LeMaoult and colleagues have identified a challenging novel mechanism that effector T cells can use to immediately acquire regulatory function. The mechanism relies on the direct and rapid exchange of membrane fragments between APC and T cells, a process called trogocytosis. Uptake of immune-tolerogenic HLA-G by some resting but most activated CD4 and CD8 T cells leads to the instant generation of a new type of regulatory cells that initially act through cell-surface molecules that they temporarily display but do not express themselves.
Cell-to-cell contact-dependent transfer of membrane fragments and associated molecules from one cell to another was first noticed in the early 1980s.1 This phenomenon has recently been named trogocytosis and meanwhile described in a number of cells in mice and humans, in most cases immune cells at the antigen-presenting cell (APC)–T-cell interface.2
The hallmarks of trogocytosis are the requirement of cell-to-cell contact and possibly immune synapse formation. Transfer via trogocytosis is fast, efficient, requires no more than a few minutes, and includes cell-surface, intramembrane, adaptor, and attached intracytoplasmic molecules (nonselective process). Various cell subsets can interact with each other (APCs–T cells, target cells–natural killer [NK] cells, peripheral cells–APCs); in most instances a predominant ligand-receptor interaction facilitates the exchange of material (MHC-TCR, CD28-B7, CD54-LFA, MHC-I–KIR). The specific physical environment seems to dictate the process, which can be antigen-specific. Most of the work known to date concerns APC–to–T-cell transfer in the murine system: CD4 and CD8 T cells acquired APC MHC class II and MHC class I molecules in an antigen-specific manner. TCR engagement seems necessary for trogocytosis to occur in most systems, since T-cell activation or anti-CD3 treatment increases efficiency of membrane exchange. In some systems, trogocytosis depends on CD28 engagement, and it was shown that costimulatory molecules (eg, B7-1 or ICAM-1) are acquired by murine antigen-specific T cells.
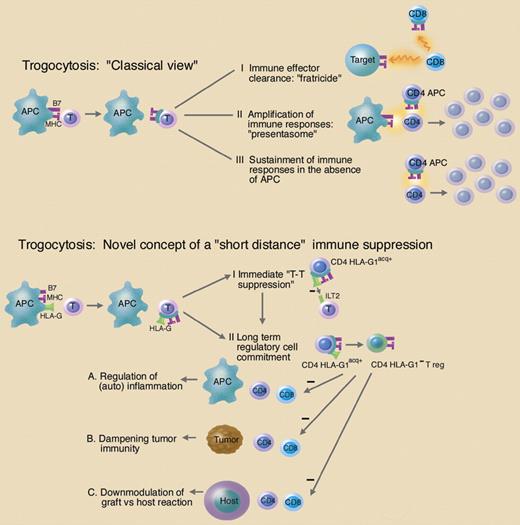
The detailed mechanisms by which these transfers occur, and even more so their overall functional and physiological significance, have remained largely elusive. Three principal possibilities are being considered (see figure): (1) immune effector clearance: acquisition of cognate MCH class I ligands by CD8 T cells predisposes cytotoxic T lymphocytes (CTLs) to “fratricide” antigen-specific cytolysis, thereby contributing to the clearance of CD8 effector cells; (2) amplification of immune responses: trogocytic transfer of stimulatory membrane portions to CD4 T cells (especially MHC class II and B7-costimulatory molecules, also called “presentasomes”) might constitute an efficient way to increase the immune system's antigen-presentation and stimulation capabilities by generating APC-like T cells; and (3) sustainment of immune responses in the absence of APCs: MHC class II and CD80 acquired from APCs regulate T-cell proliferation and sustain their activation in the absence of APCs.
Critics of this concept allege that trogocytosis—albeit possibly playing a role from a quantitative standpoint—should have no major physiological relevance, since it would not induce qualitative changes of immune responses under in vivo conditions (ie, changing the repertoire of immune cells).
Starting from this point, LeMaoult and colleagues reasoned that trogocytosis might be useful if “unusual” molecules limited in their expression in time and space and/or equipped with unusual function came into play instead of MHC molecules expressed by every APC. HLA-G, a nonclassical MHC molecule characterized by strong immunosuppressive function and highly restricted tissue expression under physiological conditions, was chosen as a paradigm. HLA-G plays a key role in mediating immune tolerance at the maternal-fetal interface. Ectopic or neoexpression has been described in cancers, transplantation, inflammatory/autoimmune disorders, and some viral infections, where HLA-G contributes to immunopathology via its overall negative immune-regulatory functions (such as inhibiting allogeneic proliferation of T cells, NK-cell cytotoxicity, and antigen-specific cytotoxicity).3
Functional consequences of trogocytosis. Top: The “classical view” on trogocytosis assumes that membrane fragments including the immunological synapse (MHC molecules and costimulatory molecules; MHC and B7, pink) are exchanged with or acquired by T cells. “Trogocytic” acquisition of immune-related molecules on T cells (CD8 or CD4) may have the following functional consequences: (I) immune effector clearance by CD8 T cells via fratricide; (II) amplification of immune responses via acquisition of APC function by CD4 T cells (CD4 APC); (III) sustainment of immune responses by CD4 APC in the absence of APC. Lower part: The novel concept of trogocytosis as proposed by LeMaoult and colleagues assumes that the transfer of the immune-tolerogenic MHC molecule HLA-G—together with other molecules of the immunological synapse—provides a rapid “short distance” immune suppression. (I) In case of the immediate display, this suppressive function is exerted by the immunetolerogenic HLA-G molecule itself (immediate T-T suppression). (II) Trogocytosis of HLA-G induces the development of CD4 effector cells into HLA-G1 negative regulatory cells (CD4 HLA-G1− Treg). These cells could have major impact in the immune regulation under physiological as well as pathological conditions (A, B, C). Illustration by Marie Dauenheimer.
Functional consequences of trogocytosis. Top: The “classical view” on trogocytosis assumes that membrane fragments including the immunological synapse (MHC molecules and costimulatory molecules; MHC and B7, pink) are exchanged with or acquired by T cells. “Trogocytic” acquisition of immune-related molecules on T cells (CD8 or CD4) may have the following functional consequences: (I) immune effector clearance by CD8 T cells via fratricide; (II) amplification of immune responses via acquisition of APC function by CD4 T cells (CD4 APC); (III) sustainment of immune responses by CD4 APC in the absence of APC. Lower part: The novel concept of trogocytosis as proposed by LeMaoult and colleagues assumes that the transfer of the immune-tolerogenic MHC molecule HLA-G—together with other molecules of the immunological synapse—provides a rapid “short distance” immune suppression. (I) In case of the immediate display, this suppressive function is exerted by the immunetolerogenic HLA-G molecule itself (immediate T-T suppression). (II) Trogocytosis of HLA-G induces the development of CD4 effector cells into HLA-G1 negative regulatory cells (CD4 HLA-G1− Treg). These cells could have major impact in the immune regulation under physiological as well as pathological conditions (A, B, C). Illustration by Marie Dauenheimer.
In an elegant series of in vitro experiments, the authors show that (1) polyclonal CD4 and CD8 T cells acquire HLA-G from APCs (HLA-G1–expressing LCL-721.221, IFN-gamma–stimulated monocytes) by membrane exchange and (2) that acquisition of HLA-G through membrane transfer immediately reverses their function from effectors to regulatory cells. HLA-G1 was transferred along with HLA-DR, CD86, CD54, and ILT-2 (but not ILT-3) and involved some resting but mostly activated T cells. Efficiency of membrane exchange greatly depended on the activation status of the acquirer cells, and maximal trogocytic capability was reached only during the late stages of activation. T cells that acquired HLA-G (CD4+HLA-G1acq+) showed no measurable phenotypical changes. Displayed HLA-G1 was lost within 24 hours from the cell surface—no endogenous changes in HLA-G expression were noticed. Nonetheless, biology of CD4+HLA-G1acq+ T cells changed significantly: these cells no longer responded to proliferation stimuli and obtained regulatory function immediately after trogocytic acquisition of HLA-G. Consequently, HLA-G was identified as the putative mediator of proliferation suppression. Thus far, LeMaoult and colleagues evidenced that trogocytic transfer of HLA-G to T cells occurs under the given conditions and that HLA-G as a transferred protein is functionally suppressive (“short term” acquisition of T-suppressive function). Interestingly, however, despite the loss of detectable cell surface HLA-G (within 24 hours), the nature of the acquirer T cells remained continuously altered: these cells—still expressing significant amounts of HLA-DR, CD86, CD54, and ILT2—showed suppressive function in MLR, indicating that they somehow have differentiated into HLA-G1− “regulatory” cells. While this differentiation could be blocked by masking trogocytosis of HLA-G with mAbs in the early stages, suppressive function was unaltered in the presence of HLA-G mAbs after successful acquisition (and loss) of HLA-G.
The molecular mechanisms underlying the genesis of HLA-G1− regulatory cells by HLA-G trogocytosis remain and clearly warrant further investigation (eg, by applying protein or mRNA array technologies). However, LeMaoult et al's work already provides some far-reaching implications that deserve attention. The findings nicely link together some aspects that are currently under extensive discussion in the field of immunology. Of importance, the paper proposes a new hypothesis on a peculiar mechanism of local (or peripheral) immune regulation. The process of trogocytosis gains a totally new aspect (see figure): the membrane exchange between APCs and T cells might contribute to a rapid and very local “emergency immune suppression,” which serves in a physiological setting—as a mechanism to counteract inflammatory responses (of autoimmune or infectious origin or at the maternal-fetal interface) or—in pathological situations—as a mechanism to downregulate, for example, antitumoral or graft-versus-host immune responses. Another aspect is the novel hypothesis of how “regulatory T cells” could be generated in situ. Regulatory T cells are critically involved in all kinds of immune reactions. However, it is under lively debate whether and how regulatory T cells could be generated at sites of immune reactions and what molecular events are involved (eg, Taams et al4 ).
Thus far, the generation of regulatory cells is considered a process involving several operational steps including cytokine induction, proliferation, cell migration, protein neosynthesis, and others. LeMaoult and colleagues provide a challenging new concept of “direct” regulatory T-cell education with interesting implications on current views of how this might affect immune regulation in situ.
Last but not least, the field of HLA-G research gets new momentum: trogocytosis might play a role in various conditions, where HLA-G exerts its role in providing immune-tolerogenic signals (eg, in the placenta, tumors, transplantation, autoimmune inflammation; eg, Wiendl et al5 ). It remains to be shown by subsequent studies to what extent trogocytosis of HLA-G contributes to the pathophysiology of any of the mentioned examples.
The author declares no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal