Abstract
Following allogeneic hematopoietic stem cell transplantation (alloHSCT), children are at risk of life-threatening pneumococcal infections. Whereas vaccination with polysaccharide vaccines fails to elicit protective immunity in most alloHSC transplant recipients, pneumococcal conjugate vaccines may effectively prevent invasive disease by eliciting T-cell–dependent antibody responses. Here, we report safety and immunogenicity in 53 children immunized with a regimen of 3 consecutive doses of a heptavalent pneumococcal conjugate vaccine (7vPCV) in monthly intervals starting 6 to 9 months after alloHSCT. Immunization was well tolerated with no vaccine-related serious adverse events. Serologic response rates evaluable in 43 patients ranged from 41.9% to 86.0% and 58.1% to 93.0% after 2 and 3 vaccinations, respectively, with 55.8% and 74.4% of patients achieving protective antibody levels to all 7 vaccine serotypes. Our study provides the first evidence that vaccination with 7vPCV is safe and elicits protective antipneumococcal antibody responses in pediatric recipients of related or unrelated donor alloHSC transplants within the first year following transplantation. This trial was registered at www.clinicaltrials.gov as NCT00169728.
Introduction
Patients following allogeneic hematopoietic stem cell transplantation (alloHSCT) are at significant risk of life-threatening infections despite leukocyte engraftment.1,2 This is based on the loss of protective immunity to vaccine-preventable diseases and the relative immaturity of the emerging immune system.3–9 In this setting, infections with encapsulated bacteria, particularly pneumococci, are associated with significant rates of morbidity and mortality.10,11 Epidemiologic studies have shown a cumulative incidence of invasive pneumococcal disease between 1% and 10% with a median onset at 1 year following transplantation.12,13 Therefore, effective prevention, including chemoprophylaxis and active immunization, is warranted. This is particularly true for children who on reintegration into day care and school become highly exposed to pathogens.
Current guidelines of the Centers for Disease Control and Prevention (CDC) and the European Blood and Marrow Transplantation group (EBMT) generally recommend vaccination of all alloHSC transplant recipients with the standard 23-valent pneumococcal polysaccharide vaccine starting at 12 months after transplantation.14,15 Because of immaturity of the immune system, however, vaccination with polysaccharide vaccines fails to elicit protective immunity in the majority of pediatric alloHSC transplant recipients, with response rates ranging from 20% to 30% in the first to 50% in the second year after alloHSCT.16,17 In contrast, in the heptavalent pneumococcal conjugate vaccine (7vPCV), serotype-specific polysaccharides are conjugated to an immunogenic protein carrier and hence elicit T-cell–dependent antibody responses. 7vPCV contains the 7 most prevalent pneumococcal serotypes and provides effective protection against invasive disease in infants and young children who, like alloHSC transplant recipients, only weakly respond to polysaccharide vaccines as a result of immunologic immaturity.18–20 Currently, there is a dearth of data on the use of 7vPCV in pediatric alloHSC transplant recipients. Here, we report our data on immunogenicity and tolerability of 7vPVC vaccination in 53 pediatric alloHSC transplant recipients and show for the first time that 7vPCV is well tolerated and provides early protective antipneumococcal antibody responses in children following alloHSCT from both related and unrelated donors.
Patients, materials, and methods
Following alloHSCT from a related or unrelated donor with standard intensity conditioning children and adolescents up to 16 years of age with stable engraftment and remission from underlying malignant disease were recruited into the prospective, multicenter IKAST trial after informed consent was obtained in accordance with the Declaration of Helsinki. Approval for the IKAST trial was obtained from the ethics committees of all participating study sites. Patients who received a transplant for primary immunodeficiency or having uncontrolled graft-versus-host disease (GvHD) (Lansky score < 60%) or known intolerance to study vaccines were excluded. Patient-, disease-, and transplant-characteristics are detailed in Table 1. Patients were immunized with a primary series of 3 doses of the 7-valent pneumococcal conjugate vaccine (7vPCV; Prevenar; Wyeth Pharma, Münster, Germany) in conjunction with a hexavalent combination vaccine (Infanrix hexa; GlaxoSmithKline, Munich, Germany) in monthly intervals starting at 6 to 9 months following alloHSCT with a minimum interval of 2 months from last intravenous immunoglobulin application or transfusion. Serum was drawn for assessment of antibody concentrations prior to the first vaccine and 4 to 6 weeks after the second and third vaccines. Serotype-specific antibody concentrations to the pneumococcal antigens 4, 6B, 9V, 14, 18C, 19F, and 23F were determined by an enzyme-linked immunoabsorbent assay technique as previously described using the reference serum lot 89-SF (Center for Biologics, Rockville, MD) for assay standardization.21,22 An antibody concentration of at least 0.5 |gmg/mL was considered protective as suggested by efficacy data from a large trial of 7vPCV vaccination in healthy children.20 Local and systemic adverse events were prospectively collected by a standardized patient's/guardian's diary and physician's assessment for the duration of 1 month following each administration of study vaccine.
Characteristics of the study cohort
| Characteristic . | Values . |
|---|---|
| Patients, no. | 53 |
| Median patient age, y (range) | 8.3 (1.4-16.9) |
| 1-5 y, no. (%) | 18 (34) |
| 6-16 y, no. (%) | 35 (66) |
| Male, no. (%) | 29 (55) |
| Diagnosis, no. (%) | |
| ALL | 20 (38) |
| AML | 8 (15) |
| MDS/JMML | 9 (17) |
| CML | 4 (8) |
| SAA | 4 (8) |
| Other* | 8 (15) |
| Conditioning regimen, no. (%) | |
| TBI-based | 24 (45) |
| Busulfan-based | 25 (47) |
| Cyclophosphamide only† | 4 (8) |
| Donor type, no. (%) | |
| Related | 24 (45) |
| Unrelated | 29 (55) |
| Stem cell source, no. (%) | |
| BM | 36 (68) |
| PBSC | 16 (30) |
| BM and PBSC | 1 (2) |
| T-cell depletion, no. (%) | |
| No | 17 (32) |
| In vivo (ATG) | 34 (64) |
| In vitro (CD34+ selection) | 2 (4) |
| GvHD prophylaxis, no. (%) | |
| CSA | 9 (17) |
| CSA + MTX | 42 (79) |
| CSA + MTX + MMF | 2 (4) |
| IVIG after transplantation, no. (%) | 46 (87) |
| Ongoing IST at vaccination‡, no. (%) | 10 (19) |
| Evaluable,§ no. (%) | |
| Efficacy | 53 (100) |
| Safety | 43 (81) |
| Characteristic . | Values . |
|---|---|
| Patients, no. | 53 |
| Median patient age, y (range) | 8.3 (1.4-16.9) |
| 1-5 y, no. (%) | 18 (34) |
| 6-16 y, no. (%) | 35 (66) |
| Male, no. (%) | 29 (55) |
| Diagnosis, no. (%) | |
| ALL | 20 (38) |
| AML | 8 (15) |
| MDS/JMML | 9 (17) |
| CML | 4 (8) |
| SAA | 4 (8) |
| Other* | 8 (15) |
| Conditioning regimen, no. (%) | |
| TBI-based | 24 (45) |
| Busulfan-based | 25 (47) |
| Cyclophosphamide only† | 4 (8) |
| Donor type, no. (%) | |
| Related | 24 (45) |
| Unrelated | 29 (55) |
| Stem cell source, no. (%) | |
| BM | 36 (68) |
| PBSC | 16 (30) |
| BM and PBSC | 1 (2) |
| T-cell depletion, no. (%) | |
| No | 17 (32) |
| In vivo (ATG) | 34 (64) |
| In vitro (CD34+ selection) | 2 (4) |
| GvHD prophylaxis, no. (%) | |
| CSA | 9 (17) |
| CSA + MTX | 42 (79) |
| CSA + MTX + MMF | 2 (4) |
| IVIG after transplantation, no. (%) | 46 (87) |
| Ongoing IST at vaccination‡, no. (%) | 10 (19) |
| Evaluable,§ no. (%) | |
| Efficacy | 53 (100) |
| Safety | 43 (81) |
ALL indicates acute lymphoblastic leukemia; AML, acute myeloblastic leukemia; MDS, myelodysplastic syndrome; JMML, juvenile myelomonocytic leukemia; CML, chronic myelogenous leukemia; SAA, severe aplastic anemia; TBI, total body irradiation; BM, bone marrow; PBSC, peripheral blood stem cells; ATG, anti–thymocyte globulin; GvHD, graft-versus-host disease; CSA, cyclosporin A; MTX, methotrexate; MMF, mycophenolate mofetil; IVIG, intravenous immunoglobulin; IST, immunosuppressive therapy.
Other diagnoses are beta-thalassemia (n = 2), Fanconi anemia (n = 1), unspecified myeloproliferative disease (n = 1), congenital amegakaryocytic thrombocytopenia (n = 1), familial hemophagocytic lymphohistiocytosis (n = 1), mucopolysaccharidosis type I (n = 1), and Diamond-Blackfan anemia (n = 1).
Patients with SAA undergoing BM transplantation from identical sibling donors received cyclophosphamide + ATG for conditioning.
Indications for IST at vaccination were chronic GvHD (n = 9) and mixed hematopoietic chimerism (n = 1). Patients received IST at all 3 vaccinations (n = 8) or at first and second vaccination only (n = 2).
All patients receiving at least one dose of study vaccine were evaluated for toxicity. For analysis of efficacy, patients with complete data on pneumococcal serotype-specific antibody concentrations were included. Reasons for incomplete serologic data were (1) dropping out during the primary vaccination series because of relapse (n = 2) or persistent thrombocytopenia (n = 1), (2) insufficient or delayed serum sampling (n = 6), and (3) administration of intravenous immunoglobulin for varicella zoster virus contact during primary vaccination series (n = 1).
The primary study end point was a serologic response to primary immunization defined as seroconversion to protective antibody levels or, in patients exhibiting protective antibody levels prior to first vaccination, a greater than 2-fold increase from baseline antibody concentration. Accordingly, the primary study aim used for sample size calculation was defined as detection of serologic response to at least 3 pneumococcal serotypes in greater than 60% of study participants. Secondary end points included serologic responses to booster immunization scheduled 1 year after baseline immunization as well as safety of the vaccine with a particular focus on tolerability in the older age group (6-16 years) for which 7vPCV is currently not licensed. Geometric mean antibody concentrations (GMCs) were calculated after log-transformation and compared by 2-tailed t test. Frequency of adverse events and proportions of patients exhibiting protective antibody concentration to all 7 vaccine serotypes were compared using Fisher exact test. The projected total enrollment was 100 patients with a prespecified interim analysis scheduled after complete primary vaccination of 50 patients. For the interim analysis, 53 patients were recruited because 3 patients dropped out during the primary vaccination series due to relapse (n = 2) or thrombocytopenia (n = 1). Of the 50 patients who completed the primary vaccination series, 7 patients were not evaluable for serologic analysis (Table 1). Because in the interim analysis the primary study aim was prematurely achieved at a high significance level (P < .001), further recruitment to the study was concluded in agreement with the ethics committee and the safety data and monitoring board.
Results and discussion
The age of study participants ranged from 1.4 to 16.9 years (median, 8.3 years), thus including a considerable number of older children and adolescents. According to general trends in pediatric alloHSCT, the majority of patients (55%) received transplants from unrelated donors, and in 68% of transplant procedures T cells were depleted either in vivo (64%) or in vitro (4%).
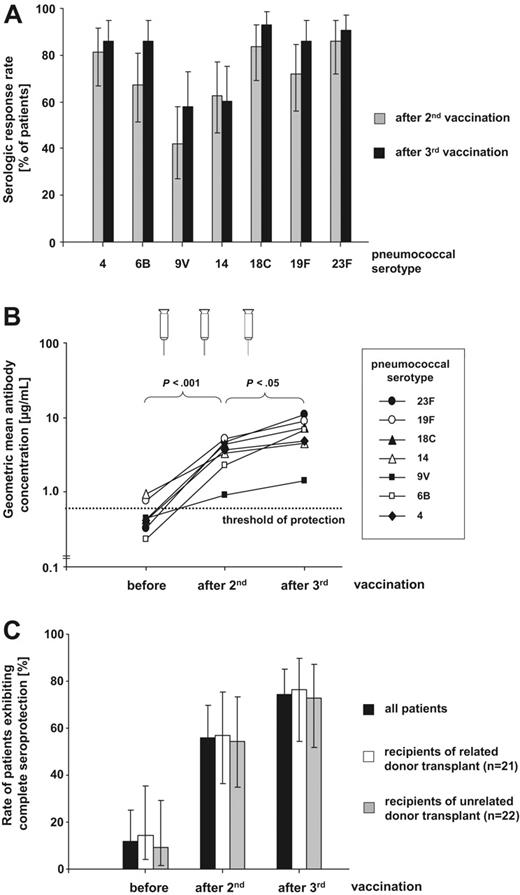
Serologic response rates to the different pneumococcal serotypes ranged from 41.9% to 86.0% after the second vaccine at a median time of 10 months (range, 8-12 months) following transplantation. The lowest response rate was observed against serotype 9V (Figure 1A). Following the third vaccine, 58.1% to 93.0% of patients responded to the individual vaccine serotypes. This is paralleled by a significant rise in geometric mean antibody concentration for each serotype (3.2- to 34.1-fold increase from baseline after third vaccination) with a major increase observed after 2 vaccinations (P < .001) and a further substantial increase after the third (P < .05; Figure 1B). Thus, following the third vaccination, mean pneumococcal antibody concentrations for all serotypes were at least as high as those observed in a large 7vPCV vaccination trial in healthy infants where efficient protection from invasive pneumococcal disease has been convincingly demonstrated.19 Of note, even the weakest serologic response against serotype 9V provided a mean antibody concentration well above the presumed protective threshold. As the clinically most critical parameter, we determined the proportion of patients exhibiting protective antibody levels against the individual vaccine serotypes: 69.8% to 100% and 81.4% to 100% of patients showed antibody concentrations above the protective threshold of ≥ 0.5 μmg/mL following 2 and 3 vaccinations, respectively, demonstrating a similar response as 7vPCV vaccination in healthy children.20 Complete seroprotection against all 7 vaccine serotypes was achieved in 55.8% (95% CI, 41.1%-69.6%) of patients after the second and 74.4% (95% CI, 59.6%-85.2%) of patients after the third vaccine independent of donor type (Figure 1C), recipient age (1-5 years versus 6-16 years; P > .25), and time from transplantation to first vaccination (< versus ≥ median; P > .5). This rate of protective titers was achieved despite that prior to vaccination, only 11.6% (95% CI, 4.6%-24.9%) of patients had already titers associated with protection and this rate again was irrespective of donor type (Figure 1C). In children receiving immunosuppressive therapy (IST) at vaccination 3 (42.9%) of 7 evaluable patients achieved protective antibody levels to all 7 vaccine serotypes compared with 29 (80.6%) of 36 patients without immunosuppresion (P = .06). However, even in the small subgroup of children receiving IST, 5 (71.4%) of 7 patients attained protective antibody concentrations to at least 6 of 7 pneumococcal serotypes.
Efficacy of 7vPCV vaccination in pediatric alloHSC transplant recipients. (A) Serologic response rates given in percentage of patients (n = 43) either showing seroconversion or achieving a greater than 2-fold increase of prevaccination antibody level against the 7 vaccine serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F) following 2 and 3 7vPCV vaccinations. Error bars indicate 95% confidence intervals (95% CI). (B) Geometric mean antibody concentrations (GMCs) for the 7 vaccine serotypes before, after 2, and after 3 7vPCV vaccinations. Increases of GMCs following 2 vaccinations (P <.001) and further rises of GMCs following the third vaccination (P <.05) are statistically significant for all serotypes as determined by 2-tailed t test. The presumed threshold of seroprotection (≥ 0.5 μmg/mL) is indicated by the horizontal line. (C) Rates of patients exhibiting protective antibody concentrations (≥ 0.5 μmg/mL) to all 7 vaccine serotypes before, after 2, and after 3 7vPCV vaccinations for the entire study cohort as well as recipients of related (n = 21) and unrelated (n = 22) hematopoietic stem cell transplants. Differences between the related and unrelated donor group were not statistically significant (Fisher exact test).
Efficacy of 7vPCV vaccination in pediatric alloHSC transplant recipients. (A) Serologic response rates given in percentage of patients (n = 43) either showing seroconversion or achieving a greater than 2-fold increase of prevaccination antibody level against the 7 vaccine serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F) following 2 and 3 7vPCV vaccinations. Error bars indicate 95% confidence intervals (95% CI). (B) Geometric mean antibody concentrations (GMCs) for the 7 vaccine serotypes before, after 2, and after 3 7vPCV vaccinations. Increases of GMCs following 2 vaccinations (P <.001) and further rises of GMCs following the third vaccination (P <.05) are statistically significant for all serotypes as determined by 2-tailed t test. The presumed threshold of seroprotection (≥ 0.5 μmg/mL) is indicated by the horizontal line. (C) Rates of patients exhibiting protective antibody concentrations (≥ 0.5 μmg/mL) to all 7 vaccine serotypes before, after 2, and after 3 7vPCV vaccinations for the entire study cohort as well as recipients of related (n = 21) and unrelated (n = 22) hematopoietic stem cell transplants. Differences between the related and unrelated donor group were not statistically significant (Fisher exact test).
Our results compare well with data from a prospective study evaluating the use of 7vPCV in predominantly adult patients after receiving alloHSCT from related donors: in the 25 patients completing the entire series of 3 7vPCV vaccinations at 3, 6, and 12 months after transplantation without prior donor vaccination, a significant rise in antibody concentration was observed only after the third vaccine with 64% of patients protected to all 7 vaccine serotypes.23 Our report extends these observations to an entirely pediatric cohort with the typical spectrum of transplantation indications for this age group. It demonstrates that an early intensive vaccination schedule with 3 vaccinations administered in monthly intervals provides protective antibody responses not only to recipients of related but also unrelated donor transplants, in whom immune reconstitution is often delayed because of in vitro or in vivo T-cell depletion.2 With an intensified vaccination regimen analogous to newborn vaccination schemes, protective antibody levels were achieved after the second vaccine as early as 10 months (median; range, 8-12 months) after transplantation; protection was further improved by the third vaccine resulting in seroprotection in 74% of patients at 11 months (range, 9-13 months). In the Molrine study of 7vPCV vaccination following matched-related donor transplantation, this level of protection was achieved as early as 6 months after transplantation when, in addition to patients, sibling donors were immunized before stem cell harvest.23 As there is a continuous increase in unrelated donor transplantation, our vaccination schedule offers a strategy to achieve early protection in the majority of pediatric patients without the need for donor vaccination.
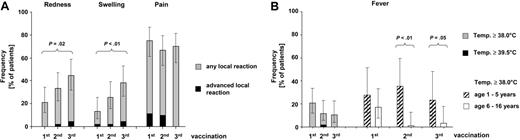
Although a total of 4 serious adverse events were observed, none of them was related to the study vaccine. The frequency of injection site reaction was 13.4% to 44.7% for redness and swelling and 66.7% to 75.0% for pain (Figure 2A). Advanced local reactions as well as pain interfering with limb movement were exceedingly rare events. The same is true for fever as a generalized adverse event with 10.5% to 20.8% of patients showing febrile reactions (Figure 2B). Only one single case of high-grade fever (≥ 39.5°) was observed despite that 7vPCV was administered in conjunction with the hexavalent combination vaccine Infanrix hexa. Of note, febrile reactions following the second and third vaccines were mostly limited to the younger age group (< 6 years) for which 7vPCV is currently licensed.
Safety of 7vPCV vaccination in pediatric alloHSC transplant recipients. (A) Frequency of local reactions and advanced local reactions (≥ 5 cm in diameter, pain interfering with limb movement) following 7vPCV vaccination. Error bars indicate 95% CIs. Frequencies were significantly different for redness and swelling between the first and third vaccination (P ≤ .05) as determined by Fisher exact test. No significant differences were found between age groups except for swelling after the first vaccination, which was more frequent in the younger (1-5 years) age group (27.8% versus 5.9%; P = .04) (B) Frequency of fever as the most common systemic adverse event. The left panel shows the overall rate of fever (body temperature ≥ 38.0°) and high-grade fever (body temperature ≥ 39.5°) following the first, second, and third 7vPCV vaccine for the entire group. The right panel compares fever rates between the younger (1-5 years; n = 18) and older (6-16 years; n = 35) age group with significant differences following the second and third vaccination (P <.05) as determined by Fisher exact test. No other significant differences were found. Additional systemic adverse events considered potentially related to study medication by the investigators include gastroenteritis (n = 2), unspecific exanthema (n = 1), herpes labialis (n = 1), and persistent thrombocytopenia leading to drop out from the study (n = 1).
Safety of 7vPCV vaccination in pediatric alloHSC transplant recipients. (A) Frequency of local reactions and advanced local reactions (≥ 5 cm in diameter, pain interfering with limb movement) following 7vPCV vaccination. Error bars indicate 95% CIs. Frequencies were significantly different for redness and swelling between the first and third vaccination (P ≤ .05) as determined by Fisher exact test. No significant differences were found between age groups except for swelling after the first vaccination, which was more frequent in the younger (1-5 years) age group (27.8% versus 5.9%; P = .04) (B) Frequency of fever as the most common systemic adverse event. The left panel shows the overall rate of fever (body temperature ≥ 38.0°) and high-grade fever (body temperature ≥ 39.5°) following the first, second, and third 7vPCV vaccine for the entire group. The right panel compares fever rates between the younger (1-5 years; n = 18) and older (6-16 years; n = 35) age group with significant differences following the second and third vaccination (P <.05) as determined by Fisher exact test. No other significant differences were found. Additional systemic adverse events considered potentially related to study medication by the investigators include gastroenteritis (n = 2), unspecific exanthema (n = 1), herpes labialis (n = 1), and persistent thrombocytopenia leading to drop out from the study (n = 1).
In summary, our data provide the first evidence that in pediatric alloHSC transplant recipients, vaccination with 7vPCV elicits early protective antibody responses within the first year following transplantation from both related and unrelated donors. Furthermore, 7vPCV was well tolerated in all pediatric age groups. Thus, early vaccination with 7vPCV should be introduced into routine reimmunization programs for pediatric alloHSC transplant recipients. The long-term course of antibody levels and functionality as well as the response to booster immunization with 7vPCV or the 23-valent polysaccharide vaccine are the subject of further study. Because the introduction of 7vPCV vaccination into routine reimmunization programs may lead to a shift in pneumococcal serotype distribution, continuous and comprehensive surveillance of pneumococcal serotype distribution in alloHSC transplant recipients developing invasive pneumococcal disease is warranted.24,25
Authorship
Contribution: R.M., U.D., H.S., S.Z., C.O., H.-J.L., and D.D. designed the research; R.M., R.S., B.G., G.S., K.B., A.H.G., U.D., R.B.-S., W.H., T.F., H.-P.G., S.Z., and D.D. performed the research; R.M., L.K., U.D., R.S., B.G., G.S., K.B., A.H.G., U.D., R.B.-S., W.H., T.F., H.-P.G., S.Z., and D.D. collected the data; R.M., L.K., C.O., H.-J.L., and D.D. analyzed the data; and R.M. and D.D. wrote the paper.
Conflict-of-interest disclosure: S.Z. serves as a member of the scientific advisory board and gives lectures for Wyeth Pharma GmbH, Münster, Germany. The remaining authors declare no competing financial interests.
A complete list of the members of the Impfung von Kindern nach allogener Stammzelltransplantation (IKAST) Study Group appears as a data supplement to the online version of this article click the Supplemental Document link at the top of the online article.
Correspondence: Roland Meisel, Universitäatsklinikum Düsseldorf, Zentrum für Kinder- und Jugendmedizin, Klinik für Kinder-Onkologie, -Häamatologie und -Immunologie, Moorenstrasse 5, D-40225 Düusseldorf, Germany; e-mail: meisel@med.uni-duesseldorf.de.
Presented orally at the 47th annual meeting of the American Society of Hematology, Atlanta, GA, December 10-13, 2005 (abstract 84).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ursula Creutzig (Hannover, Germany), Rüdiger von Kries (Institute for Social Pediatrics and Adolescence Medicine, Ludwig-Maximilians-Universität, München, Germany), and Heinz-Josef Schmitt (University Children's Hospital, Mainz, Germany) for their contribution as members of the safety and data monitoring board and Ulrich Göobel (Clinic for Pediatric Oncology, Hematology and Immunology, University Clinic Düsseldorf, Germany) for critical review of the manuscript.
This work was supported in part by research funding from Wyeth Pharma, Münster, Germany (R.M., S.Z., C.O., and D.D.), GlaxoSmithKline, Munich, Germany (R.M., C.O., and D.D.), and the Elterninitiative Kinderkrebsklinik Düsseldorf e.V.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal