In this issue, Yaccoby and colleagues demonstrate that antibodies directed against the canonical Wnt inhibitor Dickkopf-1 (DKK1) suppress tumor-induced bone resorption in a murine model of multiple myeloma. This study represents the first attempt to assess the feasibility of anti-DKK1 therapy for the treatment of bone resorption in malignant bone diseases.
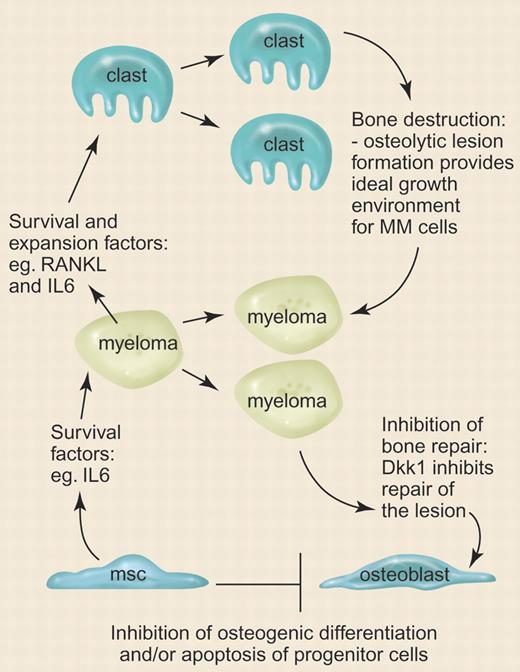
Multiple myeloma (MM) is a fatal malignancy involving the plasma cells of the blood. One of the hallmark characteristics associated with the disease are osteolytic lesions (OLs) that occur throughout the skeleton, causing intractable bone pain and pathological fractures. It has been known for some time that the formation of OLs occurs through hyperactivation of bone-resorbing osteoclasts through the action of cytokines that are secreted by the MM cells. The MM cells reside and flourish within the nutrient- and cytokine-enriched cavities of the lesions, continuing the promotion of osteolysis and establishing a destructive cycle that is the major cause of morbidity associated with the disease (see the figure).
Scheme depicting the proposed role of DKK1 in osteolytic lesion formation and maintenance in multiple myeloma. msc indicates mesenchymal stem cell; clast, osteoclast. Illustration by Paulette Dennis.
Scheme depicting the proposed role of DKK1 in osteolytic lesion formation and maintenance in multiple myeloma. msc indicates mesenchymal stem cell; clast, osteoclast. Illustration by Paulette Dennis.
For years, the treatment of OLs has focused on the inhibition of osteoclastogenesis by administration of bisphosphonates, but even when osteoclast activity is controlled, the lesions never repair, suggesting that MM cells disrupt the anabolic axis of bone formation too. Recently, Tian et al1 observed that secretion of an inhibitor of the canonical wingless (Wnt) signaling pathway, DKK1, by MM cells strongly correlated with the severity of osteolytic lesions. Given that signaling by the canonical Wnt pathway is critical for the differentiation of progenitors into osteoblasts, it seemed clear that the bone repair deficit in MM lies in the osteoinhibitory properties of DKK1 (see figure). The best evidence for the importance of Wnt signaling in osteogenesis is the finding that mutations in the gene that encodes the Wnt receptor, LRP5, can cause 2 contrasting diseases of bone: one that results in a form of osteoporosis2 and the other, abnormally high bone mass.3 Mutations that cause complete loss of function of LRP5 result in a form of osteoporosis. Conversely, abnormally high bone mass can be caused by mutations in LRP5 localized to the DKK1 binding domain that prevents negative regulation of the Wnt pathway.
Yaccoby and colleagues demonstrate here that administration of DKK1-binding antibodies prevents inhibition of osteogenesis in a model of MM. This was demonstrated by coimplantation of rabbit bones and fresh human MM cells into immunocompromised mice. After some time, the implants of control animals showed signs of MM-induced resorption, whereas treatment of recipient mice with anti-DKK1 antibodies blunted resorption and improved the bone mineral density of the implants. As the authors of this article state, aberrant regulation of Wnt signaling may affect bone repair in other malignant diseases of the skeleton. Indeed, the osteoinhibitory properties of DKK1 have been reported in an animal model of prostate cancer,4 and abnormally high levels of DKK1 have been detected in the serum of individuals with osteosarcoma.5 Anti-DKK1 agents may therefore represent the next generation of therapeutic options for the enhancement of bone repair in some malignant and degenerative bone diseases.
The author declares no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal