Abstract
Plasma procarboxypeptidase B (proCPB) is activated by the endothelial thrombin-prothrombomodulin complex. Activated (CPB) functions as a fibrinolysis inhibitor, but it may play a broader role by inactivating inflammatory mediators. To test this hypothesis, C5a-induced alveolitis was studied in wild-type (WT) and proCPB-deficient mice (proCPB−/−). C5a-induced alveolitis, as measured by cell counts and total protein contents in bronchoalveolar lavage fluids, was markedly enhanced in the proCPB−/− mice. E229K thrombin, a thrombin mutant with minimal clotting activity but retaining its ability to activate protein C and proCPB, attenuated C5a-induced alveolitis in WT but not in proCPB−/− mice, indicating that its beneficial effect is mediated primarily by its activation of proCPB. Lung tissue histology confirmed these cellular inflammatory responses. Delayed administration of E229K thrombin after the C5a instillation was ineffective in reducing alveolitis in WT mice, suggesting that the beneficial effect of E229K thrombin is due to the direct inhibition of C5a by CPB. Our studies show that thrombin-activatable proCPB, in addition to its role in fibrinolysis, has intrinsic anti-inflammatory functions. Its activation, along with protein C, by the endothelial thrombin-TM complex represents a homeostatic response to counteract the inflammatory mediators generated at the site of vascular injury.
Introduction
The complement system is part of the innate immune system and plays a major role in the host defense against pyrogenic bacterial infection.1,2 It is also involved in a wide variety of acute and chronic inflammatory disorders, including sepsis, acute respiratory distress syndrome, rheumatoid arthritis, and glomerulonephritis. The complement system consists of multiple proteins in plasma and on cell surfaces and can be activated by 3 distinct pathways, all converging on the cleavage of C3 into C3a and C3b. C3b in turn becomes an essential component of the C5 convertase enzyme complex, leading to cleavage of C5 into C5a and C5b. Both C3a and C5a are strong chemoattractants.1,2 They cause oxidative burst and enhance phagocytosis and granule enzyme release in neutrophils. They are vasodilators at sites of inflammation. C3a and C5a exert their effects through binding to cellular C3a receptors (C3aR) and C5a receptors (C5aR), respectively. C5aR are found on circulating myeloid cells, including neutrophils, monocytes, eosinophils, and basophils, as well as nonmyeloid cells in many organs, especially in the lung and the liver.2–4 Widespread up-regulation of the C5aR occurs during sepsis, and mice deficient in C5aR succumbed to bacterial pneumonias in animal models, indicating that C5a/C5aR signaling is essential in host defenses in the lung and other organs.5,6 Both C3a and C5a are inhibited by carboxypeptidase N (CPN), a constitutively active plasma carboxypeptidase.
Thrombin-activatable procarboxypeptidase B (proCPB), also known as procarboxypeptidase U, procarboxypeptidase R, and thrombin-activatable fibrinolysis inhibitor (TAFI), is a latent plasma procarboxypeptidase and is activated by thrombin when thrombin is bound on thrombomodulin (TM) on the endothelial cell surface.7–11 The thrombin-TM complex also activates protein C to activated protein C (aPC), which has well-established antithrombotic and anti-inflammatory properties.12–14 Activated proCPB (CPB) may function as a fibrinolysis inhibitor. By cleaving plasmin-exposed lysines on partially digested fibrin clot, it limits tissue plasminogen activator binding and reduces further activation of plasminogen, thereby stabilizing the fibrin clot.15–17 Thus, aPC and CPB may play complementary roles in hemostasis, with aPC dampening the clotting cascade and preventing excessive thrombin generation, whereas CPB serves to protect the clot already formed at the site of injury from premature clot lysis.
However, CPB may play a broader role in regulating inflammation, similar to aPC's role in counteracting vascular inflammation elicited by thrombin. CPB inactivates bradykinin, C3a and C5a.18,19 We have demonstrated that CPB is much more efficient than CPN, the constitutively active plasma anaphylatoxin inhibitor, in cleaving these inflammatory peptides. CPB abrogated C5a-induced neutrophil activation in vitro, and, using an engineered anticoagulant thrombin, E229K, which activates plasma proCPB in mice, we showed that E229K thrombin blocked bradykinin-induced hypotension in wild-type (WT), but not in proCPB-deficient (proCPB−/−) mice in vivo.20 proCPB−/− mice, after priming by LPS, showed increased mortality to infusion of cobra venom factor, a potent nonspecific activator of complement proteins, suggesting that CPB is important in regulating complement-mediated vascular inflammation in vivo.21 In this study, we provide direct evidence demonstrating that CPB is important in regulating C5a activity in vivo.
Materials and methods
Reagents
Human C5a and trypan blue were obtained from Sigma, St Louis, MO. The C5a was free of endotoxin. All chemicals used were reagent grade. The recombinant thrombin mutant E229K was expressed in Chinese hamster ovary (CHO) cells and purified to homogeneity as described previously.22,23 The BD mouse Th1/Th2 cytokine cytometric bead array kit and BD mouse inflammation cytometric bead array kit were purchased from BD Biosciences (San Diego, CA). The mouse KC/N51 and MIP-3α sandwich enzyme-linked immunoabsorbent assay (ELISA) kits are from R&D Systems (Minneapolis, MN), and the thrombin-antithrombin (TAT) complex ELISA kit was purchased from Cederlane Laboratories (Hornby, ON, Canada).
Methods
Animal model and study design.
We performed 3 studies in WT and proCPB-deficient mice (proCPB−/−). These were C5a dose-dependent alveolitis (study I), pretreatment of E229K thrombin on C5a-induced alveolitis (study II), and delayed administration of E229K thrombin on C5a-induced alveolitis (study III). This model was adapted from an FMLP-induced acute alveolitis mouse model.22 In each study 13-week-old male, 24 to 30 g, pathogen-free C57BL/6 WT mice and age-matched proCPB−/− mice (C57BL/6 background) were studied. WT mice were obtained from Jackson Laboratories (Bar Harbor, ME). The proCPB−/− mice, which have been extensively backcrossed into the C57BL/6 background (by Jackson Laboratories), were a gift from Dr John Morser and Dr Mariko Nagashima (Berlex, Richmond, CA). These were maintained at the Stanford University Medical School mouse colony facility following approved institutional guidelines.
Study I: C5a-induced alveolitis in WT and proCPB−/− mice.
Mice were randomly assigned to 1 of 6 groups; vehicle, low-dose C5a, and high-dose C5a in either WT or proCPB−/− mice (each group, n = 6). Mice in the low-dose C5a group received 75 μL C5a (0.05 mg/mL) instilled intratracheally. Mice in the high-dose C5a group received 75 μL C5a (0.10 mg/mL). Mice in the vehicle group received saline (75 μL).
Study II: pretreatment of E229K thrombin on C5a-induced alveolitis in WT and proCPB−/− mice.
WT or proCPB−/− mice were randomly assigned and pretreated with either vehicle or E229K thrombin at 5 minutes before endotracheal instillation of C5a (0.10 mg/mL) (4 treatment groups, n = 6 in each group).
Study III: effect of delayed administration of E229K thrombin on C5a-induced alveolitis in WT mice.
WT mice were randomly assigned to delayed treatment with either vehicle or E229K thrombin at 2 hours after initiating C5a (0.10 mg/mL)–induced alveolitis (n = 6 in each group).
Operational procedures
Endotracheal intubation and C5a instillation.
At time 0, after induction of anesthesia with isofluorene (2%), mice were placed in a supine position. Following oral endotracheal intubation with a 25-gauge catheter, anesthesia was maintained with inhalation of isofluorene (1%) administered via a Harvard rodent ventilator (Type 683; Harvard Apparatus, Boston, MA) with the following parameters: tidal volume, 0.4 mL; FiO2 = 1.0; respiratory rate, 100 per minute; and PEEP, 1 cm H2O. Vehicle (sterile saline, 75 μL) or C5a (0.05 or 0.10 mg/mL, 75 μL) was instilled endotracheally via a 25-gauge catheter inserted into the trachea.
Intravenous administration of E229K thrombin.
At time 0, after induction of anesthesia as described, a midline incision in the upper thorax was made, and E229K thrombin [5 mg/(g/min), 8 minutes] or vehicle [sterile saline; 5 mL/(g/min), 8 minutes] was administered by a Harvard pump through the right jugular vein with a catheter (PE10). During intervention, the mice were placed on a constant temperature heating pad (36°C to 38°C; Braintree Scientific, Braintree, MA). After interventions, mice were allowed to recover.
Bronchoalveolar lavage (BAL).
Six hours after the C5a instillation, mice were again anesthetized and placed in a supine position. A midline incision in the upper thorax was made, and BAL was performed twice with 750-μL aliquots of heparinized (10 U/mL) saline. BAL fluids were placed on ice until white blood cell counts and protein assays were done. Recovery of BAL fluid was quantitative in all groups.
Analysis of BAL.
White blood cell (WBC) counts of the BAL fluids were performed using a counting chamber after mixing the BAL fluids with trypan blue. BAL fluids were centrifuged (1500g, 5 minutes) to pellet the cells, and, after removing the cell pellets, the supernatants were stored at −80°C until the determination of protein contents. Protein contents were determined by Bradford assay.
Wet-to-dry lung ratio.
Wet-to-dry lung ratio was calculated as an index of lung injury (lung edema). Lungs were separated; the left lung was for histology and the right lung was divided into lobes, and the wet weights were measured. The right upper lobe was dried at 78°C for 3 days to obtain the dry weight.
General animal care.
All animals received humane care in compliance with National Society for Medical Research and National Academy of Sciences animal care guidelines. The study was approved by the Stanford Administrative Panel on Laboratory Animal Care, and was conducted in compliance with Stanford University regulations.
Lung pathology and tissue preparation
The right lung was weighed and then fixed in 10% neutral-buffered formalin. The lung was axially sectioned, processed, and embedded in paraffin wax. Five-micron sections were prepared and stained with hematoxylin and eosin (HE) and elastin–van Gieson (EVG).
Determination of cytokine levels in BAL fluids
Levels of various cytokines were determined in mouse BAL fluids (clarified by centrifugation) by flow cytometry using either the BD mouse Th1/Th2 cytokine cytometric bead array kit (IL-2, IL-4, IL-5, IFNγ, and TNF) or the BD mouse inflammation cytometric bead array kit (IL-6, IL-10, MCP-1, IFNγ, TNF, and IL12p70) as described by the manufacturer's instructions (BD Biosciences). The mouse KC/N51 and MIP-3α levels were determined using sandwich ELISA assays (R&D Systems). The TAT complex levels were determined using a human TAT sandwich ELISA kit from Cederlane Laboratories.
Statistical analysis
Data are presented as means ± SDs. Differences between groups were determined using the Student t test, with statistical significance indicated by P values less than .05.
Results
C5a-induced alveolitis was enhanced in proCPB−/− mice
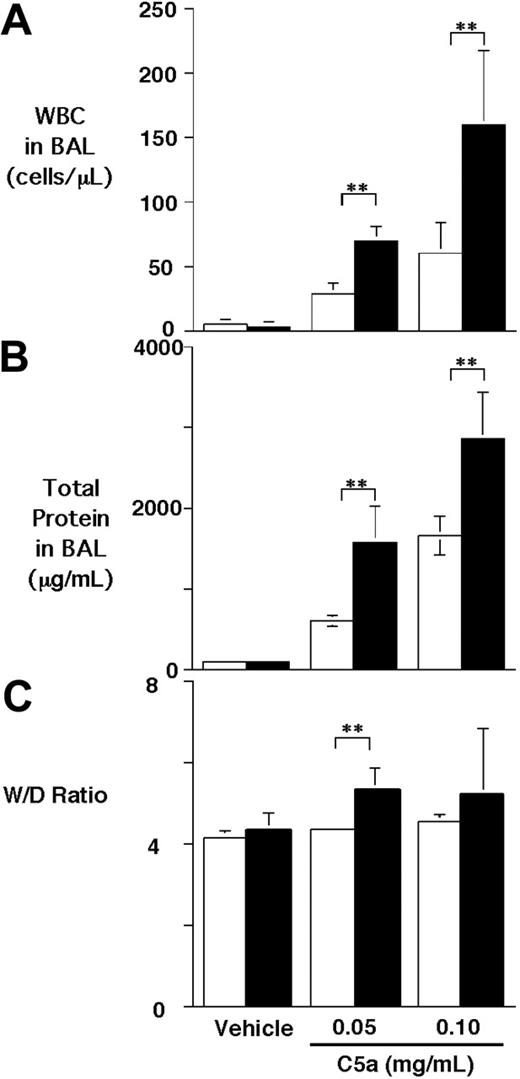
To test the effect of CPB on C5a activity in vivo, we used a C5a-induced alveolitis model in WT and proCPB−/− mice. C5a was instilled into the trachea of anesthetized mice, which were then allowed to recover. Six hours later, BAL was performed, and the extent of pulmonary inflammation was determined. C5a caused a dose-dependent increase in cell counts in the BAL fluids in WT mice (∼ 5-fold and 11-fold increase for low-dose and high-dose C5a, respectively) as compared with saline control. Similarly, a significant increase in BAL total proteins following C5a instillation was noted (Figure 1A-B). Compared with WT mice, the proCPB−/− mice had significantly enhanced inflammatory response to C5a stimulation (∼ 2.4- to 2.6-fold in cell counts, and 2.5- to 1.7-fold increase in total proteins at low-dose and high-dose C5a, respectively). Determinations of the wet-to-dry lung weight ratios, an index of pulmonary edema, showed similarly significant results (Figure 1C). Our data indicate that, in the absence of CPB, the chemotactic and inflammatory effects of C5a in pulmonary alveolar inflammation were significantly enhanced.
C5a-induced alveolitis in WT and proCPB−/− mice. C5a at 2 concentrations or saline control (vehicle) were instilled endotracheally in WT (□) or proCPB−/− (▪) mice. Six hours later, BAL was performed, and total WBC counts (A) and total protein contents (B) in the BAL fluids were determined. (C) The wet-to-dry weight ratio of the right upper lobe was determined as described in “Materials and Methods” (n = 6 in each group; ** P < .05). Data are presented as mean ± SD.
C5a-induced alveolitis in WT and proCPB−/− mice. C5a at 2 concentrations or saline control (vehicle) were instilled endotracheally in WT (□) or proCPB−/− (▪) mice. Six hours later, BAL was performed, and total WBC counts (A) and total protein contents (B) in the BAL fluids were determined. (C) The wet-to-dry weight ratio of the right upper lobe was determined as described in “Materials and Methods” (n = 6 in each group; ** P < .05). Data are presented as mean ± SD.
E229K thrombin attenuated C5a-induced alveolitis in WT but not in proCPB−/− mice
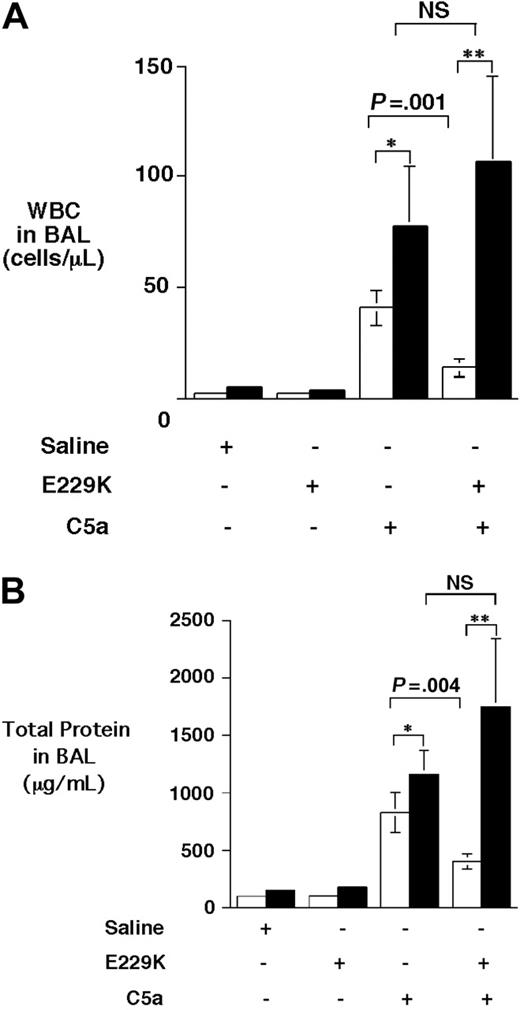
E229K thrombin was an anticoagulant thrombin that was initially identified for its selective anticoagulant property from a large panel of thrombin mutants generated based on alanine-scanning mutagenesis and was subsequently optimized by saturation mutagenesis.23,24 Compared with WT thrombin, it has less than 1% fibrinogen clotting activity and approximately 5% platelet activating activity, and approximately 50% activity to activate PC and proCPB when bound to TM. We have previously shown that E229K thrombin efficiently activated both PC and proCPB when infused in mice, without any significant consumption of either fibrinogen or platelets in vivo.20 E229K thrombin infusion following C5a instillation significantly ameliorated the alveolitis inflammatory response in WT mice, with a significant reduction in both cell counts (∼ 64% reduction) and total protein (∼ 53% reduction) in BAL fluids (Figure 2). In contrast, E229K thrombin infusion had no beneficial effect on proCPB−/− mice. In fact, both the cell counts and protein contents in the BAL fluids were slightly increased following E229K thrombin infusion, although they did not reach statistical significance. The data indicated that the beneficial effect of E229K thrombin in attenuating C5a-induced alveolitis in WT mice was mediated primarily by its activation of proCPB and not by its activation of PC.
E229K thrombin attenuated C5a-induced alveolitis in WT but not in proCPB−/− mice. C5a (0.1 mg/mL) or saline control was instilled endotracheally in WT (□) or proCPB−/− (▪) mice. E229K thrombin or saline control was administered intravenously into the right jugular vein 5 minutes before the C5a instillation. Six hours later, BAL was performed and total WBC counts (A) and protein contents (B) in the BAL fluids were determined. (n = 6 in each group). Data are presented as mean ± SD. *P < .05; **P < .01.
E229K thrombin attenuated C5a-induced alveolitis in WT but not in proCPB−/− mice. C5a (0.1 mg/mL) or saline control was instilled endotracheally in WT (□) or proCPB−/− (▪) mice. E229K thrombin or saline control was administered intravenously into the right jugular vein 5 minutes before the C5a instillation. Six hours later, BAL was performed and total WBC counts (A) and protein contents (B) in the BAL fluids were determined. (n = 6 in each group). Data are presented as mean ± SD. *P < .05; **P < .01.
Enhanced alveolitis in proCPB−/− mice and its amelioration by E229K thrombin in WT mice by histology
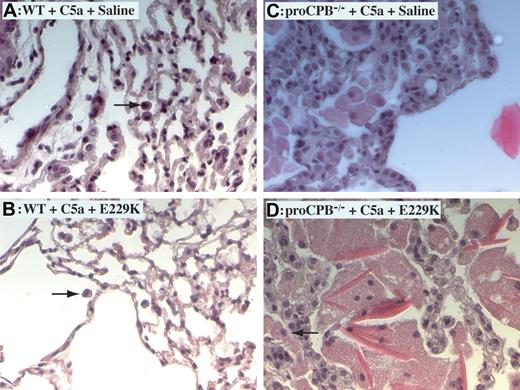
Histology of lung tissues demonstrated markedly increased inflammation in C5a-induced alveolitis in proCPB−/− mice as compared with WT mice, with thickened alveolar septum, more infiltration of neutrophils and macrophages, and extensive hyaline material deposition (Figure 3). The hyaline material was positive for fibrin(ogen) staining (data not shown). E229K thrombin reduced inflammation in WT but not in proCPB−/− mice, consistent with the findings in the BAL fluids.
C5a-induced alveolitis in WT and proCPB−/− mice. Representative lung tissue samples of C5a-induced alveolitis from WT mice in the absence (A) or presence (B) of pretreatment with E229K thrombin and from proCPB−/− mice in the absence (C) or presence (D) of pretreatment with E229K thrombin. Black arrows show macrophages. Images at 200× total magnification were visualized using a Zeiss Axiovert 40 CFL microscope equipped with an A-plan 20×/0.30 numerical aperture ph1 objective lens (Zeiss, Dublin, CA). Images were captured and rendered using a Zeiss AxioCam camera and AxioVision AC software.
C5a-induced alveolitis in WT and proCPB−/− mice. Representative lung tissue samples of C5a-induced alveolitis from WT mice in the absence (A) or presence (B) of pretreatment with E229K thrombin and from proCPB−/− mice in the absence (C) or presence (D) of pretreatment with E229K thrombin. Black arrows show macrophages. Images at 200× total magnification were visualized using a Zeiss Axiovert 40 CFL microscope equipped with an A-plan 20×/0.30 numerical aperture ph1 objective lens (Zeiss, Dublin, CA). Images were captured and rendered using a Zeiss AxioCam camera and AxioVision AC software.
Inflammatory cytokine levels in BAL fluids
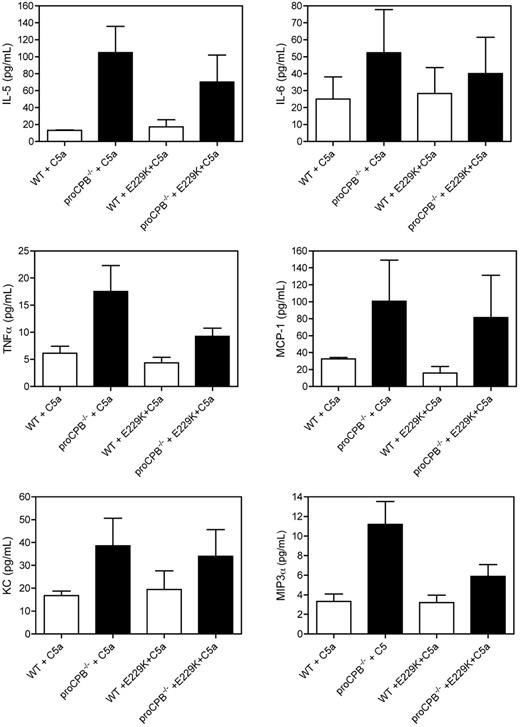
Several inflammatory cytokines and chemokines were markedly increased in the BAL fluids in proCPB−/− mice as compared with WT, including IL-5 (P = .009), TNF-α (P = .025), KC (P = 0.05), and MIP-3α (P = .006). IL-6 and MCP-1 showed a similar trend but did not reach significance (Figure 4). These results are consistent with the observed cellular inflammatory response. KC, an α- or CXX-chemokine and a mouse functional homologue of IL-8, acts primarily on neutrophils and is synthesized by a variety of cell types, including pulmonary macrophages.25,26 However, MIP-3α is a β- or CC-chemokine which is relatively specific for monocytes and lymphocytes. Neutrophils activated by TNF-α have been found to express CCR6, the receptor for the MIP-3α, suggesting MIP-3α can interact with TNF-α–activated neutrophils, leading to the acquisition of new functional phenotypes in neutrophils.27
Chemokine and cytokine levels in BAL fluids. Cytokine levels for IL-5, IL-6, TNFα, and MCP-1 were determined from BAL fluids clarified by centrifugation by flow cytometry using the BD mouse Th1/Th2 and BD mouse inflammation cytometric bead array (CBA) kits using the methods outlined by the manufacturers. The levels of mouse KC and MIP-3α were determined by sandwich ELISA according to the manufacturer's instructions. Data are presented as mean ± SD.
Chemokine and cytokine levels in BAL fluids. Cytokine levels for IL-5, IL-6, TNFα, and MCP-1 were determined from BAL fluids clarified by centrifugation by flow cytometry using the BD mouse Th1/Th2 and BD mouse inflammation cytometric bead array (CBA) kits using the methods outlined by the manufacturers. The levels of mouse KC and MIP-3α were determined by sandwich ELISA according to the manufacturer's instructions. Data are presented as mean ± SD.
However, following E229K thrombin administration, there was no significant decrease in these cytokine levels in the WT mice, which did not parallel the observed reduced inflammation by E229K thrombin as evidenced by cell counts, protein content in BAL fluids, or histology. However, a trend toward reduced inflammatory cytokine levels following E229K thrombin was seen in the proCPB−/− mice, which also was in contrast to the lack of improvement in cellular inflammation described previously. There were no differences in IL-2, IL-4, and IFN-γ levels in the BAL fluids between WT and proCPB−/− mice, with or without E229K thrombin treatment (data not shown). The reason for this discrepancy between the BAL cytokine levels and the cellular inflammatory response in response to E229K thrombin administration is unclear. The slight decrease in TNF-α, IL-5, and MIP-3α following E229K treatment in proCPB−/− mice might be due to inhibition of the T helper 2 (Th2)–type response by aPC in the airway, which would be expected to be activated by E229K thrombin in the proCPB−/− mice.28
We also determined the TAT complex levels in the BAL fluids. No significant differences were found between the WT and proCPB−/− mice, with or without E229K thrombin infusion, following saline or C5a stimulation (data not shown).
Delayed administration of E229K thrombin was ineffective in ameliorating C5a-induced alveolitis in WT mice
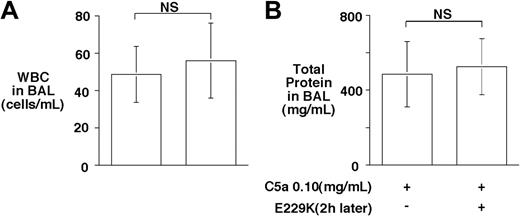
In addition to inhibiting C5a, CPB inhibits bradykinin and thrombin-cleaved osteopontin, suggesting that it may have a broad anti-inflammatory function.20 Thus, we were interested to determine whether delayed administration of E229K thrombin would retain its beneficial effect on C5a-induced alveolitis in the WT mice. Administration of E229K thrombin 2 hours after the C5a instillation was ineffective in reducing alveolitis in WT mice (Figure 5). The data suggest that E229K thrombin needs to be present at the initial instillation of C5a, and that the CPB effect was directed primarily toward inhibiting C5a and minimizing its acute inflammatory effects in this alveolitis model. Also, E229K thrombin-mediated PC activation did not have a significant beneficial effect in this acute lung injury model.
Delayed administration of E229K thrombin did not rescue C5a-induced alveolitis in WT mice. E229K thrombin or saline control was administered intravenously 2 hours after the initiation of C5a (0.10 mg/mL) induced alveolitis in this study (n = 6 in each group). Data are presented as mean ± SD.
Delayed administration of E229K thrombin did not rescue C5a-induced alveolitis in WT mice. E229K thrombin or saline control was administered intravenously 2 hours after the initiation of C5a (0.10 mg/mL) induced alveolitis in this study (n = 6 in each group). Data are presented as mean ± SD.
Discussion
Our study provided direct evidence demonstrating that CPB modulates C5a activity in vivo, in support of the hypothesis that CPB may have a broad anti-inflammatory role in vivo. Although there is evidence supporting CPB's role as a fibrinolysis inhibitor in general, for example, CPB has been shown to prevent the premature lysis of clots from hemphilic plasma,17 such a role in mice remains to be fully established. ProCPB deficiency did not influence occlusion time in either an arterial or a venous injury model in mice.29 Enhanced fibrinolysis was observed in a pulmonary clot lysis mouse model but that required the proCPB deficiency superimposed on a partial hemizygous plasminogen-deficient state.30 In a recently published Escherichia coli–induced peritonitis model in proCPB−/− mice, there was no evidence of perturbed fibrinolysis, as measured by D-dimer levels in plasma or the extents of fibrin deposition in lung and liver tissues.31 Thus, the role of CPB as a fibrinolysis inhibitor in mice does not appear to be as significant as that in humans.
It is unlikely that the enhanced C5a-induced alveolitis observed in proCPB−/− mice in our study was due to perturbation in fibrinolysis. With proCPB deficiency, one would expect enhanced fibrinolysis and thus less fibrin deposition in the alveoli following C5a-induced inflammation. Because fibrin is considered inflammatory in general, enhanced fibrinolysis should lead to less inflammation.32 But the opposite was observed. The amount of fibrin deposition and inflammation was significantly enhanced in proCPB−/− mice compared with WT. Our data are consistent with the notion that proCPB does not play a significant role in modulating fibrinolysis in vivo in mice, at least in this model of acute lung injury.
The most straightforward interpretation of our study is that CPB is important in regulating C5a activity in vivo; thus, in its absence, C5a-induced alveolitis is enhanced. This effect appeared to be directly mediated by CPB cleavage and inhibition of C5a and not mediated by CPB's effect on other potential inflammatory mediators, such as bradykinin or thrombin-cleaved osteopontin, because delayed administration of E229K thrombin did not confer a beneficial effect. Activation of proCPB in the WT mice by E229K thrombin clearly occurred in the vascular compartment following E229K thrombin binding to the endothelial TM. Only a trace amount of proCPB was detectable in the BAL fluids of WT mice under normal conditions. On the basis of Western blot analysis, the concentration of proCPB in BAL in WT mice was estimated to be approximately 130 ng/mL (T.M. and L.L.K.L., unpublished observation, June 2006). Taking into account the dilution factor (5- to 10-fold) during the lavage procedure, it is likely that the proCPB concentration in the alveolar fluids may approximate that of the plasma (∼ 2.5 μg/mL). However, activated CPB was not detectable in the BAL, suggesting that the activation of proCPB following acute lung injury in vivo occurs primarily intravascularly at a site in close proximity to the lung injury site.
It is notable that in the E coli–induced peritonitis model, pro-CPB deficiency appeared to correlate with a reduction of sepsis-induced liver necrosis.31 Studies have shown that C3a and C5a are capable of priming hepatocytes for regeneration, which is mediated by C3a and C5a binding to the C3aR and C5aR on Kupfer cells, a role distinct from the usual inflammatory role ascribed to C3a and C5a.33,34 The result is consistent with the interpretation that there is more local C5a generation in the liver in the proCPB−/− mice in the peritonitis model, thus leading to enhanced liver regeneration and reduced liver necrosis.
C3a and C5a are major anaphylatoxins generated at the site of acute inflammation, and, from a physiologic standpoint, it will be important that they be regulated locally. In this scenario, thrombin is generated at the site of vascular injury, providing hemostasis locally by the formation of platelet-fibrin thrombi. However, thrombin has multiple proinflammatory effects via binding to its cellular thrombin receptors (PARs) on a variety of cell types, including endothelial cells, monocytes, and smooth muscle cells.35 The binding of thrombin to TM on the neighboring intact endothelial cell surface thus represents a pivotal homeostatic loop, leading to the generation of aPC as well as CPB. aPC has well-established anticoagulant as well as anti-inflammatory effects, as demonstrated by the clinical efficacy of recombinant aPC in reducing mortality in patients with severe sepsis.12,13 Part of this salutary effect of aPC is mediated by its induction of an anti-inflammatory and prosurvival phenotype on endothelial cells, although the cellular receptor(s) involved in this process remains controversial.36–39 Our previous data demonstrating CPB inhibition of bradykinin in vivo20 and the current data with C5a inhibition support the thesis that CPB is a carboxypeptidase with broad substrate specificity and has intrinsic anti-inflammatory functions, in addition to its role in modulating fibrinolysis. Its activation by the endothelial thrombin-TM complex represents a vascular injury-induced anti-inflammatory response to augment the constitutively active plasma CPN to regulate the anaphylatoxins and other inflammatory mediators generated at the site of vascular injury.
E229K thrombin has been shown to activate PC and has an antithrombotic effect in vivo.22,23 Recombinant aPC has proven to be clinically useful in the treatment of advanced sepsis.13 In a recent randomized, double-blinded, placebo-controlled trial, the use of recombinant soluble complement receptor 1 (sCR1), which functions as a complement inhibitor by inhibiting C3 and C5 convertases, has been shown to lead to early extubation and a significant reduction in the duration of mechanical ventilation in patients who received a lung transplant.40 The current data show that E229K thrombin, in addition to activating aPC, is also effective in activating proCPB and thus may have a broad antithrombotic and anti-inflammatory effect in vivo. Selective activation of thrombin, enhancing its antithrombotic and anti-inflammatory functions by combined activation of PC and proCPB, although minimizing its prothrombotic properties, may represent another avenue of developing new antithrombotic agents.41–43
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lawrence Leung, Medical Service, VA Palo Alto Health Care System, 3801 Miranda Ave (111), Palo Alto, CA 94304; e-mail: lawrence.leung@stanford.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grant RO1 HL57530).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal