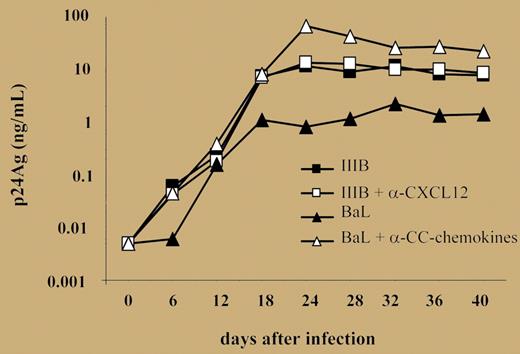
Kinetics of R5 and X4 virus production by primary cultures established from 3 HTLV-2/HIV-1MEU persons. See the complete figure in the article beginning on page 1850.
Kinetics of R5 and X4 virus production by primary cultures established from 3 HTLV-2/HIV-1MEU persons. See the complete figure in the article beginning on page 1850.
More studies are needed to determine how and why HTLV-IIb alters the cell programming apparatus to induce production of CCL3L1, protective cytokines, and a protective status against HIV. Eventually, this might result in a better understanding of the interactions between the virus and components of the immune system. It could also help in an understanding of how to induce such a protective staus.
The author declares no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal