Abstract
Bone marrow transplantation (BMT) is the only known cure for the hematologic manifestations of Fanconi anemia (FA). Potential benefits of unrelated donor BMT for FA, however, have been severely limited by graft rejection and treatment-related mortality with resultant poor survival. Therefore, we evaluated the impact of potential prognostic factors on hematopoietic recovery, graft-versus-host disease (GVHD), and mortality in 98 recipients of unrelated donor BMT who received transplants between 1990 and 2003. Probabilities of neutrophil (89% vs 69%; P = .02) and platelet (74% vs 23%; P < .001) recovery were higher after fludarabine-containing regimens than nonfludarabine-containing regimens. Risks of acute GVHD (relative risk [RR], 4.29; P < .001) were higher with non–T-cell–depleted grafts. The day-100 mortality rate was significantly higher after nonfludarabine-containing regimens than fludarabine-containing regimens (65% vs 24%, respectively; P < .001). Corresponding 3-year adjusted overall survival rates were 13% versus 52% (P < .001). In addition, mortality was higher in recipients who were older (> 10 years), who were cytomegalovirus (CMV) seropositive, and who received more than 20 blood product transfusions before BMT. Based on these results, significant practice changes are suggested: use of a fludarabine-containing conditioning regimen in the context of T-cell–depleted marrow allografts, and earlier referral for transplantation prior to excessive transfusions in patients with marrow failure.
Introduction
Fanconi anemia (FA) is a genetically and phenotypically heterogeneous disorder characterized by congenital malformations, progressive marrow failure, and marked predisposition to leukemia and epithelial malignancies.1–7 While the basic genetic and biochemical defects responsible are now being elucidated, allogeneic hematopoietic stem cell transplantation still remains the only known treatment modality that can correct the hematologic defects associated with FA. Importantly, hematologic abnormalities occur in virtually all patients with FA at a median age of 7 years (range, birth to 41 years).4,5 Based on clinical data in the International Fanconi Anemia Registry (IFAR; n = 754 patients), the cumulative incidence of bone marrow failure by age 40 years is 90%. Initial hematologic findings are most commonly pancytopenia; however, some patients present with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML). The cumulative incidence of MDS or AML by age 40 is 33%.4,5
In 2000, the results of a retrospective multicenter study of 69 unrelated donor bone marrow transplantations (BMTs) facilitated through the European Group for Blood and Marrow Transplantation (EBMT) and the European FA Registry were reported by Guardiola et al.8 The 3-year probability of survival was 33%. The primary causes of death were acute graft-versus-host disease (GVHD; n = 18), primary or secondary graft failure (n = 13), chronic GVHD (n = 4), infections (n = 7), and veno-occlusive disease of the liver (n = 1). In this study, presence of 3 or more extramedullary congenital malformations, use of androgens prior to BMT, positive recipient cytomegalovirus (CMV) serology, and use of a female donor were independent risk factors associated with poor survival. Because of the high risk of transplantation-related mortality (TRM) from graft failure and GVHD, unrelated donor transplantation has not been generally recommended until failure of other treatment modalities, such as use of androgens and transfusions. However, early reports2,9,10 suggest improved outcome in patients with FA undergoing alternative donor hematopoietic stem cell transplantation with use of fludarabine as part of the conditioning regimen. To determine the impact of fludarabine and identify risk factors associated with mortality in patients with FA transplanted in the United States, a retrospective analysis of data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) was performed.
Patients, materials, and methods
Data collection
A formal affiliation of the research division of the National Marrow Donor Program (NMDP; established in 1986) and the International Bone Marrow Transplant Registry (IBMTR; established in 1972) led to the establishment of the CIBMTR in 2004. The CIBMTR is a working group of more than 500 voluntary transplantation centers worldwide that contribute detailed patient, disease, and, transplantation characteristics and outcome data on allogeneic transplant recipients to the Statistical Center at the Medical College of Wisconsin. Participating centers are required to register consecutive transplantations. Detailed demographic and clinical data are collected on a representative sample of registered patients and all unrelated donor transplantations facilitated by the NMDP in the United States. Patients are followed longitudinally. Computerized error checks, physician review of submitted data, and on-site audits of participating centers ensure data quality. Data on diepoxybutane (DEB) sensitivity (mean chromosome breaks per cell), DEB T-cell mosaicism (ie, presence of DEB-resistant T lymphocytes), and complementation group assignment were obtained from the IFAR database (established at the Rockefeller University in 1982).
Inclusion criteria
Patients with FA who received transplants of volunteer unrelated donor bone marrow were included in this analysis. Transplantations were performed between January 1, 1990, and December 31, 2003, and were facilitated by the NMDP. Excluded were recipients of peripheral blood or umbilical cord blood grafts. Human leukocyte antigen (HLA) typing was prospectively performed at transplantation centers using intermediate- to high-resolution molecular typing methods for HLA-A and HLA-B, and with high-resolution typing for HLA-DRB1 in all cases. The matching process identified all specificities that were recognized by the World Health Organization at the time of transplantation. Recipients of HLA-matched and 1-locus HLA-mismatched transplants were included.
The NMDP retrospectively obtained consent for study participation from surviving patients or their parent/legal guardian for transplantations it facilitated during the study period; the NMDP institutional review board (IRB) waived consent for patients who had died before soliciting consent. To address introduction of bias by inclusion of only a proportion of surviving patients (those consenting) but all deceased recipients, a sample of deceased patients was selected using a weighted randomized scheme that adjusts for overrepresentation of deceased patients in the consented cohort.11 This weighted randomized scheme was developed based on all survivors in the NMDP database. A logistic regression model was fit to identify the factors that predicted whether a patient had consented to use of data collected by the NMDP. This analysis found that the following factors were associated with the likelihood of a patient consenting: age at transplantation, disease, race, sex, CMV serologic status, and country of transplantation (US vs non-US). Using estimated consenting probabilities from this model based on characteristics of dead patients, the biased-coin method of randomization was performed to determine which of the dead patients likely would have consented to participate had they been alive. Thus, this procedure includes the preconsented dead patients at the same probability as surviving patients who consented to participate. Thus, of the 120 patients who met the eligibility criteria for this study, 98 were included after this adjustment.
End points
Neutrophil recovery was defined as achieving an absolute neutrophil count (ANC) of at least 0.5 × 109/L for 3 consecutive days; platelet recovery was defined as achieving at least a platelet count of 20 × 109/L unsupported by transfusions for at least 7 days. Diagnosis of acute and chronic GVHD was based on local institutional criteria, with overall grade of acute GVHD assigned retrospectively by the CIBMTR based on stage of involvement reported for each individual organ.12,13 Transplantation-related mortality was defined as any cause of death other than recurrent leukemia. Surviving patients were censored at date of last contact.
Statistical analysis
Variables related to patient, disease, and transplantation characteristics are shown in Table 1. The probability of day-100 mortality and overall survival were calculated using the Kaplan-Meier estimator.14 For analysis of survival, death from any cause was considered an event, and outcome data on living patients were censored at 36 months after transplantation. The probabilities of neutrophil and platelet recovery, acute and chronic GVHD, and transplantation-related mortality were calculated using the cumulative-incidence function method.15 For neutrophil and platelet recovery and GVHD, death without an event (hematopoietic recovery or GVHD) was the competing event. For transplantation-related mortality, recurrent leukemia was the competing event. Confidence intervals (CIs) were calculated with the use of a log transformation.15 The P value for comparison of probabilities of hematopoietic recovery, GVHD, transplantation-related mortality, day-100 mortality, and overall survival at any given time point was calculated using the point-wise P value estimator. Cox regression models were built for analysis of risk factors for GVHD, early mortality (within 100 days after transplantation), transplantation-related and overall mortality, and logistic regression models were built for analysis of risk factors for neutrophil and platelet recovery at day 28 and at 1 year, respectively.15,16 Of the 98 patients, 96 were evaluable for analysis of risk factors for transplantation-related, early, and overall mortality. Two patients were excluded because data on GVHD prophylaxis were not available. Multivariable models were built with the use of stepwise forward selection, with a P value of .05 or less considered to indicate statistical significance. All variables met the proportional hazards assumptions. Results are expressed as relative risk (RR)—the relative rate of occurrence of an event and corresponding P values obtained from Cox regression models. Variables considered in multivariate models included recipient age (≤ 10 years vs > 10 years), reason for transplantation (marrow aplasia vs MDS or AML), prior therapy (androgens with or without other, including steroids vs steroids with or without other, not androgens vs none), red blood cell and platelet transfusion prior to transplantation (0-20 transfusions vs more than 20 transfusions), DEB T-cell mosaicism (absent vs any percentage of mosaicism), DEB sensitivity (≤ 9 mean chromosome breaks per cell vs > 9 mean chromosome breaks per cell), performance score, serologic status of the donor and recipient with respect to CMV before transplantation, sex of the donor and recipient, conditioning regimen and GVHD prophylaxis (fludarabine-based conditioning vs nonfludarabine-based conditioning with T-cell depletion vs nonfludarabine-based conditioning with no T-cell depletion), and HLA disparity (matched at HLA-A, HLA-B, and HLA-DRB1 vs 1-locus mismatch). There were no first-order interactions. P values are 2-sided. Analyses were completed with the use of SAS software, version 9.1 (SAS Institute, Cary, NC).
Characteristics of patients, disease, and transplantation procedures
| Variable . | Value . |
|---|---|
| Total | 98 |
| Male | 52 (53) |
| Age | |
| 10 y or younger | 38 (39) |
| 11-20 y | 43 (44) |
| Older than 20 y | 17 (17) |
| Bone marrow status prior to transplantation | |
| Aplastic anemia | 75 (77) |
| MDS | 14 (14) |
| AML | 7 (7) |
| Unknown | 2 (2) |
| Complementation group | |
| A | 37 (38) |
| C | 12 (12) |
| D1 | 1 (1) |
| D2 | 2 (2) |
| G | 4 (4) |
| Not determined | 42 (43) |
| DEB mosaicism | |
| Absent | 34 (35) |
| Present | 45 (46) |
| Not determined | 19 (19) |
| DEB sensitivity, median (range) | 9.1 (0.5-23.9) |
| No. of prior blood transfusions | |
| None | 5 (5) |
| 1-20 | 35 (36) |
| More than 20 | 24 (24) |
| Unknown | 34 (35) |
| Therapy prior to transplantation | |
| None | 19 (19) |
| Androgens ± other including steroids | 54 (55) |
| Steroids ± other (without androgens) | 11 (11) |
| Unknown | 14 (14) |
| Year of transplantation | |
| 1990-1998 | 51 (52) |
| 1999-2003 | 47 (48) |
| Conditioning regimen* | |
| Nonfludarabine regimens | 52 (53) |
| TLI + cyclophosphamide | 2 (4) |
| TAI + cyclophosphamide + ATG | 6 (12) |
| TLI + busulfan ± ATG | 1 (1) |
| TBI + cyclophosphamide ± ATG† | 43 (83) |
| Fludarabine-containing regimens | 46 (47) |
| Cyclophosphamide + ATG | 1 (2) |
| Cyclophosphamide + busulfan + ATG | 1 (2) |
| TAI + cyclophosphamide + ATG | 1 (2) |
| TBI + cyclophosphamide ± ATG‡ | 43 (93) |
| GVHD prophylaxis | |
| Cyclosporine ± other | 16 (16) |
| Cyclosporine + methotrexate ± other | 8 (8) |
| T-cell depletion + other | 70 (71) |
| Tacrolimus ± other | 2 (2) |
| Unknown | 2 (2) |
| Donor-recipient CMV status | |
| Both donor, recipient negative | 45 (46) |
| Donor positive/recipient negative | 20 (20) |
| Donor negative/recipient positive | 24 (24) |
| Donor positive/recipient positive | 9 (9) |
| Donor-recipient sex match | |
| Female donor to male recipient | 22 (22) |
| Other | 76 (78) |
| Donor-recipient HLA match | |
| Matched | 76 (78) |
| One-antigen mismatched | 22 (22) |
| Variable . | Value . |
|---|---|
| Total | 98 |
| Male | 52 (53) |
| Age | |
| 10 y or younger | 38 (39) |
| 11-20 y | 43 (44) |
| Older than 20 y | 17 (17) |
| Bone marrow status prior to transplantation | |
| Aplastic anemia | 75 (77) |
| MDS | 14 (14) |
| AML | 7 (7) |
| Unknown | 2 (2) |
| Complementation group | |
| A | 37 (38) |
| C | 12 (12) |
| D1 | 1 (1) |
| D2 | 2 (2) |
| G | 4 (4) |
| Not determined | 42 (43) |
| DEB mosaicism | |
| Absent | 34 (35) |
| Present | 45 (46) |
| Not determined | 19 (19) |
| DEB sensitivity, median (range) | 9.1 (0.5-23.9) |
| No. of prior blood transfusions | |
| None | 5 (5) |
| 1-20 | 35 (36) |
| More than 20 | 24 (24) |
| Unknown | 34 (35) |
| Therapy prior to transplantation | |
| None | 19 (19) |
| Androgens ± other including steroids | 54 (55) |
| Steroids ± other (without androgens) | 11 (11) |
| Unknown | 14 (14) |
| Year of transplantation | |
| 1990-1998 | 51 (52) |
| 1999-2003 | 47 (48) |
| Conditioning regimen* | |
| Nonfludarabine regimens | 52 (53) |
| TLI + cyclophosphamide | 2 (4) |
| TAI + cyclophosphamide + ATG | 6 (12) |
| TLI + busulfan ± ATG | 1 (1) |
| TBI + cyclophosphamide ± ATG† | 43 (83) |
| Fludarabine-containing regimens | 46 (47) |
| Cyclophosphamide + ATG | 1 (2) |
| Cyclophosphamide + busulfan + ATG | 1 (2) |
| TAI + cyclophosphamide + ATG | 1 (2) |
| TBI + cyclophosphamide ± ATG‡ | 43 (93) |
| GVHD prophylaxis | |
| Cyclosporine ± other | 16 (16) |
| Cyclosporine + methotrexate ± other | 8 (8) |
| T-cell depletion + other | 70 (71) |
| Tacrolimus ± other | 2 (2) |
| Unknown | 2 (2) |
| Donor-recipient CMV status | |
| Both donor, recipient negative | 45 (46) |
| Donor positive/recipient negative | 20 (20) |
| Donor negative/recipient positive | 24 (24) |
| Donor positive/recipient positive | 9 (9) |
| Donor-recipient sex match | |
| Female donor to male recipient | 22 (22) |
| Other | 76 (78) |
| Donor-recipient HLA match | |
| Matched | 76 (78) |
| One-antigen mismatched | 22 (22) |
Values in table represent number of patients (%) unless otherwise indicated. ± indicates with or without; TLI, total lymphoid irradiation; TAI, total abdominal radiation; ATG, antithymocyte globulin.
Fludarabine was used exclusively after 1998.
TBI doses: 200 cGy, n = 1; 300 cGy, n = 1; 400 cGy, n = 8; 450 cGy, n = 21; 500 cGy, n = 1; and 600 cGy, n = 11.
TBI dose: 450 cGy, n = 43.
Results
Patients
Patient, disease, and transplantation characteristics are shown in Table 1. The median age at transplantation was 12 years (range, 0.8-33 years). Data were available on complementation group for 56 (57%) of 98 patients and on DEB T-cell mosaicism for 79 (81%) of 98 patients. Approximately 60% of patients received red blood cell and platelet transfusions prior to transplantation. The most common indication for transplantation was marrow aplasia. All patients received bone marrow grafts, and most received grafts from donors matched at HLA-A, HLA-B, and HLA-DRB1. Nearly all (44 of 46) recipients of the fludarabine-based conditioning regimen received T-cell–depleted grafts; the remaining 2 patients received non–T-cell–depleted grafts. Half (26) of the 52 recipients of nonfludarabine regimens received T-cell–depleted grafts; 24 received non–T-cell–depleted grafts, and GVHD prophylaxis was not reported in 2 patients. The median total nucleated cell dose infused for recipients of non–T-cell–depleted transplants was 3.65 × 108/kg (range, 2.29-9.72 × 108/kg). The cell dose infused was lower in recipients of T-cell–depleted grafts (0.23 × 108/kg [range, 0.01-6.17 × 108/kg]). The median follow-up of survivors who received a fludarabine-containing conditioning regimen was 41 months (range, 10-69 months), and 135 months (range, 63-163 months) in survivors of nonfludarabine conditioning regimens. In order to adjust for differences in follow-up between recipients of fludarabine-containing (used exclusively after 1998) and nonfludarabine conditioning regimens, all patients were censored at 36 months for analysis of transplantation outcomes.
Patient, disease, and transplantation characteristics of recipients of fludarabine-based and nonfludarabine conditioning regimens were similar, except that recipients of fludarabine-containing regimens were more likely to have a good performance score (90-100) at transplantation (91% vs 64%; P = .002) and received T-cell–depleted grafts (96% vs 51%; P < .001). Potential differences in the proportion of patients with DEB T-cell mosaicism between groups could not be determined, as data on DEB mosaicism were more likely available (93%) in recipients of fludarabine and less likely available (60%) in recipients of nonfludarabine regimens.
Neutrophil and platelet recovery
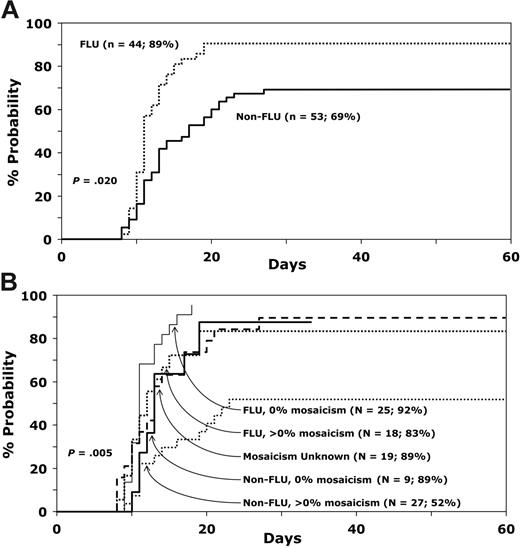
Most (77 of 98) patients achieved neutrophil recovery. Median time to recovery was 11 days (range, 8-27 days), with an overall probability of recovery of 78% (95% CI, 68-85) by day 28. Among the 79 patients for whom data on DEB T-cell mosaicism were available, the probability of neutrophil recovery differed by type of conditioning regimen and DEB T-cell mosaicism (Table 2; Figure 1A-B). The day-28 probability of recovery was higher in recipients of a fludarabine-containing regimen versus a nonfludarabine-containing regimen (89% vs 69%; P = .02). Among recipients of fludarabine-containing regimens, probability of recovery was similar in patients with and without DEB T-cell mosaicism. In contrast, the probability of recovery differed in recipients of nonfludarabine regimens, with probable greater engraftment in those without DEB T-cell mosaicism (89% vs 52%; odds ratio [OR], 0.14; 95% CI, 0.02-1.23; P = .076).
Results of multivariable analysis for predictors of neutrophil recovery at 28 days after transplantation in patients tested for DEB T-cell mosaicism
| Variable . | N1/N2 . | Odds ratio (95% CI) . | P . |
|---|---|---|---|
| Conditioning regimen/DEB mosaicism | .005* | ||
| FLU regimen, 0% DEB mosaicism | 23/25 | 1.00 | |
| FLU regimen, more than 0% DEB mosaicism | 15/18 | 0.44 (0.07-2.92) | .391 |
| Non-FLU regimen, 0% DEB mosaicism | 8/9 | 0.70 (0.06-8.75) | .778 |
| Non-FLU regimen, more than 0% DEB mosaicism | 14/27 | 0.09 (0.02-0.48) | .004 |
| Variable . | N1/N2 . | Odds ratio (95% CI) . | P . |
|---|---|---|---|
| Conditioning regimen/DEB mosaicism | .005* | ||
| FLU regimen, 0% DEB mosaicism | 23/25 | 1.00 | |
| FLU regimen, more than 0% DEB mosaicism | 15/18 | 0.44 (0.07-2.92) | .391 |
| Non-FLU regimen, 0% DEB mosaicism | 8/9 | 0.70 (0.06-8.75) | .778 |
| Non-FLU regimen, more than 0% DEB mosaicism | 14/27 | 0.09 (0.02-0.48) | .004 |
N1 indicates number of patients with neutrophil recovery; N2, number of evaluable patients; FLU, fludarabine.
Three-degree freedom test.
Probability of neutrophil recovery. (A) Comparison of neutrophil recovery with fludarabine and nonfludarabine conditioning regimens. (B) Effect of DEB mosiacism on neutrophil recovery with fludarabine and nonfludarabine conditioning regimens.
Probability of neutrophil recovery. (A) Comparison of neutrophil recovery with fludarabine and nonfludarabine conditioning regimens. (B) Effect of DEB mosiacism on neutrophil recovery with fludarabine and nonfludarabine conditioning regimens.
Nearly half (44 of 90) of the evaluable patients achieved platelet recovery. Median time to recovery was 36 days (range, 15-148 days), with an overall probability of recovery of 49% (95% CI, 38%-59%) at 1 year. The probability of platelet recovery also differed by conditioning regimen (Table 3). The day-100 probability of platelet recovery was higher in recipients of fludarabine-containing regimens than nonfludarabine-containing regimens (74% vs 23%; P < .001). An effect of DEB T-cell mosaicism was not observed on platelet recovery. Recovery was more likely in patients who had received 20 or fewer blood product transfusions prior to transplantation.
Results of multivariable analysis for predictors of platelet recovery at 3 months after transplantation
| Variable . | N1/N2 . | Odds ratio (95% CI) . | P . |
|---|---|---|---|
| Conditioning regimen | |||
| FLU-containing regimen | 35/46 | 1.00 | |
| Non–FLU-containing regimen | 9/44 | 0.07 (0.02-0.25) | < .001 |
| Prior transfusions | |||
| 20 or less | 22/39 | 1.00 | |
| More than 20 | 5/24 | 0.17 (0.06-0.92) | .014 |
| Variable . | N1/N2 . | Odds ratio (95% CI) . | P . |
|---|---|---|---|
| Conditioning regimen | |||
| FLU-containing regimen | 35/46 | 1.00 | |
| Non–FLU-containing regimen | 9/44 | 0.07 (0.02-0.25) | < .001 |
| Prior transfusions | |||
| 20 or less | 22/39 | 1.00 | |
| More than 20 | 5/24 | 0.17 (0.06-0.92) | .014 |
N1 indicates number of patients with platelet recovery; N2, number of evaluable patients; and FLU, fludarabine.
Acute and chronic GVHD
Of the 96 patients, 30 developed grades 2 to 4 acute GVHD. Of these 30, 28 developed acute GVHD within 100 days, for an overall day-100 probability of acute GVHD of 29% (95% CI, 20-38). The occurrence of grades 2 to 4 acute GVHD was statistically significantly higher in recipients of a nonfludarabine-containing regimen who received a non–T-cell–depleted graft (RR, 4.29; 95% CI, 1.82-10.10; P < .001). The day-100 probabilities of the occurrence of grades 2 to 4 acute GVHD were 70% and 21% after transplantation of non–T-cell–depleted and T-cell–depleted bone marrow grafts after a nonfludarabine-containing regimen, respectively. The corresponding probability of acute GVHD after a fludarabine-containing regimen was 16%. A number (17) of patients developed grades 3 to 4 acute GVHD. Most patients (n = 13) who developed grades 3 to 4 acute GVHD received non–T-cell–depleted grafts. Of 55 patients, 16 patients surviving beyond 90 days developed chronic GVHD for an overall 3-year probability of chronic GVHD of 31% (95% CI, 19-44). There were no statistically significant differences in risks of chronic GVHD by conditioning regimen or GVHD prophylaxis.
Transplantation-related mortality
Risks of transplantation-related mortality were statistically significantly higher after a nonfludarabine-containing regimen with a T-cell–depleted graft (RR, 3.69; 95% CI, 1.76-7.68; P < .001) or non–T-cell–depleted graft (RR, 3.87; 95% CI, 1.94-7.73; P < .001) compared with fludarabine-containing regimens. Transplantation-related mortality rates were higher in patients who were CMV positive by serology and who received bone marrow grafts from donors who were also CMV positive (RR, 5.01; 95% CI, 2.08-12.09; P < .001), and in patients who received more than 20 blood product transfusions prior to transplantation (RR, 2.48; 95% CI, 1.30-4.75; P = .006). The 3-year probabilities of treatment-related mortality were 47% (95% CI, 31-60) and 81% (95% CI, 67-89) in recipients of fludarabine-containing and nonfludarabine-containing regimens, respectively (P < .001).
Early mortality
Risks of early mortality were statistically significantly higher after a nonfludarabine-containing regimen in patients who were CMV seropositive and in patients aged 10 years or older at transplantation (Table 4). The day-100 probability of mortality after a fludarabine-containing regimen versus a nonfludarabine-containing regimen was 24% and 65%, respectively (P < .001). Early death occurred in 11 patients after fludarabine-containing regimens and in 34 patients after nonfludarabine-containing regimens. Causes of early mortality in recipients of fludarabine-containing regimens and nonfludarabine-containing regimens were graft failure (n = 5 and n = 13), GVHD (n = 0 and n = 9), interstitial pneumonitis (n = 1 and n = 1), opportunistic infection (n = 2 and n = 8), and organ failure (n = 3 and n = 3).
Results of multivariable analysis for predictors of early mortality
| Variables . | N1/N2 . | RR (95% CI) . | P . |
|---|---|---|---|
| Conditioning regimen | < .001* | ||
| FLU-containing regimen | 11/46 | 1.00 | |
| Non–FLU-containing regimen, T-cell–depleted grafts | 17/26 | 4.19 (1.88-9.39) | < .001 |
| Non–FLU-containing regimen, non–T-cell-depleted grafts | 15/24 | 5.11 (2.21-11.84) | < .001 |
| Age at transplantation | |||
| 10 years or younger | 12/37 | 1.00 | |
| Older than 10 years | 31/59 | 2.07 (1.03-4.15) | .041 |
| Donor-recipient CMV status | .006† | ||
| Donor negative/recipient negative | 19/45 | 1.00 | |
| Donor positive/recipient negative | 5/18 | 1.11 (0.40-3.09) | .839 |
| Donor negative/recipient positive | 13/24 | 2.20 (1.07-4.52) | .032 |
| Donor positive/recipient positive | 6/9 | 4.91 (1.83-13.19) | .002 |
| Variables . | N1/N2 . | RR (95% CI) . | P . |
|---|---|---|---|
| Conditioning regimen | < .001* | ||
| FLU-containing regimen | 11/46 | 1.00 | |
| Non–FLU-containing regimen, T-cell–depleted grafts | 17/26 | 4.19 (1.88-9.39) | < .001 |
| Non–FLU-containing regimen, non–T-cell-depleted grafts | 15/24 | 5.11 (2.21-11.84) | < .001 |
| Age at transplantation | |||
| 10 years or younger | 12/37 | 1.00 | |
| Older than 10 years | 31/59 | 2.07 (1.03-4.15) | .041 |
| Donor-recipient CMV status | .006† | ||
| Donor negative/recipient negative | 19/45 | 1.00 | |
| Donor positive/recipient negative | 5/18 | 1.11 (0.40-3.09) | .839 |
| Donor negative/recipient positive | 13/24 | 2.20 (1.07-4.52) | .032 |
| Donor positive/recipient positive | 6/9 | 4.91 (1.83-13.19) | .002 |
N1 indicates number of early deaths; N2, number of evaluable patients; FLU, fludarabine.
Two-degree freedom test.
Three-degree freedom test.
Overall survival
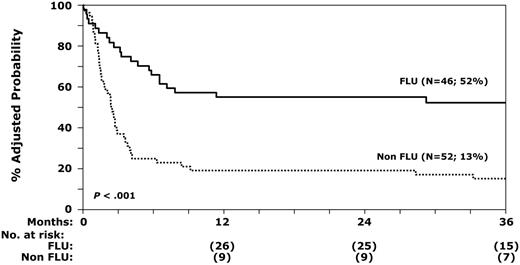
Risks of overall mortality were statistically significantly higher after a nonfludarabine-containing regimen in patients who were CMV seropositive and received bone marrow grafts from donors who were also CMV seropositive and in patients who received more than 20 blood product transfusions prior to transplantation (Table 5). The 3-year probabilities of overall survival after fludarabine-containing and nonfludarabine-containing regimens were 52% and 13%, respectively (P < .001), after adjusting for number of prior red blood cell transfusions and CMV serostatus (Figure 2). Beyond day 100, 21 patients died; 10 received fludarabine-containing regimens and 11 received nonfludarabine-containing regimens. Causes of mortality in recipients of fludarabine-containing and nonfludarabine-containing regimens were graft failure (n = 2 and n = 6), GVHD (n = 5 and n = 3), interstitial pneumonitis (n = 0 and n = 1), organ failure (n = 2 and n = 0), and recurrent leukemia (n = 1 and n = 1). There were no statistically significant differences in causes of death by conditioning regimen or HLA disparity.
Probability of overall survival with fludarabine and nonfludarabine regimens after adjusting for prior red blood cell transfusions and CMV serostatus.
Probability of overall survival with fludarabine and nonfludarabine regimens after adjusting for prior red blood cell transfusions and CMV serostatus.
Results of multivariable analysis for predictors of overall mortality
| Variables . | N1/N2 . | RR (95% CI) . | P . |
|---|---|---|---|
| Conditioning regimen | < .001* | ||
| FLU-containing regimen | 21/46 | 1.00 | |
| Non–FLU-containing regimen, T-cell–depleted grafts | 24/26 | 3.44 (1.72-6.91) | < .001 |
| Non–FLU-containing regimen, non–T-cell-depleted grafts | 19/24 | 3.57 (1.80-7.06) | < .001 |
| Prior red blood cell transfusions | |||
| 20 or less | 26/40 | 1.00 | |
| More than 20 | 22/24 | 2.49 (1.34-4.66) | .004 |
| Donor-recipient CMV status | .007† | ||
| Donor negative/recipient negative | 30/45 | 1.00 | |
| Donor positive/recipient negative | 11/18 | 1.09 (0.53-2.26) | .817 |
| Donor negative/recipient positive | 15/24 | 1.29 (0.68-2.44) | .437 |
| Donor positive/recipient positive | 8/9 | 4.52 (1.90-10.74) | < .001 |
| Variables . | N1/N2 . | RR (95% CI) . | P . |
|---|---|---|---|
| Conditioning regimen | < .001* | ||
| FLU-containing regimen | 21/46 | 1.00 | |
| Non–FLU-containing regimen, T-cell–depleted grafts | 24/26 | 3.44 (1.72-6.91) | < .001 |
| Non–FLU-containing regimen, non–T-cell-depleted grafts | 19/24 | 3.57 (1.80-7.06) | < .001 |
| Prior red blood cell transfusions | |||
| 20 or less | 26/40 | 1.00 | |
| More than 20 | 22/24 | 2.49 (1.34-4.66) | .004 |
| Donor-recipient CMV status | .007† | ||
| Donor negative/recipient negative | 30/45 | 1.00 | |
| Donor positive/recipient negative | 11/18 | 1.09 (0.53-2.26) | .817 |
| Donor negative/recipient positive | 15/24 | 1.29 (0.68-2.44) | .437 |
| Donor positive/recipient positive | 8/9 | 4.52 (1.90-10.74) | < .001 |
N1 indicates number of deaths; N2, number of evaluable patients; and FLU, fludarabine.
Two-degree freedom test.
Three-degree freedom test.
Discussion
BMT is the only current treatment with curative potential for the marrow failure syndrome that characterizes FA. Early experiences with BMT for the treatment of marrow failure were poor, primarily the result of excessive regimen-related toxicity, graft failure, and severe acute GVHD.2,8,17–19 Early conditioning regimens were modeled after those for patients with acquired aplastic anemia, administering cyclophosphamide alone or in combination with busulfan or total body irradiation (TBI). Severe toxicities, including cardiac myopathy, gastrointestinal hemorrhage, hemorrhagic cystitis, and radiation skin burns, were commonly reported. This clinical experience prompted in vitro laboratory studies that confirmed the hypersensitivity of FA cells to cyclophosphamide and TBI.20–22 As a result of these in vitro studies, Gluckman et al22,23 proposed the use of low-dose cyclophosphamide and thoracoabdominal irradiation in the treatment of patients with FA. This approach subsequently proved to be a major advance in the treatment of patients with FA who had HLA-matched sibling donors. Unfortunately, this therapy was not sufficient for consistent engraftment in recipients of unrelated donor BMT, with graft rejection exceeding 20% in most series.2,8,18,23
To date, there have only been a few registry reports on the efficacy of unrelated donor BMT in FA.8,17 In an attempt to extend these observations as well as to assess specifically the impact of fludarabine, which could not be evaluated in the European report8 that in general predated the use of fludarabine, we evaluated the impact of unrelated donor BMT for FA at US centers as facilitated by the NMDP. In contrast to the EBMT report,8 in which a higher mortality was observed in patients with 3 or more congenital malformations, prior exposure to androgens, and a female donor, the current series identified 3 different factors associated with improved survival: use of fludarabine in the preparative therapy, younger recipient age at BMT (10 years or younger), and exposure to fewer than 20 blood product transfusions. Recipient CMV-negative serostatus was found to be associated with improved survival in both analyses. We were not able to access the impact of congenital malformations due to incomplete data.
Importantly, this is the first report to document the benefit of fludarabine in patients with FA in terms of hematopoietic recovery and survival. Fludarabine is optimal in patients with FA as it is an antimetabolite with profound immunosuppressive properties. Because FA cells are hypersensitive to DNA cross-linking agents, agents like cyclophosphamide have a relatively tight therapeutic window associated with a high rate of excess nonhematopoietic toxicities. As such, low-dose cyclophosphamide with or without limited field irradiation or low-dose TBI has been inadequate for consistent engraftment. As reported by Guardiola et al8 and others,2,10,18,19 we observed an excess rate of graft failure in recipients not treated with fludarabine. Incorporation of fludarabine into the preparative regimen has markedly improved the probability of engraftment without increasing the risk of regimen-related end-organ toxicity, leading to improved survival.
In the past 5 years, there has been increasing recognition of somatic mosaicism in patients with FA. While it has been postulated that somatic mosaicism may delay the onset of hematopoietic failure in patients with FA, it is clear that it does not always prevent marrow failure or progression to MDS or leukemia.24–26 While it remains to be proven whether the presence of DEB-resistant cells generally reflects a genetic reversion in a stem cell, there is some data that suggest that the presence of any DEB-resistant cells may be associated with a higher risk of graft failure.2,10 In the present series, neutrophil recovery was not influenced by the presence of DEB-resistant cells. However, when stratified by use of fludarabine, DEB mosaicism had a significant effect in patients not treated with fludarabine as part of the preparative therapy. Neutrophil recovery was less likely in patients with DEB mosaicism compared with patients without DEB mosaicism (ie, presence of DEB-sensitive cells only). While not proof, this observation suggests that the high frequency of graft rejection observed in recipients of low-dose cyclophosphamide and radiation may be due to incomplete ablation of DEB-resistant lymphocytes, and that the addition of fludarabine overcame this obstacle.
In reports by Guardiola et al8 and others,2,17,18 a major limitation to the success of unrelated donor BMT has been GVHD. In this series, T-cell depletion of the marrow graft reduced the risk of acute GVHD, and in recipients of fludarabine-based preparatory regimens, T-cell depletion did not have an adverse effect on hematopoietic recovery or overall survival. As 96% of recipients of a fludarabine-based preparatory regimen received a T-cell–depleted bone marrow graft, it is possible that the additional immunosuppression from fludarabine may have overcome any adverse effect on hematopoietic recovery often observed in recipients of a T-cell–depleted allograft.27 Because a history of GVHD has been associated with an increased risk of malignancy in patients with FA at a median of 8 years,28 strategies to reduce the risk of GVHD and improve overall survival, such as T-cell depletion with fludarabine-based preparatory regimens, are an important part of the treatment plan.
Although excess transfusion exposure was associated with higher mortality, we did not observe a greater risk of graft failure. It is possible that transfusion exposure is a surrogate marker for factors that go along with delayed transplantation (eg, infection exposure and hemochromatosis), and may explain its association with higher mortality. Data on transfusion exposure are unknown for approximately a third of patients, and we do not have data on pretransplantation infections or hemochromatosis. Thus, insufficient data are available to explain the observed association of excess transfusion exposure and greater risk of mortality. Nonetheless, the data would argue for earlier transplantation in those with marrow failure, prior to excess transfusion exposures.
As in other transplantation studies, age at BMT has an effect on survival. Older recipients (older than 10 years) had higher early mortality. This effect, however, was not observed beyond the first 100 days after transplantation. CMV seropositivity of the recipient also had an adverse effect on both early and overall survival. Guardiola et al8 also observed higher mortality in patients who were seropositive for CMV and, as in the current series, were unable to provide a clear explanation.
We did not observe a significant correlation between complementation groups and hematopoietic recovery or mortality. Data on complementation groups, however, were available for only 56% of patients, and small numbers may have limited our ability to detect a meaningful effect. In this series, androgens and HLA disparity had no demonstrable effect on transplantation outcomes. It is also possible that the use of fludarabine may have sufficiently improved outcomes to mask any deleterious effect of androgens and HLA mismatch that has been observed previously. However, despite this observation, larger studies in unrelated donor BMT clearly demonstrate the negative effect of HLA mismatch on survival.29–33 Therefore, matching between recipient and donor using high-resolution allele-level HLA typing represents the current standard of care.32,33 Because fludarabine has been used almost exclusively since 1998, we were unable to evaluate the effect of year of transplantation, a surrogate for advances in supportive care, on mortality after transplantation.
The combined use of a fludarabine-based preparative therapy and T-cell depletion has resulted in a significant improvement in both early and overall survival. Higher rates of hematopoietic recovery and lower rates of GVHD have markedly reduced the principal obstacles reported in prior series. Furthermore, a major limitation of previous reports has been marked heterogeneity in the treatment of this rare disease. Although this is a multicenter registry–based report, approximately half of the patients received fludarabine in the preparative therapy; of these, 93% received a fairly uniform regimen (single-fraction TBI at a dose of 450 cGy in combination with cyclophosphamide at a dose of 40 mg/kg [n = 35] or 20 mg/kg [n = 8]). While not initially constructed as a cooperative trial, it is clear that measures to promote conformity have permitted more rapid evaluation of new treatments, such as addition of fludarabine. Future cooperative studies should be encouraged when testing new treatment strategies, particularly in rare diseases such as FA. In the meantime, early transplantation before excessive transfusions and use of fludarabine in the preparative therapy should become standard practice for patients with FA who have marrow failure.
Authorship
Contribution: All authors contributed equally to the conception, design, and interpretation of data, and the final manuscript. A.D.A. provided data on DEB sensitivity and complement group assignment; M.E. and M.-J.Z. performed the statistical analysis; and J.E.W. had primary responsibility for drafting the manuscript.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: John E. Wagner, Department of Pediatrics, Mayo Mail Code 366, 420 Delaware St SE, Minneapolis, MN, 55455; e-mail: wagne002@umn.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to the work of many clinical investigators who have advanced the field and the many physicians and nurses who have diligently cared for these complex patients. In addition, we gratefully acknowledge the work of the many search coordinators and the dedicated staff of the National Marrow Donor Program.
This work was supported by Public Health Service Grants U24-CA76518-08 (M.E.) from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; R37 HL32987 (A.D.A.) from the National Heart, Lung and Blood Institute; and the Fanconi Anemia Research Fund (J.E.W., M.L.M.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal