Abstract
Overcoming imatinib mesylate (IM) resistance and disease persistence in patients with chronic myeloid leukemia (CML) is of considerable importance to the issue of potential cure. Here we asked whether autocrine signaling contributes to survival of BCR/ABL+ cells in the presence of IM and nilotinib (NI; AMN107), a novel, more selective Abl inhibitor. Conditioned media (CM) of IM-resistant LAMA84 cell clones (R-CM) was found to substantially protect IM-naive LAMA cells and primary CML progenitors from IM- or NI-induced cell death. This was due to an increased secretion of the granulocyte-macrophage colony-stimulating factor (GM-CSF), which was identified as the causative factor mediating IM resistance in R-CM. GM-CSF elicited IM and NI drug resistance via a BCR/ABL-independent activation of the janus kinases 2 (JAK-2)/signal transducer and activator of transcription 5 (STAT-5) signaling pathway in GM-CSF receptor α receptor (CD116)–expressing cells, including primary CD34+/CD116+ GM progenitors (GMPs). Elevated mRNA and protein levels of GM-CSF were detected in IM-resistant patient samples, suggesting a contribution of GM-CSF secretion for IM and NI resistance in vivo. Importantly, inhibition of JAK-2 with AG490 abrogated GM-CSF–mediated STAT-5 phosphorylation and NI resistance in vitro. Together, adaptive autocrine secretion of GM-CSF mediates BCR/ABL-independent IM and NI resistance via activation of the antiapoptotic JAK-2/STAT-5 pathway. Inhibition of JAK-2 overcomes GM-CSF–induced IM and NI progenitor cell resistance, providing a rationale for the application of JAK-2 inhibitors to eradicate residual disease in CML.
Introduction
Imatinib mesylate, or IM (Gleevec; Novartis Pharma, Basel, Switzerland), has demonstrated a remarkable efficacy in the treatment of BCR/ABL+ leukemias, with most patients in early chronic phase achieving complete cytogenetic remissions (CCRs).1 Despite this, BCR/ABL+ disease remains detectable in essentially all patients with chronic-phase chronic myelogenous leukemia (CML), and CML always recurs after cessation of IM treatment.2 This reflects disease persistence under IM therapy. Mechanisms of stem and progenitor cell persistence are presumably progenitor cell quiescence,3,4 BCR/ABL overexpression,5 BCR/ABL kinase mutations,6 and influx and efflux pump expression7,8 regulating intracellular concentrations of IM. However, additional aberrations may be required to cause a fully drug-resistant phenotype.9
Clinically manifest IM resistance primarily occurs in advanced stages of CML and in BCR/ABL+ acute lymphoblastic leukemia (ALL), and is frequently associated with mutations10–17 in the causative oncogene of CML, BCR/ABL.18,19 BCR/ABL kinase mutations are today the best-characterized IM resistance mechanism.
To overcome mutation-dependent IM resistance, more selective second-generation ABL kinase inhibitors such as nilotinib (NI; AMN107)20 and dasatinib (DA),21 a dual-kinase inhibitor of Abl and Src kinases, were developed and recently also introduced into clinical practice.22,23 However, DA and NI failed to achieve sustained responses in IM-resistant CML blast crisis and ALL.22–24 Interestingly, neither the response nor the depth of response to NI (hematologic or cytogenetic) depended on the presence or absence of kinase mutations in IM-resistant CML and BCR/ABL+ ALL22 . This implies that BCR/ABL-independent resistance mechanisms25,26 contribute to IM and NI resistance in a major cohort of patients.
Using clonal populations of LAMA cells, we have previously shown that a BCR/ABL-independent activation of the PI3K/Akt signaling cascade precedes kinase mutation–dependent IM resistance development in vitro,27 and that this autonomous pathway activation mediates early BCR/ABL-independent IM resistance. Others have shown a growth factor–dependent, BCR/ABL-independent activation of the Ras-signaling pathway in CD34+ CML progenitors under IM exposure.28 Here we were seeking to identify putative factors present in the culture supernatant of IM-exposed BCR/ABL+ cells that confer IM resistance.
Materials and methods
Drugs and reagents
IM and NI was kindly supplied by Drs E. Buchdunger and P. Manley (both of Novartis Pharma). NI was dissolved in dimethyl sulfoxide (DMSO) as a 10-mM stock solution and stored in aliquots at −20°C; IM was stored at −20°C at 10 mM in distilled water. AG49029 was obtained from Calbiochem-Novabiochem (Bad Soden, Germany). Stock solutions (50 mM) were prepared in DMSO and stored in aliquots at −20°C. Fresh working solutions were prepared in RPMI-1640 for each experiment. The recombinant human (rh) cytokines interleukin-3 (IL-3), IL-6, stem cell factor (SCF), granulocyte colony-stimulating factor (G-CSF) (all from PeproTech, London, United Kingdom), rh granulocyte-macrophage CSF (GM-CSF), and macrophage inhibitory protein 1α (MIP-1α) were purchased from Calbiochem-Novabiochem, and neutralizing antibodies to recombinant human GM-CSF were purchased from R&D Systems (Wiesbaden-Nordenstadt, Germany).
Patient samples
Primary CML progenitor cells were obtained from newly diagnosed patients in the chronic phase of CML (n = 6) after written informed consent during routine bone marrow aspirations performed at diagnosis. Peripheral blood samples (20-40 mL) were obtained during routine diagnostic venal punctures at diagnosis (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) or at the time of IM-resistant disease (Tables S2–S3) from patients treated within multicenter CML studies in the Mannheim or Marburg University Clinic after the patients' written informed consent, with approval of the local ethics committee in Mannheim and Marburg. RNA extraction and cDNA synthesis were performed as previously described.12 Mononuclear cells (MNCs) were isolated from peripheral blood or bone marrow using Ficoll-Hypaque (Seromed, Biochrom Berlin, Germany) density gradient centrifugation as previously described.27
CD34+ enrichment
Primary CD34+ CML progenitors were enriched from 16 to 24 mL of heparinzed bone marrow using the magnetic-activated cell sorting (MACS) procedure (Miltenyi Biotech, Bergisch Gladbach, Germany). Briefly, bone marrow MNCs were washed with ice-cold phosphate-buffered saline (PBS; Gibco-BRL, Eggenstein, Germany) containing 0.2 M EDTA and 0.25 g/mL bovine serum albumin (MACS buffer) and labeled with 100 μL anti-CD34+ beads per 108 to 109 MNCs. After labeling for 20 minutes at 4°C, cells were washed 2 times with cold MACS buffer, and separation was performed through 1 LS column as recommended by the manufacturer (Miltenyi Biotech).
In vitro treatment with cytokines and inhibitors
BCR/ABL+, IM-resistant, and IM-sensitive LAMA-84 (LAMA) cell clones were maintained as recently described.27 To assess apoptosis or intracellular signal transduction, LAMA cells were treated with 1 μM IM, 1 μM NI, and 100 μM AG490 for 24 to 48 hours in the presence or absence of GM-CSF (5 ng/mL). Primary CD34+-enriched cells were cultured for 48 hours in the presence or absence of 3 μM NI with or without 100 μM AG490 in Iscove modified Dulbecco medium (IMDM; HyClone Laboratories, Logan, UT) containing 4 mM l-glutamine, 0.1 mM HEPES, and 20% fetal calf serum (FCS; Gibco-BRL) plus 5 growth factors: SCF (0.2 ng/mL), IL-6 (1 ng/mL), GM-CSF (0.2 ng/mL), G-CSF (1 ng/mL), and MIP-1α (0.2 ng/mL).
Intracellular staining
For intracellular staining, 1 to 5 × 105 stimulated parental LAMA cells or primary CD34+-enriched CML samples were fixed at 37°C for 10 minutes using Fix Buffer I (BD Biosciences, Heidelberg, Germany), and then were immediately stored at −80°C for future analysis or permeabilized on ice for 30 minutes in 1 mL BD Phosflow Perm Buffer III (BD Biosciences), washed twice with BD Pharmingen Stain Buffer, and resuspended in 100 μL BD Pharmingen Stain Buffer; fluorochrome-conjugated antibodies were added as indicated: 5 μL anti-CD34 PE-Cy7 (8G12), 5 μL antiphospho–signal transducer and activator of transcription 5 (STAT-5)–PE (Y694), 5 μL biotinylated mouse anti–human GM-CSFR (CD116, 4H1) (all from BD Biosciences), 2.5 μL antiphospho-CrkL (Y207; Cell Signaling Technologies, Beverly, MA), and 2.5 μL antiphospho–janus kinase 2 (JAK-2) (Cell Signaling Technologies). Labeling occurred for 30 minutes at room temperature in the dark. After first staining, cells were washed twice with BD Pharmingen Stain Buffer, and secondary staining was performed with 3 μL fluorescein isothiocyanate (FITC)–labeled goat anti–rabbit immunoglobulin G (IgG) and 3 μL streptavidin-APC (BD Biosciences). Cells were again washed and resuspended in 500 μL of BD Pharmingen Stain Buffer for fluorescence-activated cell sorter (FACS) analysis on a LSR II FACS analyzer (BD Biosciences). Data were analyzed using FlowJo software (Treestar, Portland, OR).
Generation of conditioned media
Conditioned media (CM) was harvested from R- and UR-LAMA cell clones by culturing 1 × 106/mL LAMA cells in serum-free RPMI-1640 medium (Gibco-BRL) containing 4 mM l-glutamine. CM of primary cells was generated by culturing 1 × 106/mL peripheral blood mononuclear cells (PBMCs) in serum-free IMDM containing 4 mM l-glutamine and 0.1 mM HEPES. CM was saturated by keeping cells for 24 hours in a humidified atmosphere at 37°C with 5% CO2. Supernatant was cleared from contaminating cells by centrifuging twice at 425g for 5 minutes. Supernatant was eventually aliquoted and stored at −80°C for later use.
CFC assays
First, 5 × 105 CD34+-enriched primary CML progenitors of untreated patients with chronic-phase CML (patients no. 3-8; Table S1) were grown in CM of LAMA cell clones 25R (or 25UR as control) and treated for 72 hours as indicated in the Figure 1 legend in the presence or absence of 10 μM NI. In some experiments, CD34+-enriched primary CML progenitors were treated with 10 μM NI for 72 hours in CM derived from primary PBMCs of IM-resistant patients. CM was always supplemented with a 5–growth factor cocktail (5GF) containing GM-CSF (0.2 ng/mL), SCF (0.2 ng/mL), IL-6 (1 ng/mL), G-CSF (1 ng/mL), and MIP-1α (0.2 ng/mL). Alternatively, CD34+-enriched primary CML progenitors were cultured in IMDM (4 mM l-glutamine, 0.1 mM HEPES) containing the 5GF plus 20% FCS (Gibco-BRL), and treated with 10 μM NI with or without an additional AG490 (100 μM) for 72 hours. To test the effect of GM-CSF, G-CSF, or IL-3 (all from PeproTech) on colony-forming cell (CFC) formation, these cytokines were each individually supplemented at 10 ng/mL. After treatment, cells were transferred into semisolid Methocult Medium (H4230; StemCell Technologies, Vancouver, BC, Canada) and incubated in triplicate for 14 days in 35 × 10-mm plates (Greiner Bio-One, Frickenhausen, Germany) at 37°C in a humidified atmosphere containing 5% CO2 saturation. Colonies were counted under a microscope.
Quantitative GM-CSF PCR
GM-CSF mRNA expression levels were analyzed on an ABI Prism 7700 sequence detector (Applied Biosystems, Foster City, CA) using standardized primers for GM-CSF (QuantiTect Primer Assay; Qiagen, Hilden, Germany) validated for use with the QuantiTect SYBR Green PCR Kit (Qiagen). GMCSF expression was normalized by comparison with the expression of the housekeeping gene GAPDH (primer pair: 5′-GAA GGT GAA GTT CGG AGT C-3′ and 5′-GAA GAT GGT GAT GGG ATT TC-3′). The amplification conditions were 15 minutes at 95°C to activate HotStarTaq DNA polymerase (Applied Biosystems), then 45 cycles of 94°C for 15 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 30 seconds. The polymerase chain reaction (PCR) efficiencies of the target and endogenous reference gene were comparable, so that the normalized GMCSF expression was calculated by using using the ΔΔCT method.
GM-CSF ELISA
GM-CSF concentration in total protein cell lysates generated from the peripheral blood cells of IM-sensitive and IM-resistant patients (Table S3) was quantitated using the human GM-CSF enzyme-linked immunosorbent assay (ELISA) kit from Diaclone (Besançon, France); the detection range was 8 to 500 pg/mL, and the sensitivity was less than 4.4 pg/mL. Briefly, microtiter wells were washed twice with washing buffer. Cell lysate (30 μg) was added per well for each sample in duplicate. Horseradish peroxidase (HRP) conjugate (HRP-conjugated anti–GM-CSF monoclonal antibody) was added followed by an incubation on a plate shaker for 3 hours at room temperature. Subsequently, wells were washed, 100 μL TMB substrate solution was added, and the plate was incubated for 20 minutes at room temperature. Finally, 100 μL stop solution was added, and the absorbance was measured on an ELISA Reader (Molecular Devices, Ismaning, Germany) at 450 nm. The absorbance values of the samples were converted into concentration values based on the calibration curve.
Cytokine antibody array
A cytokine antibody array was performed using the RayBio Human Cytokine Antibody Array C Series 1000 Kit (Ray Biotech, Norcross, GA) according to the manufacturer's recommendations. Briefly, 2 membranes each consisting of 60 cytokine antibodies spotted in duplicates onto the membranes were blocked with 2 mL 1 × blocking buffer at room temperature for 30 minutes. Membranes were then incubated with 2 mL serum-free conditioned media derived from LAMA subclones 25UR and 25R, respectively, at room temperature for 90 minutes. Membranes were washed 3 times for 5 minutes at room temperature with 2 mL of 1 × wash buffer I, and 2 times with 2 mL of 1 × wash buffer II according to the manufacturer's recommendations (Ray Biotech). Membranes were then exposed for 90 minutes at room temperature to 1 mL of a 1:500 dilution of biotin-conjugated antibodies. Following a thorough wash as before, membranes were incubated with 2 mL 1:1000 diluted HRP-conjugated streptavidin at room temperature for 120 minutes. Membranes were washed again and exposed for 2 minutes to the peroxidase substrate, which was constituted by mixing 1 × detection buffer C and 1 × Buffer D in a 1:1 ratio. Membranes were exposed to BioMax-MR films (Eastman Kodak, Rochester, NY) for the appropriate times.
Cell proliferation
Cell proliferation was assessed by using the Promega CellTiter 96 cell proliferation assay (Madison, WI) using the MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) colorimetric reduction method as previously described.27 Briefly, 7.5 × 105/mL LAMA cells were cultured in 25R-CM or 25UR-CM and treated with NI (1 μM) or not in the presence or absence of GM-CSF with or without neutralizing anti–GM-GSF antibodies. Later (24 hours), 20 μL CellTiter 96 Aqueous One Solution reagent (Promega, Madison, WI) was added, and cells were cultured for a further 4 hours at 37°C, 5% CO2. Absorbance was then measured at 492 nm in an OptiMax microplate reader (Molecular Devices).
Apoptosis measurement
Apoptosis was measured using the annexin V–FITC apoptosis detection kit (Alexis Biochemicals, Grünberg, Germany) according to the manufacturer's recommendations and essentially as previously described.30 Briefly, 5 × 105 cells were washed with ice-cold PBS, resuspended in 195 mL 1 × binding buffer, and stained for 10 minutes at room temperature with 5 mL FITC-conjugated anti–annexin V antibody. Unbound annexin V antibody was removed by washing with 1 × binding buffer. Cells were stained immediately prior to FACS measurement on a FACScan (BD Biosciences, Heidelberg, Germany) with 20 μg/mL propidium iodide (PI). Data were analyzed using CellQuest Pro software v4.0.2 (BD Biosciences). PI and annexin V double-negative cells were counted as viable.
IM and NI resistance induction assay
Medium (200 mL) containing 4 × 105/mL parental LAMA cells were cultured in 96-well plates in the presence of IM or NI at 2 μM in the absence or presence of GM-CSF at 0.1, 0.25 and 0.5 ng/mL. The concentrations of IM and NI were increased to 3 μM after 72 hours and to 4 μM after 96 hours. Colonies that formed on the bottom of the wells 10 to 14 days after initiation of treatment were counted.
Western blotting
Protein gel electrophoresis and Western blotting was performed essentially as previously described27,30 using the following purchased antibodies: antiphosphotyrosine antibody (4G10; Santa Cruz Biotechnology, Heidelberg, Germany), antiphospho–STAT-5, clone 8-5-2 (Y694/Y699; Upstate, Lake Placid, NY), antiphospho-CrkL (Y207; Cell Signaling Technologies) and anti–β-actin (AC-74; Sigma, Munich, Germany).
Statistical analysis
Analysis of significance of differences in treatment groups were performed using the GraphPad Prism 4.02 software (San Diego, CA). One-way ANOVA analysis was used with Dunnett or Bonferroni adjustments for multiple comparisons, or with the Mann-Whitney U test for comparison of less than 2 treatment groups. A P value below .05 was considered significant.
Results
CM of IM-resistant LAMA clones mediates IM and NI resistance
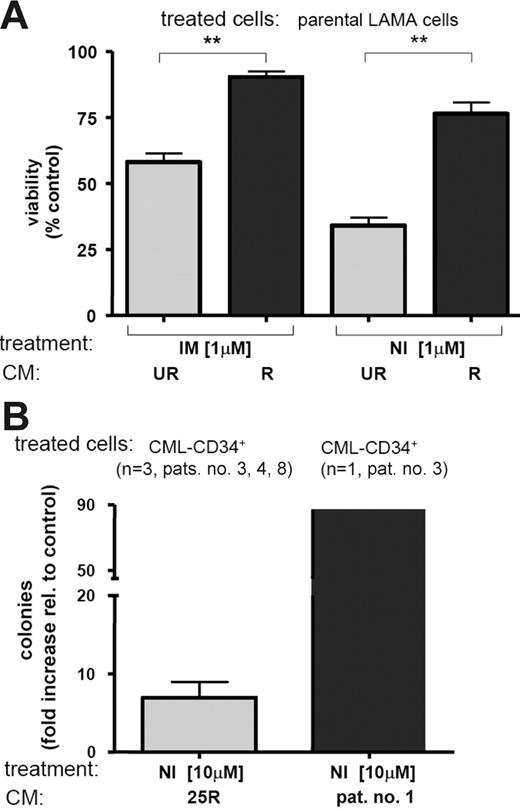
IM at 1 μM induces almost complete apoptosis of parental LAMA cells.27 To test the effect of culture supernatant (CM) of IM-resistant LAMA-84 cell clones27 (R-CM) on IM- or NI-induced apoptosis, R-CM was applied to IM-naive LAMA-84 cells in the presence of 1 μM IM or NI. Intriguingly, R-CM of the IM-resistant LAMA cell clones, 14R (harboring a Q252H point mutation), 10R, and 25R, potently protected against IM-induced apoptosis, but also against NI-induced apoptosis (P < .001; Figure 1A). In contrast, plain FCS or sensitive CM (UR-CM) derived from IM-naive homolog clones (10UR, 14UR, and 25UR) or R-CM of another IM-resistant LAMA-clone (2R) did not protect against IM-induced apoptosis.
Conditioned medium of IM-resistant LAMA cells confers IM and NI resistance to IM-naive LAMA cells. (A) CM of IM-resistant (R) clones protects IM-naive LAMA cells from IM- and NI-induced apoptosis. Percentage of viable cells after treatment is shown relative to no treatment controls. Bars represent mean ± SD of 3 independent experiments using CM of the 3 R and 3 UR clones (10, 14, and 25, respectively); ** P < .001 (1-way ANOVA, Bonferroni adjustment for multiple comparisons). (B) 25R-CM and patient no. 1–derived CM protects IM-naive, CD34+-enriched progenitors of patients with first-diagnosis CML (n = 3; patients no. 3, 4, and 8). Primary progenitor cells were exposed to 10 μM NI for 72 hours in the presence of 25UR-CM (as control), 25R-CM, or IM-resistant patient no. 1–derived CM (▪) and then placed into semisolid Methocult medium. Emerging colonies were counted. Bars represent fold increased colony formation ± SD by 25R-CM (⊡), or patient no. 1–derived CM (▪) relative to 25UR control treatment.
Conditioned medium of IM-resistant LAMA cells confers IM and NI resistance to IM-naive LAMA cells. (A) CM of IM-resistant (R) clones protects IM-naive LAMA cells from IM- and NI-induced apoptosis. Percentage of viable cells after treatment is shown relative to no treatment controls. Bars represent mean ± SD of 3 independent experiments using CM of the 3 R and 3 UR clones (10, 14, and 25, respectively); ** P < .001 (1-way ANOVA, Bonferroni adjustment for multiple comparisons). (B) 25R-CM and patient no. 1–derived CM protects IM-naive, CD34+-enriched progenitors of patients with first-diagnosis CML (n = 3; patients no. 3, 4, and 8). Primary progenitor cells were exposed to 10 μM NI for 72 hours in the presence of 25UR-CM (as control), 25R-CM, or IM-resistant patient no. 1–derived CM (▪) and then placed into semisolid Methocult medium. Emerging colonies were counted. Bars represent fold increased colony formation ± SD by 25R-CM (⊡), or patient no. 1–derived CM (▪) relative to 25UR control treatment.
To ask whether R-CM induced IM and NI resistance exclusively in LAMA cells, we next also tested R-CM on K562 cells and primary CD34+ CML progenitors. It has previously been established that IM and DA selectively inhibit proliferation of CML CFCs in vitro5,31,32 Although inefficient in K562 cells (K562 cells do not express CD116; not shown), 25R-CM clearly overcame NI-mediated growth inhibition of primary CD34+-enriched progenitor samples from patients with first-diagnosis chronic-phase CML (n = 3). This was illustrated by a 6.9-fold (± 3.5 standard deviation [SD]) increase in the number of CML CFCs surviving NI exposure through the presence of 25R-CM instead of 25UR-CM (Figure 1B; Table S1). Notably, CM derived from an IM-resistant patient (patient no. 1; Table S1) was (87-fold) more potent than 25UR control CM in overcoming CFC inhibition by NI (Figure 1B). Together, these experiments suggested that factors were secreted by IM-resistant LAMA cells and primary cells that promoted IM and NI resistance.
R-CM mediates BCR/ABL-independent NI resistance
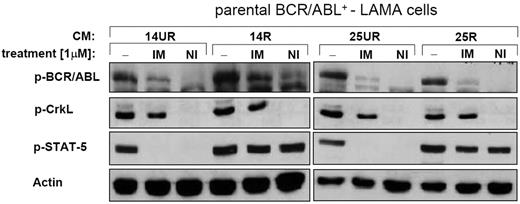
Next, we asked whether 14R and 25R-CM–induced IM/NI resistance was BCR/ABL dependent. BCR/ABL kinase activation status was assessed using Western blotting for detection of phosphorylated BCR/ABL (p-BCR/ABL) and Crk-like protein (p-CrkL). Activation of another key BCR/ABL substrate, STAT-5, was also evaluated (Figure 2). Neither R- nor UR-CM interfered with the inhibition of BCR/ABL by IM or NI (Figure 2). In fact, NI completely dephosphorylated BCR/ABL (and CrkL), indicating that 14R/25R-CM–mediated resistance was BCR/ABL independent. In contrast, STAT-5 phosphorylation was exclusively blocked in the presence of 14UR/25UR-CM (Figure 2), but not 14R/25R-CM. Thus, 14R/25R-CM–induced IM and NI resistance was associated with a BCR/ABL-independent activation of STAT-5.
R-CM mediates BCR/ABL-independent activation of STAT-5. Western blotting. 14R-CM and 25R-CM causes a BCR/ABL-independent activation of STAT-5 in the presence of IM or NI. Activated (phosphorylated) BCR/ABL kinase (p-BCR/ABL) was demonstrated by staining for p-BCR/ABL and the phosphorylated direct substrate of BCR/ABL, p-CrkL. Activation of the BCR/ABL-substrate, STAT-5, was shown using phospho-STAT-5 (p-STAT-5)–specific antibodies. Actin served as loading control.
R-CM mediates BCR/ABL-independent activation of STAT-5. Western blotting. 14R-CM and 25R-CM causes a BCR/ABL-independent activation of STAT-5 in the presence of IM or NI. Activated (phosphorylated) BCR/ABL kinase (p-BCR/ABL) was demonstrated by staining for p-BCR/ABL and the phosphorylated direct substrate of BCR/ABL, p-CrkL. Activation of the BCR/ABL-substrate, STAT-5, was shown using phospho-STAT-5 (p-STAT-5)–specific antibodies. Actin served as loading control.
GM-CSF is causal for NI resistance induction by 25R-CM
STAT-5 is a common antiapoptotic and transforming target of BCR/ABL and JAKs.33–35 Cytokines, upon binding to their receptors, activate members of the JAK family (eg, JAK-1, JAK-2, JAK-3, and tyrosine kinase 2 [Tyk2]), and, subsequently, STAT-5.35 We hypothesized that the resistance-mediating factor in R-CM could be a cytokine. A number (120) of cytokines were screened by cytokine array for differential expression between 25UR- and 25R-resistant medium. The concentrations of GM-CSF, IL-6, monocyte chemoattractant protein 1 (MCP-1), IL-8, and vascular endothelial growth factor (VEGF) were substantially increased in 25R-CM compared with 25UR-CM (Figure 3A). However, only GM-CSF, when supplemented into 25UR-CM, conferred NI resistance to parental LAMA cells (Figure 3B), indicating that GM-CSF was sufficient to mediate NI resistance. In fact, adding anti–rhGM-CSF antibodies, which neutralize GM-CSF in 25R-CM, caused a dose-dependent reduction of the capability of 25R-CM to confer NI resistance to LAMA cells (Figure 3C). Moreover, addition of GM-CSF into 25UR-CM dose-dependently restored the biochemical phenotype of 25R-CM, namely, a BCR/ABL-independent STAT-5 phosphorylation of parental LAMA cells in the presence of NI (Figure 3D). Together, GM-CSF was identified as being involved in conferring BCR/ABL-independent IM and NI resistance by 25R-CM.
GM-CSF mediates BCR/ABL-independent IM and NI resistance. (A) Cytokine array. Different cytokines (120) were screened for differential expression in the CM of 25UR (left 2 membranes) versus 25R (right 2 membranes). Different cytokines (60) are spotted in duplicate on each membrane. Positive controls (4 dots) are shown in the top left corners. Darker spots indicate higher expression. Cytokines with increased expression in 25R-CM (bold-lined squares) compared with 25UR-CM (thin-lined squares) are indicated. (B) The viability of parental LAMA cells after 24 hours of treatment with NI in the presence of 25UR-CM and GM-CSF was assessed relative to the control treatment (no NI or GM-CSF) using the MTS colorimetric assay. Boxes display data points located in the middle 2 quartiles of all data points. Lines in boxes indicate medians; whiskers extend to the 2 extreme values of all data points. Data points of 3 independent experiments are depicted. (C) Reversal of 25R-CM–mediated NI resistance of parental LAMA cells by addition of increasing concentrations of neutralizing anti–human GM-CSF antibodies as indicated. Data points of 3 independent experiments are depicted. Bars and lines are as in panel B. (D) IM-naive parental LAMA cells were treated with NI and increasing concentrations of GM-CSF as indicated in the presence of 25R-CM or 25UR-CM. After 24 hours of treatment, cells were harvested and cell lysates were separated using PAGE and blotted with the indicated antibodies. Actin served as loading control.
GM-CSF mediates BCR/ABL-independent IM and NI resistance. (A) Cytokine array. Different cytokines (120) were screened for differential expression in the CM of 25UR (left 2 membranes) versus 25R (right 2 membranes). Different cytokines (60) are spotted in duplicate on each membrane. Positive controls (4 dots) are shown in the top left corners. Darker spots indicate higher expression. Cytokines with increased expression in 25R-CM (bold-lined squares) compared with 25UR-CM (thin-lined squares) are indicated. (B) The viability of parental LAMA cells after 24 hours of treatment with NI in the presence of 25UR-CM and GM-CSF was assessed relative to the control treatment (no NI or GM-CSF) using the MTS colorimetric assay. Boxes display data points located in the middle 2 quartiles of all data points. Lines in boxes indicate medians; whiskers extend to the 2 extreme values of all data points. Data points of 3 independent experiments are depicted. (C) Reversal of 25R-CM–mediated NI resistance of parental LAMA cells by addition of increasing concentrations of neutralizing anti–human GM-CSF antibodies as indicated. Data points of 3 independent experiments are depicted. Bars and lines are as in panel B. (D) IM-naive parental LAMA cells were treated with NI and increasing concentrations of GM-CSF as indicated in the presence of 25R-CM or 25UR-CM. After 24 hours of treatment, cells were harvested and cell lysates were separated using PAGE and blotted with the indicated antibodies. Actin served as loading control.
BCR/ABL-independent activation of STAT-5 by GM-CSF in LAMA cells—role of CD116 expression
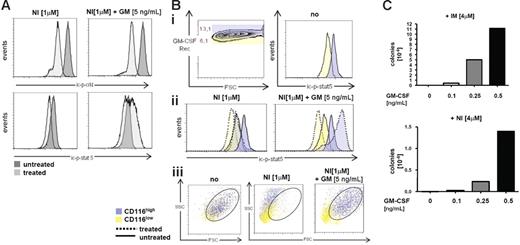
GM-CSF is a hematopoietic cytokine regulating proliferation and survival of normal GMPs,36,37 and its aberrant secretion may also be implicated in the pathogenesis of CML.38,39 The data shown in Figures 2 and 3 indicated that GM-CSF–induced STAT-5 activation substitutes for the loss of BCR/ABL-mediated STAT-5 activation by NI. We sought to verify this on the single-cell level by multicolor FACS using a combined intracellular and extracellular staining. Intracellular p-CrkL levels (ic-p-CrkL) as a marker of BCR/ABL activation and the ic-p-STAT-5 levels were concomitantly measured. It was confirmed that NI effectively blocked BCR/ABL in the presence or absence of GM-CSF (Figure 4A; top histograms), and that STAT-5 inhibition by NI was overcome by GM-CSF (Figure 4A; bottom histograms). We also noted that GM-CSFR α chain (CD116) expression was critical for the regulation of STAT-5 by GM-CSF. An arbitrary discrimination between CD116low- (yellow) and CD116high (blue)–expressing LAMA cells revealed higher basal STAT-5 activation in the CD116high population (Figure 4Bi; right histogram) and a further increase of ic-p-STAT-5 by GM-CSF exclusively in CD116high cells (Figure 4Bii; right histogram). On the contrary, CD116 expression did not influence NI-mediated p-STAT-5 inhibition in the absence of GM-CSF (Figure 4Bii; left histogram). The scatter characteristics would support the conclusion that GM-CSF maintained viability (oval gate) in the presence of NI, preferably in the CD116high (blue), but not the CD116low population (yellow) (Figure 4Biii), demonstrating that GM-CSF via binding to its receptor CD116 protected against NI-induced apoptosis. In an attempt to address whether GM-CSF supports long-term survival of BCR/ABL+ cells in the presence of NI or IM, we adapted a previously reported cell-based in vitro resistance induction assay.40 In this assay, higher levels of IM (4 μM) are used to provoke resistance development.27,40 Therefore, GM-CSF was added at variable concentrations to 96-well plates each containing 4 × 105 cells in the presence of NI or IM at 4 μM. Readout after 14 to 30 days was the number of colonies that emerged (Figure 4C). This showed that GM-CSF dose-dependently maintained survival, but also the growth of LAMA cells in the presence of high doses of IM or NI (Figure 4C).
GM-CSF-receptor signaling mediates BCR/ABL-independent NI resistance through activation of STAT-5. (A) Intracellular staining of p-STAT-5 (ic-p-STAT-5) and ic-p-CrkL in LAMA-cells before (dark histogram) and after treatment (light histogram) with NI or GM-CSF as indicated. (B) Analysis of ic-p-STAT-5 according to GM-CSFR α (CD116) expression levels; (Bi) Blue denotes high CD116 expressers; and yellow, low CD116 expressers. (Bii) ic-p-STAT-5 regulation according to high and low CD116 expression levels (blue and yellow) before (full lines) and after (dotted lines) treatment with NI or NI plus GM-CSF. (Biii) Viability of CD116high and CD116low populations of LAMA cells after exposure for 24 hours to NI with or without GM-CSF. The circles indicate viable cells according to the typical scatter characteristics of viable LAMA cells. (C) GM-CSF maintains growth and survival of LAMA-cells in the presence of high doses of IM and NI. LAMA cells (4 × 105 cells / well) were cultured in 96 well plates and exposed to a final concentration of 4μM IM or NI in the presence or absence of GM-CSF as indicated. Colonies formed after 14 to 30 days and are depicted as resistant colonies per 106 input cells at the beginning of the culture. One of 3 experiments is shown, respectively, showing very similar results.
GM-CSF-receptor signaling mediates BCR/ABL-independent NI resistance through activation of STAT-5. (A) Intracellular staining of p-STAT-5 (ic-p-STAT-5) and ic-p-CrkL in LAMA-cells before (dark histogram) and after treatment (light histogram) with NI or GM-CSF as indicated. (B) Analysis of ic-p-STAT-5 according to GM-CSFR α (CD116) expression levels; (Bi) Blue denotes high CD116 expressers; and yellow, low CD116 expressers. (Bii) ic-p-STAT-5 regulation according to high and low CD116 expression levels (blue and yellow) before (full lines) and after (dotted lines) treatment with NI or NI plus GM-CSF. (Biii) Viability of CD116high and CD116low populations of LAMA cells after exposure for 24 hours to NI with or without GM-CSF. The circles indicate viable cells according to the typical scatter characteristics of viable LAMA cells. (C) GM-CSF maintains growth and survival of LAMA-cells in the presence of high doses of IM and NI. LAMA cells (4 × 105 cells / well) were cultured in 96 well plates and exposed to a final concentration of 4μM IM or NI in the presence or absence of GM-CSF as indicated. Colonies formed after 14 to 30 days and are depicted as resistant colonies per 106 input cells at the beginning of the culture. One of 3 experiments is shown, respectively, showing very similar results.
JAK-2 inhibition antagonizes GM-CSF–mediated STAT-5 activation in LAMA cells
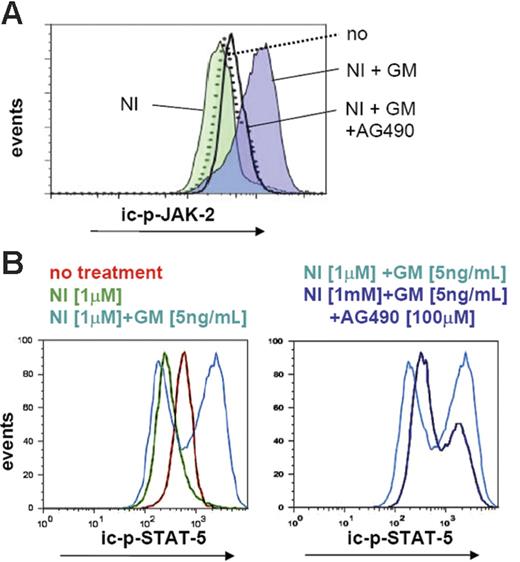
We next reasoned that blocking GM-CSF receptor signaling (eg, by inhibition of a main downstream target kinase, JAK-2) may antagonize ic-p-STAT-5 formation, thereby overcoming GM-CSF–mediated NI resistance. AG490 is a previously described JAK-2 inhibitor that is used at a concentration of 50 to 100 μM in vitro.29,41 Western blotting indicated that 100 μM AG490 caused a complete inhibition of JAK-2 activation (phosphorylation) in LAMA cells (not shown). JAK-2 activation was therefore quantitated by FACS-based intracellular staining in the presence and absence of 100 μM AG490 with or without GM-CSF. Whereas GM-CSF activated JAK-2 regardless of the presence of NI (Figure 5A), AG490 completely prevented JAK-2 activation. This translated into a reduction of GM-CSF–induced STAT-5 activation (reduction of ic-p-STAT-5; Figure 5B), and, as expected, severely reduced the potential of GM-CSF to confer IM or NI resistance.
GM-CSF–mediated JAK-2/STAT-5 activation in LAMA cells. (A) FACS histograms illustrate the levels of expression of intracellular phosphorylated JAK-2 (ic-p-JAK-2) and (B) the quantity of ic-p-STAT-5 after treatment of parental LAMA cells for 48 hours with indicated inhibitors with or without GM-CSF.
GM-CSF–mediated JAK-2/STAT-5 activation in LAMA cells. (A) FACS histograms illustrate the levels of expression of intracellular phosphorylated JAK-2 (ic-p-JAK-2) and (B) the quantity of ic-p-STAT-5 after treatment of parental LAMA cells for 48 hours with indicated inhibitors with or without GM-CSF.
GM-CSF–induced NI resistance of primary CML progenitors is associated with a BCR/ABL-independent activation of STAT-5
IM and DA selectively inhibit proliferation of CML CFCs in vitro.5,31,32 Initial experiments with R-CM suggested that primary CML progenitors were protected from IM-induced growth inhibition due to the presence of higher concentrations of GM-CSF in 25R-CM (Figure 1B). GM-CSF was therefore tested directly in colony assays for its ability to mediate NI resistance of primary CD34+-enriched CML progenitors derived from 4 consecutive patients with CML in chronic phase. NI at 10 μM achieved a maximum growth inhibition in this assay and was therefore used as standard concentration in colony formation experiments. GM-CSF still potently protected against NI-induced growth inhibition of BCR/ABL+ primary CML precursors (Figure 6A). Interestingly, GM-CSF was obviously more effective than IL-3 or G-CSF in overcoming NI-mediated growth inhibition (Figure 6A). The latter 2 cytokines were tested in parallel because they have been reported previously to cause abnormal progenitor cell proliferation in CML42 .
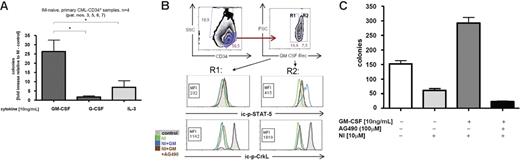
GM-CSF overcomes NI-induced proliferation inhibition of primary CD34+ progenitors by JAK-2/STAT-5 pathway activation. (A) CD34+-enriched progenitors from patients with first-diagnosis CML (n = 4) were cultured in vitro in IMDM medium (+ 5 growth factors) in the presence of 10 μM NI plus 10 ng/mL of either 1 of the 3 test cytokines (GM-CSF, IL-3, or G-CSF) as indicated. After 72 hours, cells were seeded in soft agar, and 10 to 14 days later, emerging colonies (CFCs) were counted. Bars represent mean fold increase ± SD in CFC counts obtained after NI exposure in the presence of indicated test cytokines versus a standard cytokine cocktail only. *P < .05 (1-way ANOVA Dunnett adjustment for multiple comparisons). (B) BCR/ABL-independent STAT-5 activation by GM-CSF in primary CML progenitors. CD34+-enriched primary CML progenitors of patient no. 7 (CML at diagnosis) were treated for 48 hours with NI, the JAK-2 inhibitor AG490 (100 μM) with or without GM-CSF (10 ng/mL), and were analyzed by FACS for the regulation of ic-p-STAT-5 (top 2 histograms) and ic-p-CrkL (bottom histograms) according to the indicated gating strategy: CD34high/SSClow cells were gated into CD34high/CD116low (gate R1) or CD116high (gate R2) and separately analyzed for R1 and R2. The gray curves in each histogram plot represent baseline expression levels; the colored curves represent different treatments as indicated. (C) CD34+-enriched progenitors from a patient with first-diagnosis CML (patient no. 3) were cultured in vitro in a liquid culture containing a cytokine cocktail of 5 growth factors (± GM-CSF, as indicated) in the presence of NI or not and/or AG490. After 72 hours, cells were seeded in triplicates in soft agar and emerging colonies (CFCs) were counted 10 to 14 days later. Bars represent mean CFC counts ± SD of triplicates.
GM-CSF overcomes NI-induced proliferation inhibition of primary CD34+ progenitors by JAK-2/STAT-5 pathway activation. (A) CD34+-enriched progenitors from patients with first-diagnosis CML (n = 4) were cultured in vitro in IMDM medium (+ 5 growth factors) in the presence of 10 μM NI plus 10 ng/mL of either 1 of the 3 test cytokines (GM-CSF, IL-3, or G-CSF) as indicated. After 72 hours, cells were seeded in soft agar, and 10 to 14 days later, emerging colonies (CFCs) were counted. Bars represent mean fold increase ± SD in CFC counts obtained after NI exposure in the presence of indicated test cytokines versus a standard cytokine cocktail only. *P < .05 (1-way ANOVA Dunnett adjustment for multiple comparisons). (B) BCR/ABL-independent STAT-5 activation by GM-CSF in primary CML progenitors. CD34+-enriched primary CML progenitors of patient no. 7 (CML at diagnosis) were treated for 48 hours with NI, the JAK-2 inhibitor AG490 (100 μM) with or without GM-CSF (10 ng/mL), and were analyzed by FACS for the regulation of ic-p-STAT-5 (top 2 histograms) and ic-p-CrkL (bottom histograms) according to the indicated gating strategy: CD34high/SSClow cells were gated into CD34high/CD116low (gate R1) or CD116high (gate R2) and separately analyzed for R1 and R2. The gray curves in each histogram plot represent baseline expression levels; the colored curves represent different treatments as indicated. (C) CD34+-enriched progenitors from a patient with first-diagnosis CML (patient no. 3) were cultured in vitro in a liquid culture containing a cytokine cocktail of 5 growth factors (± GM-CSF, as indicated) in the presence of NI or not and/or AG490. After 72 hours, cells were seeded in triplicates in soft agar and emerging colonies (CFCs) were counted 10 to 14 days later. Bars represent mean CFC counts ± SD of triplicates.
We next analyzed the GM-CSF–associated signal transduction events that were associated with NI resistance in primary progenitors. Gating for CD34+/CD116high versus CD116low expression established that STAT-5 activation by GM-CSF depended on the expression of the GM-CSF receptor (CD116). The ic-p-STAT-5 levels increased exclusively in the CD116high (R2) population, but not in the CD116low (R1) population (Figure 6B). Notably, GM-CSF–induced ic-p-STAT-5 formation was not inhibited by NI (Figures 6B, S1), but only the JAK-2 inhibitor AG490 (Figures 6B, S1). In turn, NI effectively blocked BCR/ABL kinase activation as documented by a GM-CSF– and CD116 expression–independent down-regulation of ic-p-CrkL (Figures 6B, S1). Apparently, the BCR/ABL-independent STAT-5 activation translated into a significant increase in CFC numbers in the presence of NI (Figure S2). This result was confirmed in another patient sample (patient no. 3; Figure 6C). Inhibition of JAK-2 by AG490 reduced the levels of ic-p-STAT-5 (Figures 6B, S1) and abolished the protective effect of GM-CSF against NI treatment (Figure 6C). Thus, BCR/ABL-independent activation of STAT-5 by GM-CSF contributes to BCR/ABL-independent NI resistance in primary CML progenitors. Blocking JAK-2 may antagonize this type of resistance.
Overexpression of GM-CSF in IM-resistant patients
We then asked whether GM-CSF might also contribute to IM resistance development in vivo. We reasoned that if adaptive autocrine GM-CSF secretion would be a relevant resistance/persistence mechanism in vivo by improving growth or survival, GM-CSF–overexpressing clones would expand out during manifest IM resistance. Indeed, when comparing GMCSF mRNA expression in the peripheral blood (n = 24), bone marrow (n = 17), or PBMCs (n = 11) of patients with untreated chronic-phase CML with the peripheral blood samples of 29 IM-resistant patients, GMCSF mRNA was substantially overexpressed in 5 (17%) of 29 IM-resistant patients (Figure 7A; Table S2). Increased GM-CSF was independent from the kinase mutation status and was also not related to the phase of disease at the time of resistance (Figure 7B; Table S2). GM-CSF overexpression at the time of IM resistance was even more obvious when GM-CSF protein levels were studied. Elevated amounts of GM-CSF were detected in more than half of the IM-resistant patients (P = .007; Figure 7B; Table S3). Notably, CM derived from the mononuclear cells of patient no. 1 (patient marked with “#” in Figure 7A-B; Tables S1–S2) caused an even greater (8 times) expansion of IM-naive, CD34+ CML CFCs in the presence of NI than did 25R-CM (Figure 1B).
GM-CSF expression in primary CML samples. (A) GMCSF mRNA expression was quantitated relative to GAPDH expression in 52 patients with chronic-phase CML at diagnosis and 29 patients with CML at the time of clinically manifested IM resistance. The cell sources of mRNA (bone marrow, peripheral blood, or PBMCs) are indicated. *Presence of a BCR/ABL kinase mutation at the time of IM resistance. – indicates no available kinase mutation analysis; #, patient no. 1 (PBMCs). (B) GM-CSF ELISA. The GM-CSF concentration was determined in whole peripheral blood cell lysates of IM-sensitive patients with first-diagnosis CML and IM-resistant patients with CML. Bars represent means of duplicate measurements ± SD; **P = .006 (Mann-Whitney test). BCR/ABL kinase mutations are shown; # indicates patient no.1 (Table S1). (C) Different mechanisms of IM/NI resistance on the progenitor and stem cell compartment. Autocrine or paracrine secretion of GM-CSF stimulates BCR/ABL-independent growth and survival of GMPs.
GM-CSF expression in primary CML samples. (A) GMCSF mRNA expression was quantitated relative to GAPDH expression in 52 patients with chronic-phase CML at diagnosis and 29 patients with CML at the time of clinically manifested IM resistance. The cell sources of mRNA (bone marrow, peripheral blood, or PBMCs) are indicated. *Presence of a BCR/ABL kinase mutation at the time of IM resistance. – indicates no available kinase mutation analysis; #, patient no. 1 (PBMCs). (B) GM-CSF ELISA. The GM-CSF concentration was determined in whole peripheral blood cell lysates of IM-sensitive patients with first-diagnosis CML and IM-resistant patients with CML. Bars represent means of duplicate measurements ± SD; **P = .006 (Mann-Whitney test). BCR/ABL kinase mutations are shown; # indicates patient no.1 (Table S1). (C) Different mechanisms of IM/NI resistance on the progenitor and stem cell compartment. Autocrine or paracrine secretion of GM-CSF stimulates BCR/ABL-independent growth and survival of GMPs.
Discussion
The rational development and successful clinical application of second-generation ABL inhibitors such as NI and DA demonstrates that the understanding of molecular mechanisms of IM resistance is of decisive clinical value.22,23 In contrast, disease persistence and its significance for the development of outright clinical resistance is still little understood. Primary CML CD34+ progenitor cells are growth inhibited but supposedly not killed in vitro by IM4,31,32 or DA5 in vitro. This may be due to an inherent inhibitor resistance of quiescent progenitors and BCR/ABL overexpression.5 Kinase mutations6 as well as drug influx and efflux regulations leading to low intracellular concentrations of IM may also contribute to persistence.7,8 Together, most IM resistance and persistence models are in line with a concept of BCR/ABL-dependent survival. Here we identified that autosecretion of GM-CSF, a hematopoietic cytokine, conferred BCR/ABL-independent NI and IM resistance by activating JAK-2/STAT-5.
Aberrant production of hematopoietic cytokines, particularly IL-3, but also G-CSF and GM-CSF, has long been suggested to play a role in CML biology.38,42,43 IL-3 and GM-CSF were initially reported to cause autocrine growth loops in certain BCR/ABL+ cell lines.38,39 In addition, mice that received transplants of BCR/ABL-transduced bone marrow showed elevated IL-3 and GM-CSF levels,43 and GM-CSF was also elevated in the serum of patients with CML.44 Compelling evidence was then provided for IL-3 and G-CSF as mediators of autocrine growth loops in primary human CML CD34+ cells, leading to activation of STAT-5.42 This altogether established that autocrine stimulation via G-CSF, IL-3, and GM-CSF could be of pathophysiologic relevance in CML by regulating progenitor cell growth and survival.
We describe here a potential novel role for autocrine GM-CSF secretion as a counterregulatory mechanism of BCR/ABL+ cells to resist IM and NI. In different cell systems, we showed that the GM-CSF–mediated JAK-2/STAT-5 pathway activation circumvents the need for BCR/ABL signaling to maintain survival or proliferation, and hence that the leukemic population itself activates JAK-2 signaling via autocrine secretion of GM-CSF. Interestingly, only GM-CSF, and to a lesser extent also IL-3, but none of the other tested cytokines (MCP-1, IL-6, IL-8, VEGF, G-CSF), overcome NI-mediated CFC inhibition (Figure 6A). Both GM-CSF and IL-3 are similar in that they are strong inducers of JAK-2/STAT-5, which are critical antiapoptotic and transforming targets of BCR/ABL.34,45
Since the antiapoptotic and mitogenic effects of GM-CSF are strictly CD116 expression dependent (Figures 4, 6B), GMPs expressing CD116 are supposedly the protected targets in the presence of GM-CSF. This could be biologically particularly interesting because GMPs supposedly constitute a progenitor population which, after acquisition of respective mutations, constitutes the leukemic, self-renewing stem cell population in CML blast crisis (Figure 7C).46
The mechanism of adaptive GM-CSF overexpression in response to IM exposure is unclear at present. However, it is known that expression changes are easier to achieve by tumor cells than strategic genetic alterations such as de novo BCR/ABL kinase mutations.47 Thus, GM-CSF overexpression/secretion in response to IM selection pressure could be an early resistance mechanism before kinase mutations emerge. In turn, at the time of manifest IM resistance, GM-CSF may even become dispensable as a resistance factor, and its expression may be switched off. This is seen in some of the LAMA cell clones that we have studied for GM-CSF expression in a longitudinal manner.27 Lack of GM-CSF overexpression at the time of outright IM resistance in vivo (eg, in the presence of kinase mutations) would reflect this in vitro observation (Figure 7A).
Since GM-CSF is not only secreted by CML cells (before44 and in response to IM exposure), but also by BCR/ABL− cell types (Figure 7C), progenitor clones that do not themselves up-regulate GM-CSF could be protected by paracrine stimulation. In fact, we also found evidence for STAT-5 activation of LAMA cells when testing CM collected ex vivo from the PMBC samples of patients with CML in complete remission, indicating that paracrine secretion from BCR/ABL− cells could be a relevant source for GM-CSF at the time of complete remission (not shown). Thus, GM-CSF—regardless of cellular source—can protect primary CML progenitors from IM- and NI-induced proliferation inhibition.
The data presented here may have significant clinical implications. Since inhibition of JAK-2 blocks GM-CSF and supposedly also alternative auto-/paracrine signaling, JAK-2 inhibitors may overcome cytokine-dependent IM and NI resistance.
According to this model, a combined inhibition of BCR/ABL and JAK-2 may be a more effective treatment also for IM-sensitive patients by inducing deeper molecular remissions and by reducing the risk for disease progression. This extends the role of JAK-2 from a transforming target41,45 to a mediator of IM/NI resistance in BCR/ABL+ leukemias.
Authorship
Contributions: A.B. designed the research, analyzed the data, and wrote the paper. Y.W., D.C., and C.B. performed the research. P.E. extracted patient samples and analyzed patient data. P.W.M. contributed vital new reagents and discussed the data. A.H. and A.N. contributed to the design of the experiments, analyzed the data, and proofread the manuscript.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Burchert, Philipps Universität Gießen und Marburg, Standort Marburg, Klinik für Hämatologie, Onkologie und Immunologie, 35043 Marburg, Germany; e-mail: burchert@staff.uni-marburg.de.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We greatly appreciate the help of Dr Tanja Lahaye, university clinic Mannheim, in collecting patient samples and of Drs. T. Franz and XinPing Li (Proteomics Core Facility, EMBL Heidelberg) in performing complex protein analysis of conditioned media. We wish to thank Drs. Martin Eilers and Werner Lutz for fruitful discussions of the data.
Supported by the Deutsche José Carreras Leukämie-Stiftung e.V., (R04-22f to A.B.) and by the Deutsche Forschungsgemeinschaft, Transregio 17 (to A.B. and A.N.) and by the Competence Network “Acute and chronic leukemias,” sponsored by the German Bundesministerium für Bildung und Forschung (Projektträger Gesundheitsforschung; DLR e.V.-01 GI9980/6).



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal