Abstract
Polymorphonuclear leukocyte (PMN)–platelet interactions at sites of vascular damage contribute to local and systemic inflammation. We sought to determine the role of “outside-in” signaling by Src-family tyrosine kinases (SFKs) in the regulation of αMβ2-integrin–dependent PMN recruitment by activated platelets under (patho)physiologic conditions. Activation-dependent epitopes in β2 integrin were exposed at the contact sites between PMNs and platelets and were abolished by SFK inhibitors. PMNs from αMβ2−/−, hck−/−fgr−/−, and hck−/−fgr−/−lyn−/− mice had an impaired capacity to adhere with activated platelets in suspension. Phosphorylation of Pyk2 accompanied PMN adhesion to platelets and was blocked by inhibition as well as by genetic deletion of αMβ2 integrin and SFKs. A Pyk2 inhibitor reduced platelet-PMN adhesion, indicating that Pyk2 may be a downstream effector of SFKs. Analysis of PMN-platelet interactions under flow revealed that SFK signaling was required for αMβ2-mediated shear-resistant adhesion of PMNs to adherent platelets, but was dispensable for P-selectin–PSGL-1–mediated recruitment and rolling. Finally, SFK activity was required to support PMN accumulation along adherent platelets at the site of vascular injury, in vivo. These results definitely establish a role for SFKs in PMN recruitment by activated platelets and suggest novel targets to disrupt the pathophysiologic consequences of platelet-leukocyte interactions in vascular disease.
Introduction
Damage to arteries, such as occurs following percutaneous coronary interventions (PCIs) or atherosclerotic plaque erosion/rupture, results in platelet adhesion, activation, aggregation, and thrombus formation. Activated platelets recruit leukocytes that become enmeshed in platelet thrombi and/or form monolayers on top of adherent or aggregated platelets. Both platelet-monocyte and platelet–polymorphonuclear leukocyte (PMN) interactions may be important contributors to atherothrombotic vascular disease progression.1
Recent work points to a role for platelets and platelet-leukocyte interactions in progression of atherosclerosis. For example, in hypercholesterolemic animals, platelets adhere to endothelium at sites prone to plaque formation,2–4 and platelet accumulation on the luminal surface of atherosclerotic arteries has been associated with leukocyte recruitment. Injection of activated platelets into atherosclerotic-prone apo E−/− mice accelerates the formation of atherosclerosis.4 P-selectin–deficient platelets lack this effect, suggesting that promotion of atherosclerosis by activated platelets may require recruitment of leukocytes. Moreover, platelet P-selectin appears necessary for development of neointimal hyperplasia after experimental endovascular injury.5 Once recruited to sites of vascular injury, monocytes and neutrophils may release factors, such as matrix-degrading enzymes, oxygen-free radicals, and chemokines that stimulate vascular smooth muscle cell proliferation and migration, promote neointimal formation, and increase the propensity for plaque rupture. The recruitment of leukocytes by platelets and leukocyte infiltration into the vessel wall may be a crucial determinant of a local and systemic inflammatory response. Thus, leukocyte-platelet interactions may contribute to both the initiation and progression of atherosclerosis as well as clinical restenosis after PCI.
Early work from several groups established that αMβ2 integrin (CD11b/CD18, Mac-1) is required for leukocyte recruitment by activated platelets and transplatelet migration of leukocytes.6–9 Studies from our group10,11 and others12 demonstrated that platelet P-selectin signals through its leukocyte counterreceptor PSGL-1 to trigger activation of αMβ2 integrin. The molecular mechanisms that control the functional cross talk between the receptor pairs mediating the initial platelet-leukocyte attachment (P-selectin and PSGL-1) and those responsible for firm adhesion (platelet glycoprotein Ib and/or platelet-bound fibrinogen and αMβ2 integrin)13,14 are largely unknown.
Recent experiments, based on pharmacologic inhibition of members of the Src-family tyrosine kinases (SFKs), suggested that these signaling molecules may regulate αMβ2-dependent adhesion of neutrophils in suspension to activated platelets in human and mouse systems.11,15 The present study was undertaken to test this hypothesis that SFKs regulate an “outside-in”/“inside-out” signaling pathway in which αMβ2 positively modulates its own function to stabilize ligand binding under (patho)physiologically relevant conditions.
Materials and methods
Chemicals
Hydroethidine (HE) was purchased from Molecular Probes Europe (Leiden, the Netherlands); 2′,7′-bis-(2-carboxyethyl)-5(6)-carboxy-fluorescein triacetoxy methyl ester (BCECF-AM) was from Alexis (San Diego, CA). Prostaglandin E1 (PGE1), N-2 hydroxyethyl piperazine-N 1-2-ethanesulfonic acid (HEPES), ethylene glycol-bis (β-aminoethyl ether)-N, N, N′, N′,-tetraacetic acid (EGTA), and thrombin from human plasma (2000 U/mg protein; National Institute of Health [NIH, Rockville, MD]) were purchased from Sigma Chemical (St Louis, MO). Thrombin was dissolved in saline at 50 U/mL and stored at −20°C until use. BCECF-AM and HE were dissolved in DMSO at concentrations of 1 mg/mL and 8 mg/mL, respectively, stored at −20°C, and used within 4 weeks. The kinase inhibitor 4-amino-5-(-methylphenyl)-7-(tbutyl) pyrazolo [3,4-d] pyrimidine (PP1) was purchased from Alexis; 4-amino-5-(clorophenyl)-7-(t-butyl) pyrazolo [4-d] pyrimidine (PP2), the inactive analog 4-amino-7-phenilpyrazol [3,4-d] pyrimidine (PP3), SU6656, and tyrphostin A9 (AG17) were from Calbiochem (La Jolla, CA).
Antibodies
The following monoclonal antibodies (mAbs) were kindly provided: the anti–β2 KIM127 by Dr M. K. Robinson (Exploratory Research Cell Tech Therapeutics Limited, Bath Road Slough, England); 327C by D. Staunton (ICOS Corporation, Bothell, Washington); the anti–β3-integrin mAb 7E3 by Dr B. Coller (The Rockefeller University, New York, NY); and the anti–PSGL-1 and PSG3-G1 by Wyeth Research (Cambridge, MA). The anti–P-selectin WAPS 12.2, anti–E-selectin H18/7, the anti–αL-integrin TS1/22, and the anti–β2-integrin IB4 were purified from mouse ascites or hybridoma cell supernantant using protein G–Sepharose affinity chromatography. The anti–human α4β1 (2B4) was purchased from RD Systems (Minneapolis, MN). The anti–mouse β2-integrin mAb Game-46 and the anti–mouse P-selectin mAb RB40 were purchased from Pharmingen (San Diego, CA). The anti–β2 mAb M18/2 was from RDI (Concord, MA). Rat IgG control antibody was from Jackson Immunoresearch Laboratories (West Grove, PA). F(ab′)2 fragments were prepared using an ImmunoPure Preparation Kit (Pierce Biotechnology, Rockford, IL).
Preparation of human PMNs and platelets
Approval for blood donations was obtained from the institutional review board at both Consorzio Mario Negri Sud and the University of North Carolina. Platelets and PMNs were isolated and labeled as previously described.7
Mice
The generation of double (hck−/−fgr−/−) or triple (hck−/−fgr−/−lyn−/−) knock-out mice has been previously described.16 αM-integrin–deficient and P-selectin–deficient mice were obtained from Jackson Laboratories. Animals were used according to procedures approved by the Institutional Animal Care and Use Committees at both Consorzio Mario Negri Sud and The University of North Carolina.
Preparation of mouse platelets and PMNs
Whole blood from 5 animals was collected and platelets were prepared as previously described.15 Mouse bone marrow PMNs were isolated from femurs of 5 to 10 wild-type, double SFK knock-out (hck−/−fgr−/−), triple SFK knock-out (hck−/−fgr−/−lyn−/−), P-selectin−/−, or αM−/− mice weighing 18 to 25 g. Briefly, bone marrow cells were flushed from femurs using Ca2+ and Mg2+–free Hanks balanced salt solution (HBSS) containing 0.1% bovine serum albumin (BSA). Cells were aspirated through an 18-gauge needle to disrupt clumps. After removal of contaminating red blood cells by lysis in 0.15 M NH4Cl, 1 mM KHCO3, and 0.1 mM Na2EDTA, the remaining bone marrow cells were washed twice and resuspended in Ca/Mg-free HBSS with 0.1% BSA. PMNs were then purified by centrifugation on Ficoll-Hypaque gradient as described for human cells. Final cellular suspensions contained 80% PMNs as determined by light microscopy.
Platelet-leukocyte mixed cell suspension assay
PMNs alone or mixed with thrombin (0.25 U/mL)–activated, paraformaldehyde (PFA)–fixed platelets (ratio 1:10) were stirred at 1000 rpm at 37°C in siliconized glass cuvettes placed in an aggregometer as previously described.10,11,15 The reaction was stopped at different times by addition of one volume 2% PFA. A negative control was included in each experiment and consisted of the same sample prepared in the presence of 5 mM EGTA to prevent divalent cation–dependent platelet-leukocyte interactions. In particular experiments using mouse cells, 5 mM EGTA was added to the mixed cell suspension 30 seconds before PFA in order to reverse calcium-dependent P-selectin–mediated platelet-PMN interactions.15 In antibody inhibition studies to assess the contribution of individual receptors, antibodies were preincubated at a near-saturating concentration (10 μg/mL) with the appropriate cell fraction for 15 minutes at 4°C. Protein kinase inhibitors or the diluent (DMSO) was preincubated with PMNs for 2 minutes at room temperature.
Confocal microscopy
PMNs were stirred alone or with thrombin-activated, fixed platelets for 2 minutes, incubated for an additional 5 minutes at 37°C with KIM127 or 327C (10 μg/mL) without stirring, and then fixed overnight with 2% PFA. Samples were incubated for 30 minutes at 4°C with Alexa-Fluor 488–conjugated goat antimouse antibody and permeabilized with PBS containing 0.05% saponin and 0.5% BSA for 30 minutes at 4°C. Filamentous actin (F-actin) was stained with 1 μg/mL rhodamine-phalloidin in PBS containing 0.01% saponin and 0.5% BSA for 30 minutes at 4°C. Samples were resuspended in Mowiol, plated on a glass coverslip, and observed with a Zeiss LSM 510 Laser Scanning Microscope equipped with an Axiovert 100 M-BP (Carl Zeiss, Jena, Germany) using a 60×/1.2 NA oil objective (Figure 1C, 1D) or a 40×/0.75 NA objective (Figure 1B, 1E, 1F) objective. Optical Z-sections from each sample were taken with 0.3 μm Z-step from the top to the bottom of the cells. Immunofluorescence images were acquired at high confocality (pinhole = 1 Airy unit) to achieve the thinnest optical slices.
Two-color cytofluorimetric assay of PMN adhesion to platelets in suspension
Measurement of activated β2 integrins by flow cytometry
PMNs alone or with PFA-fixed or unfixed thrombin (0.25 U/mL)–activated platelets (1:10 ratio) were stirred (1000 rpm) for 0 to 10 minutes at 37°C, at which time KIM127 (10 μg/mL) was added for an additional 5 minutes at 37°C. Then cells were fixed in 2% PFA for 30 minutes, washed, and stained with Alexa-Fluor 488–conjugated goat antimouse antibody (Molecular Probes, Invitrogen-srl, Milano, Italy) at 4°C for 30 minutes. Nonspecific fluorescence was determined by incubation of PMNs with Alexa-Fluor 488–conjugated goat antimouse antibody only. For each experiment, a sample of PMNs was incubated with KIM127 at 0°C as negative sample, conditions that resulted in 1.7% ± 0.3% of PMNs binding KIM127.
Pyk2 tyrosine phosphorylation
PMNs were pretreated with 5 mM di-isopropyl fluorophosphates (DFPs) to inhibit intracellular proteases. Samples of DFP-pretreated PMNs (5 × 106) were stirred at 1000 rpm with thrombin-activated, fixed platelets (ratio 5:1) for 2 minutes at 37°C and then lysed in reducing Laemmli buffer containing protease and phosphatase inhibitors. The samples were boiled for 10 minutes, and diluted 1:20 with 1% Triton X-100 lysis buffer (1% Triton X-100, 150 mM NaCl, and 25 mM Tris-Hcl, pH 7.4) containing protease and phosphatase inhibitors. After preclearing with protein G–Sepharose, the samples were incubated at 4°C overnight with 10 μg/mL anti-Pyk2 antibody and protein G–Sepharose. The immunoprecipates were immunoblotted with either 0.1 μg/mL anti-Pyk2 or PY99 antibody.
Parallel plate flow adhesion assay
PMN adhesion to adherent platelets under physiologic flow was investigated in a parallel plate flow chamber. Platelets (3 × 107/mL) were allowed to adhere to a glass slide previously coated with APES.8 The platelet-coated slides were washed and served as the chamber's bottom plate. The chamber (width, 1.4 cm; height, 0.250 mm) was placed in a thermoregulated Plexiglas box maintained at 37°C by an electric heating element. PMNs (suspended at 5 × 106/mL in M199 medium containing 0.1% BSA) were perfused through the chamber over the platelet surface at a shear stress of 2 dynes/cm2 for 2 minutes. Then, the chamber was perfused with fresh media for an additional 2 minutes at 5 dynes/cm2, without interruption in the perfusion. The interaction of PMNs with adherent platelets was observed by contrast phase video microscopy with a 20×/0.40 NA objective (Olympus, Munich, Germany). Images were continuously recorded (Pro-Series video camera, High Performance CCD camera; Media Cybernetics, Silver Spring, MD). The fractions of rolling and firmly adhered PMNs were quantified in the last 20 seconds of flow using ad hoc software for image analysis (Image Pro-Plus for Windows; Media Cybernetics). In some experiments, human platelet P-selectin or the β3 integrins αIIbβ3 and αvβ3 were blocked by incubating the platelet-coated slide with mAb WAPS12.2 or 7E3, respectively (20 μg/mL), for 15 minutes. β2- or α4β1-integrin function was blocked by treating PMNs with mAbs IB4 or 2B4 (20 μg/mL), respectively, for 15 minutes on ice. Where indicated, PMNs were treated with chemical inhibitors, inactive analog, or DMSO for 5 minutes at room temperature.
Arterial injury
Where indicated, mice were pretreated with normal saline, PP1 (1.5 mg/kg), or SU6656 (0.015 mg) by intravenous injection 10 minutes prior to performing femoral artery injury. F(ab′)2 fragments (2 mg/kg) were administered by intraperitoneal injection 30 to 45 minutes before surgery. Endothelial denudation of femoral arteries was performed on mice anesthetized with inhaled isoflurane essentially as previously described.5 In brief, a 0.014-inch (0.36-mm) diameter angioplasty guide wire (Hi torque CROSS-IT 200XT guide wire; Guidant, Indianapolis, IN) was introduced into the femoral arterial lumen and advanced to the level of the aortic bifurcation and pulled back 3 times. Animals were killed one hour after the surgery and perfused with 60 mL 4% paraformaldehyde in PBS at 220 mL/h administered with a syringe pump via a cannula in the left ventricle. A 5-mm segment of the limb containing the femoral artery from the inguinal ligament to its division into the epigastric artery was excised en bloc, fixed, decalcified, and embedded in paraffin. Multiple serial sections were taken at 400-μm intervals and stained with hematoxylin/eosin or processed for immunohistochemistry as previously described.5 Images were acquired with a Nikon Eclipse microscope (Nikon, Melville, NY) with a 60×/1.49 NA objective. The numbers of attached white blood cells (WBCs) were manually determined in 5 serial sections taken through 2 mm injured vessel immediately proximal to the epigastric artery. Results are reported as mean ± SD. Measurements of vessel diameter were made from digitized images using Image J software (NIH).
Statistical analysis
In vitro data are presented as mean ± SEM, and were analyzed by repeated measurement ANOVA or by paired t test. P below .05 was considered statistically significant.
Results
Localization of activated β2 integrins at the adhesion sites in PMN-platelet aggregates
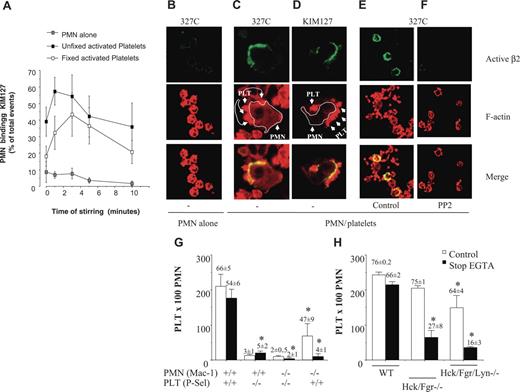
When stirred together in suspension, thrombin-activated, PFA-fixed human platelets and PMNs form mixed cell aggregates through a process that requires cross talk between platelet P-selectin and αMβ2 integrin.7 To examine the activation state of β2 integrins on PMNs interacting with activated platelets, flow cytometry and confocal microscopy were performed using 2 mAbs specific for activation-dependent epitopes on the human integrins: mAb KIM127 that recognizes epitopes in the cysteine-rich repeat region of the β2 chain that are shielded by the α subunit in the closed-inactive conformation and become exposed in the open-active conformation of the integrin17 and mAb 327C that recognizes an activation-dependent epitope in the β I–like domain.18
Incubation of PMNs with thrombin-activated platelets rapidly increased the binding of the β2-integrin activation reporter mAb KIM127. Maximum binding of KIM127 was observed at one minute in 57% ± 8.4% of PMNs stirred with thrombin-activated unfixed platelets and at 3 minutes in 43% ± 13% of PMNs stirred with thrombin-activated PFA-fixed platelets (Figure 1A). P-selectin promotes the initial interactions of activated platelets with PMNs. To determine whether P-selectin alone was capable of altering the activation state of β2 integrins, soluble P-selectin was purified by immunoaffinity chromatography from platelet lysates and incubated with PMNs. Soluble P-selectin (10 μg/mL) exposed activation-dependent epitopes in 40% ± 5% of stirred PMNs as detected by the binding of KIM127, which was maximal at 3 minutes.
SFKs are required for localization of activated β2 integrins at sites of PMN-platelet contacts and for αMβ2-dependent PMN-platelet interactions in suspension. (A) PMNs alone or with PFA-fixed or unfixed thrombin (0.25 U/mL)–activated platelets (1:10 ratio) were incubated for 0 to 10 minutes at 37°C in stirring conditions (1000 rpm). At each time point, stirring was stopped and KIM127 (10 μg/mL) was added to the cells for an additional 5 minutes at 37°C. The cells were then fixed in 2% PFA for 30 minutes, washed, and stained with Alexa-Fluor 488–conjugated goat antimouse antibody at 4°C for 30 minutes. Nonspecific fluorescence was determined by incubation of PMNs with Alexa-Fluor 488–conjugated goat antimouse antibody only. Values are reported as percentages of PMNs expressing KIM127-specific binding. (B) PMNs alone or with thrombin-activated, PFA-fixed platelets (C-D) were stirred at 37°C for 3 minutes, at which time 10 μg/mL mAb 327C (B-C, E-F) or KIM127 (D) was added. Confocal microscopy to detect KIM127 and 327C mAb binding and rhodamine-phalloidin was performed as described in “Materials and methods.” (C-D) The actin staining in these images was captured at a lower amplification than in panels B, E, or F in order to allow clear identification of platelets that contain higher concentration of F-actin than do PMNs. PMNs are identified by a white line around the membrane edge and show intense 327C (C) or KIM127 (D) binding in areas with attached platelets. (E-F) PMNs were pretreated with vehicle (E) or PP2 (F) for 5 minutes at RT before incubation with platelets. PP2 treatment dramatically reduces expression of activated β2 integrins at platelet contact sites. (G) PMNs from wild-type or αM−/− mice were stirred in the absence or in the presence of fixed-activated wild-type or P-selectin–deficient platelets. Samples were immediately fixed with an equal volume of 2% PFA (open bars) or, alternatively, 5 mM EGTA was added to mixed cell suspensions 30 seconds before fixation to disrupt P-selectin–mediated bonds (closed bars designated stop EGTA). The formation of mixed platelet–PMN conjugates was analyzed by flow cytometry, as described in “Materials and methods.” The numbers of platelets recruited by 100 PMNs are graphed and the numbers on the top of the bars are the percentage of PMNs with attached platelets. Average results (mean ± SEM) from 6 different experiments for wild-type cells and 4 experiments for αM−/− or P-selectin–deficient cells are presented. *P < .05 versus WT. (H) PMNs from wild-type or double hck−/−fgr−/− or triple hck−/−fgr−/−lyn−/− mice were stirred in the absence or in the presence of activated, fixed wild-type platelets and treated as described in panel F. The numbers of platelets recruited by 100 PMNs are graphed and the numbers on top of the bars are the percentage of PMNs with attached platelets (mean, n = 4). The figure shows average results (mean ± SEM) from 4 different experiments performed with PMNs isolated from pooled bone marrow obtained from 5 wild-type or 5 knock-out mice. *P < .05 versus WT.
SFKs are required for localization of activated β2 integrins at sites of PMN-platelet contacts and for αMβ2-dependent PMN-platelet interactions in suspension. (A) PMNs alone or with PFA-fixed or unfixed thrombin (0.25 U/mL)–activated platelets (1:10 ratio) were incubated for 0 to 10 minutes at 37°C in stirring conditions (1000 rpm). At each time point, stirring was stopped and KIM127 (10 μg/mL) was added to the cells for an additional 5 minutes at 37°C. The cells were then fixed in 2% PFA for 30 minutes, washed, and stained with Alexa-Fluor 488–conjugated goat antimouse antibody at 4°C for 30 minutes. Nonspecific fluorescence was determined by incubation of PMNs with Alexa-Fluor 488–conjugated goat antimouse antibody only. Values are reported as percentages of PMNs expressing KIM127-specific binding. (B) PMNs alone or with thrombin-activated, PFA-fixed platelets (C-D) were stirred at 37°C for 3 minutes, at which time 10 μg/mL mAb 327C (B-C, E-F) or KIM127 (D) was added. Confocal microscopy to detect KIM127 and 327C mAb binding and rhodamine-phalloidin was performed as described in “Materials and methods.” (C-D) The actin staining in these images was captured at a lower amplification than in panels B, E, or F in order to allow clear identification of platelets that contain higher concentration of F-actin than do PMNs. PMNs are identified by a white line around the membrane edge and show intense 327C (C) or KIM127 (D) binding in areas with attached platelets. (E-F) PMNs were pretreated with vehicle (E) or PP2 (F) for 5 minutes at RT before incubation with platelets. PP2 treatment dramatically reduces expression of activated β2 integrins at platelet contact sites. (G) PMNs from wild-type or αM−/− mice were stirred in the absence or in the presence of fixed-activated wild-type or P-selectin–deficient platelets. Samples were immediately fixed with an equal volume of 2% PFA (open bars) or, alternatively, 5 mM EGTA was added to mixed cell suspensions 30 seconds before fixation to disrupt P-selectin–mediated bonds (closed bars designated stop EGTA). The formation of mixed platelet–PMN conjugates was analyzed by flow cytometry, as described in “Materials and methods.” The numbers of platelets recruited by 100 PMNs are graphed and the numbers on the top of the bars are the percentage of PMNs with attached platelets. Average results (mean ± SEM) from 6 different experiments for wild-type cells and 4 experiments for αM−/− or P-selectin–deficient cells are presented. *P < .05 versus WT. (H) PMNs from wild-type or double hck−/−fgr−/− or triple hck−/−fgr−/−lyn−/− mice were stirred in the absence or in the presence of activated, fixed wild-type platelets and treated as described in panel F. The numbers of platelets recruited by 100 PMNs are graphed and the numbers on top of the bars are the percentage of PMNs with attached platelets (mean, n = 4). The figure shows average results (mean ± SEM) from 4 different experiments performed with PMNs isolated from pooled bone marrow obtained from 5 wild-type or 5 knock-out mice. *P < .05 versus WT.
In agreement with the flow cytometric results, confocal microscopic examination showed that the epitopes for both KIM127 and 327C were barely detectable in PMNs stirred in the absence of platelets (Figure 1B) but were up-regulated in clusters that appear to be specifically located at the contact sites between PMNs and thrombin-activated, PFA-fixed platelets. Soluble P-selectin also stimulated a similar pattern of KIM127 localization (data not shown). Of interest, treatment of human PMNs with the SFK inhibitor PP2, but not the control compound PP3, abolished the expression of activation-dependent epitopes recognized by 327C (Figure 1F) and by KIM127 (not shown), suggesting that SFK may play a role in sustaining the active β2-integrin conformation.
Genetic deficiency of SFK impairs Mac-1–dependent PMN-platelet adhesion in a mixed cell suspension assay
To demonstrate unequivocally that SFK activity is necessary for αMβ2-mediated PMN-platelet adhesion, we compared the ability of PMNs from wild-type mice or mice lacking the major myeloid SFK members (hck−/−fgr−/−lyn−/−) to form mixed cell aggregates with wild-type platelets. Although many adhesive features are similar, murine PMNs do differ from their human counterparts in several important ways. Like their human counterparts, murine PMN-platelet interactions require P-selectin, and the addition of EGTA to PMNs before stirring with platelets completely prevents the formation of mixed cell aggregates (under these conditions, only 3% ± 1% of PMNs attach to platelets, n = 6). However, unlike human PMN-platelet interactions, we have previously reported that P-selectin–mediated bonds can sustain murine PMN-platelet aggregates in suspension in the absence of β2 function.15 Thus, in order to detect β2-integrin–dependent interactions between murine PMNs and platelets, P-selectin bonds have to be reversed, which can be accomplished by adding 5 mM EGTA to already formed PMN-platelet aggregates.15 In agreement with our previously published findings,15 the addition of EGTA to already formed PMN-platelet aggregates had only a small effect on PMN-platelet interactions (66% ± 5% of PMNs had attached platelets without EGTA and 54% ± 6% had attached platelets after the addition of EGTA [“stop EGTA”] mean ± SEM, n = 6) (Figure 1G). In contrast, formation of mixed cell aggregates between αM-deficient PMNs and wild-type platelets was nearly completely reversed after the disruption of P-selectin–mediated bonds by EGTA (47% ± 9% of αM−/− PMNs had attached platelets without EGTA and 4% ± 1% had attached platelets after the addition of EGTA [stop EGTA] n = 4). Under the same conditions, less than 5% of PMNs formed mixed cell aggregates with P-selectin−/− platelets (Figure 1G).
As was observed with integrin αM−/− PMNs, the ability of hck−/−fgr−/− and hck−/−fgr−/−lyn−/− PMNs to form heterotypic conjugates with murine platelets was similar to that of wild-type PMNs (75% ± 1% of hck−/−fgr−/− PMNs had attached platelets, as did 64% ± 4% of hck−/−fgr−/−lyn−/− PMNs). However, after addition of EGTA to disrupt P-selectin–mediated bonds, mixed cell aggregates formed by hck−/−fgr−/− PMNs partially reversed: 27% ± 8% of PMNs retained attached platelets, and the number of platelets recruited by 100 PMNs was substantially lower than wild-type PMNs (64 ± 20 vs 243 ± 8 platelets/100 PMNs, respectively, n = 3-6) (Figure 1H). The addition of EGTA nearly completely disrupted mixed cell aggregates formed by hck−/−fgr−/−lyn−/− PMNs, with only 16% ± 3% retaining attached platelets (Figure 1H). These results establish that SFKs are required for αMβ2-mediated firm adhesion of PMNs to platelets in suspension.
Role of Pyk2 as a downstream effector of SFKs in PMN-platelet interactions
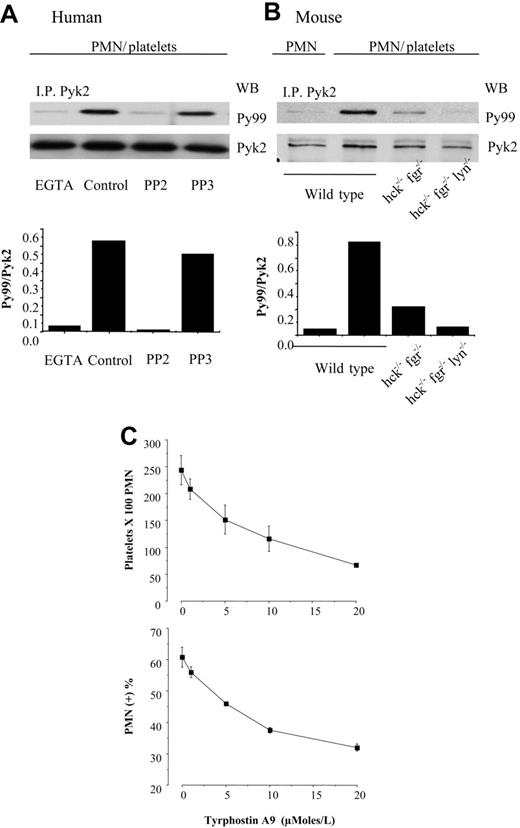
We and others have identified the tyrosine kinase Pyk2 as a possible downstream mediator of SFKs in the regulation of β2-integrin adhesion.11,19 Therefore, we analyzed Pyk2 phosphorylation in human PMNs in the mixed cell suspension assay. As shown in Figure 2A, Pyk2 became tyrosine phosphorylated in human PMNs stirred with thrombin-activated, fixed platelets. Platelet-induced Pyk2 phosphorylation in human PMNs was inhibited by approximately 70% by the anti–P-selectin mAb WAPS and by approximately 90% by the anti–β2-integrin mAb IB4, as judged by the ratio of phospho-Pyk2 to total Pyk2. In wild-type mouse PMNs, Pyk2 tyrosine phosphorylation was barely detectable in the absence of platelets (data not shown), but increased approximately 14-fold in wild-type PMNs after coincubation with activated platelets. Tyrosine phosphorylation of Pyk2 was reduced by approximately 70% in wild-type PMNs stirred with P-selectin–deficient platelets and by more than 90% in αM-deficient PMNs interacting with wild-type platelets (Figure 2B). Furthermore, Pyk2 tyrosine phosphorylation was prevented by pretreatment of human PMNs with the Src inhibitor PP2 but not by the inactive analog PP3 (Figure 3A). Likewise, hck−/−fgr−/− PMNs displayed approximately 70% of the levels of Pyk2 phosphorylation observed in wild-type PMNs after coincubation with activated platelets, and Pyk2 phosphorylation in hck−/−fgr−/−lyn−/− PMNs did not increase over baseline upon exposure to activated platelets (Figure 3B). These results suggest that Pyk2 is a potential mediator of αMβ2 and SFK signaling in both human and mouse PMNs.
PMN interaction with activated platelets stimulates αMβ2-dependent Pyk2 phosphorylation. (A) Human PMNs were preincubated with the function-blocking anti–β2-integrin mAb IB4 or with an irrelevant mouse monoclonal antibody for 15 minutes in ice. Thrombin-activated fixed platelets were preincubated with control or the anti–P-selectin mAb WAPS for 15 minutes at room temperature. PMNs and platelets were stirred at 1000 rpm at 37°C for 3 minutes. Pyk2 was immunoprecipitated from total cell lysates, and immune complexes were analyzed for phospho-Pyk2 by immunoblotting with PY99, and then the blots were reprobed with anti-Pyk2 antibody to detect total Pyk2. The bars report the ratio between optical density of PY99 andPYK2 (ie, phospho-Pyk2/total Pyk2). The figure is representative of results obtained in 3 different experiments. (B) PMNs from wild-type or αM−/− mice were stirred in the presence of fixed-activated wild-type or P-selectin−/− platelets at 37°C for 3 minutes. The interactions were stopped by the addition of an equal volume of reduced sample buffer, and samples were processed for immunoblot analysis as described for panel A. The bars indicate the ratio between optical density of PY99 and PYK2 (phospho-Pyk2/total Pyk2). The figure is representative of results obtained in 2 different experiments performed with PMNs isolated from pooled bone marrow obtained from 5 wild-type or 5 knock-out mice.
PMN interaction with activated platelets stimulates αMβ2-dependent Pyk2 phosphorylation. (A) Human PMNs were preincubated with the function-blocking anti–β2-integrin mAb IB4 or with an irrelevant mouse monoclonal antibody for 15 minutes in ice. Thrombin-activated fixed platelets were preincubated with control or the anti–P-selectin mAb WAPS for 15 minutes at room temperature. PMNs and platelets were stirred at 1000 rpm at 37°C for 3 minutes. Pyk2 was immunoprecipitated from total cell lysates, and immune complexes were analyzed for phospho-Pyk2 by immunoblotting with PY99, and then the blots were reprobed with anti-Pyk2 antibody to detect total Pyk2. The bars report the ratio between optical density of PY99 andPYK2 (ie, phospho-Pyk2/total Pyk2). The figure is representative of results obtained in 3 different experiments. (B) PMNs from wild-type or αM−/− mice were stirred in the presence of fixed-activated wild-type or P-selectin−/− platelets at 37°C for 3 minutes. The interactions were stopped by the addition of an equal volume of reduced sample buffer, and samples were processed for immunoblot analysis as described for panel A. The bars indicate the ratio between optical density of PY99 and PYK2 (phospho-Pyk2/total Pyk2). The figure is representative of results obtained in 2 different experiments performed with PMNs isolated from pooled bone marrow obtained from 5 wild-type or 5 knock-out mice.
Tyrosine phosphorylation of Pyk2 is mediated by SFKs and is required for shear-resistant PMN attachment to platelets. (A) Untreated PMNs or PMNs pretreated with DMSO, 10 μM PP2, or 10 μM PP3 were stirred for 3 minutes with activated, fixed platelets (ratio 5:1) in the presence or in the absence of 5 μM EGTA. Pyk2 was immunoprecipitated from total cell lysates, and immune complexes were analyzed for phospho-Pyk2 by immunoblotting with PY99 and then reprobed with anti-Pyk2 antibody to detect total Pyk2. The bars report the ratio between optical density of PY99 and PYK2 (ie, phospho-Pyk2/total Pyk2). The figure is representative of results obtained in 3 different experiments. (B) PMNs from wild-type, double hck−/−fgr−/−, or triple hck−/−fgr−/−lyn−/− mice were stirred in the absence or in the presence of activated, fixed wild-type platelets and processed as described in panel A. The bars indicate the ratio between optical density of PY99 and PYK2 (phospho-Pyk2/total Pyk2). The figure is representative of results obtained in 2 different experiments performed with PMNs isolated from pooled bone marrow obtained from 5 wild-type or 5 knock-out mice. (C) Human PMNs pretreated with DMSO or the indicated concentrations of the Pyk2 inhibitor tyrphostin A9 (AG17) were stirred for 3 minutes with activated, fixed platelets (ratio 5:1). Samples were immediately fixed with an equal volume of 2% PFA and the formation of mixed platelet-PMN conjugates was analyzed by flow cytometry, as described in “Materials and methods.” The number of platelets recruited by 100 PMNs and the percentage of PMNs with attached platelets are graphed (mean ± SEM, n = 3).
Tyrosine phosphorylation of Pyk2 is mediated by SFKs and is required for shear-resistant PMN attachment to platelets. (A) Untreated PMNs or PMNs pretreated with DMSO, 10 μM PP2, or 10 μM PP3 were stirred for 3 minutes with activated, fixed platelets (ratio 5:1) in the presence or in the absence of 5 μM EGTA. Pyk2 was immunoprecipitated from total cell lysates, and immune complexes were analyzed for phospho-Pyk2 by immunoblotting with PY99 and then reprobed with anti-Pyk2 antibody to detect total Pyk2. The bars report the ratio between optical density of PY99 and PYK2 (ie, phospho-Pyk2/total Pyk2). The figure is representative of results obtained in 3 different experiments. (B) PMNs from wild-type, double hck−/−fgr−/−, or triple hck−/−fgr−/−lyn−/− mice were stirred in the absence or in the presence of activated, fixed wild-type platelets and processed as described in panel A. The bars indicate the ratio between optical density of PY99 and PYK2 (phospho-Pyk2/total Pyk2). The figure is representative of results obtained in 2 different experiments performed with PMNs isolated from pooled bone marrow obtained from 5 wild-type or 5 knock-out mice. (C) Human PMNs pretreated with DMSO or the indicated concentrations of the Pyk2 inhibitor tyrphostin A9 (AG17) were stirred for 3 minutes with activated, fixed platelets (ratio 5:1). Samples were immediately fixed with an equal volume of 2% PFA and the formation of mixed platelet-PMN conjugates was analyzed by flow cytometry, as described in “Materials and methods.” The number of platelets recruited by 100 PMNs and the percentage of PMNs with attached platelets are graphed (mean ± SEM, n = 3).
In order to explore the functional role of Pyk2 as a downstream effector of SFK regulation of PMN-platelet interactions, we used tyrphostin A9 (AG17) to inhibit Pyk2 activity.20 Tyrphostin A9 dose-dependently inhibited PMN-platelet interactions in the mixed cell suspension assay (Figure 3C). The inhibition was not accompanied by a change of tyrosine phosphorylation of SFK tyrosine 416 (data not shown), indicating that Pyk2 is acting downstream of SFK.
Requirement for SFK activity in αMβ2-dependent firm adhesion of PMNs to adherent platelets under physiologic flow
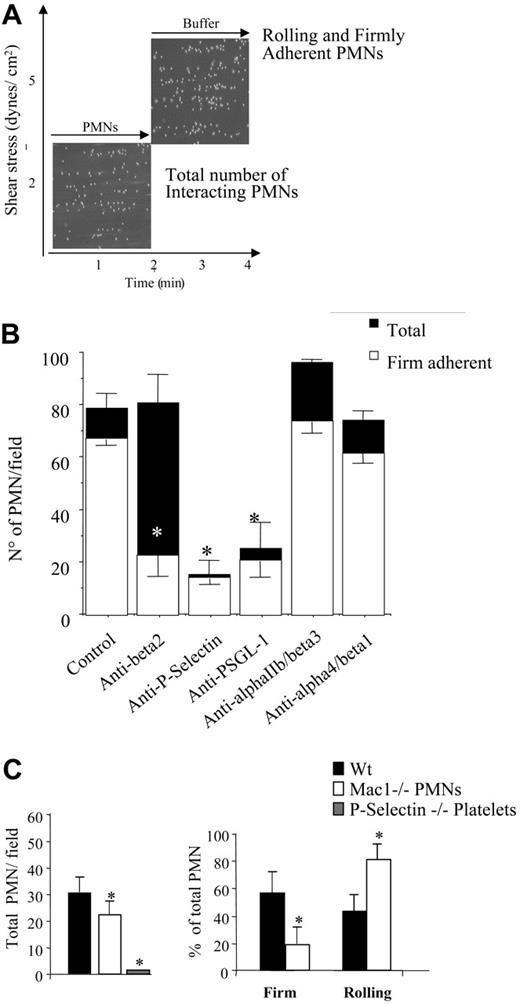
Our findings establish that SFK activity is required for firm αMβ2-mediated interactions of PMNs with platelets in a mixed cell suspension assay. In order to investigate the role of SFKs in PMN recruitment by adherent platelets under conditions of physiologic shear, we used a flow adhesion assay in which PMNs were perfused for 2 minutes across a platelet surface at a flow rate that produced 2 dynes/cm2 shear stress. To study the ability of the adherent neutrophils to resist shear—which is mediated in part by αMβ2 integrin—the chamber was perfused with fresh media for an additional 2 minutes at 5 dynes/cm2 without interruption in the perfusion (Figure 4A). The majority of recruited PMNs (78 ± 5.7 PMNs/field, mean ± SEM, n = 10) stopped immediately and firmly adhered to the platelet layer. Approximately 80% of the adherent PMNs resisted the higher shear stress and remained firmly attached. As expected, treatment of the platelet monolayer with the anti–P-selectin mAb WAPS reduced PMN recruitment by approximately 80% (15 ± 5.5 PMNs/field, n = 5). Treatment of PMNs with an anti–PSGL-1 mAb significantly reduced PMN recruitment (28 ± 10 PMN/field, n = 3). Treatment of PMNs with the anti–β2-integrin mAb IB4 did not modify the number of PMNs (80.8 ± 10.8 PMNs/field) interacting with the adherent platelets but did significantly reduce to 22.6% ± 6% the number of firmly adherent cells at high shear stress (Figure 4B). In contrast, neither blockade of αIIbβ3 and αvβ3 on platelets by mAb 7E3 nor α4β1 by mAb 2B4 in PMNs significantly affected the ability of PMNs to interact with adherent platelets under physiologic flow conditions (Figure 4B).
PMN recruitment by adherent platelets under flow requires P-selectin and αMβ2. (A) Human PMNs (5 × 106/mL) were perfused across a platelet surface at a flow rate of 2 dynes/cm2 for 2 minutes. Then, the chamber was perfused with fresh media for an additional 2 minutes at 5 dynes/cm2, without interruption in the perfusion. The interaction of PMNs with adherent platelets was observed by contrast phase video microscopy with a 20×/0.40 NA objective. The total number of cells interacting at 2 minutes and the fractions of rolling and firmly adhered PMNs in the last 20 seconds of flow (4 minutes) were measured using an ad hoc software for image analysis as described in “Materials and methods.” (B) P-selectin and αIIbβ3 and αvβ3 were blocked by incubating the human platelet–coated slide with the mAbs WAPS12.2 or 7E3, respectively (20 μg/mL) for 15 minutes. β2 or α4/1 integrins on human PMNs were blocked by treating cells with the mAbs IB4 or 2B4 (20 μg/mL) for 15 minutes in ice. Experiments were performed as described for panel A and in “Materials and methods.” The results are presented as means ± SEM for 3 to 8 different experiments performed using cells from different donors. Black bars indicate the total number of PMNs recruited per field, and white bars indicate the number of PMNs that establish firm adhesion. *P < .05 for treatment versus control. (C) PMNs were isolated from wild-type or αM−/− mice bone marrow. Platelets were isolated from wild-type or P-selectin–deficient (P-sel−/−) mice. Experiments were performed as described for panel A and in “Materials and methods.” The results presented are means ± SEM from 4 different experiments each performed using cells collected from 5 wild-type or knock-out mice. *P < .05 for wild-type versus αM−/− PMNs or P-selectin−/− platelets.
PMN recruitment by adherent platelets under flow requires P-selectin and αMβ2. (A) Human PMNs (5 × 106/mL) were perfused across a platelet surface at a flow rate of 2 dynes/cm2 for 2 minutes. Then, the chamber was perfused with fresh media for an additional 2 minutes at 5 dynes/cm2, without interruption in the perfusion. The interaction of PMNs with adherent platelets was observed by contrast phase video microscopy with a 20×/0.40 NA objective. The total number of cells interacting at 2 minutes and the fractions of rolling and firmly adhered PMNs in the last 20 seconds of flow (4 minutes) were measured using an ad hoc software for image analysis as described in “Materials and methods.” (B) P-selectin and αIIbβ3 and αvβ3 were blocked by incubating the human platelet–coated slide with the mAbs WAPS12.2 or 7E3, respectively (20 μg/mL) for 15 minutes. β2 or α4/1 integrins on human PMNs were blocked by treating cells with the mAbs IB4 or 2B4 (20 μg/mL) for 15 minutes in ice. Experiments were performed as described for panel A and in “Materials and methods.” The results are presented as means ± SEM for 3 to 8 different experiments performed using cells from different donors. Black bars indicate the total number of PMNs recruited per field, and white bars indicate the number of PMNs that establish firm adhesion. *P < .05 for treatment versus control. (C) PMNs were isolated from wild-type or αM−/− mice bone marrow. Platelets were isolated from wild-type or P-selectin–deficient (P-sel−/−) mice. Experiments were performed as described for panel A and in “Materials and methods.” The results presented are means ± SEM from 4 different experiments each performed using cells collected from 5 wild-type or knock-out mice. *P < .05 for wild-type versus αM−/− PMNs or P-selectin−/− platelets.
Similar to human cells, mouse bone marrow–derived PMNs were recruited by adherent platelets in a P-selectin–dependent manner. Essentially no wild-type PMNs adhered to platelets from P-selectin−/− mice (1.3 ± 0.5 PMNs/field; Figure 4C). Integrin αM−/− PMNs displayed a slight, but statistically significant, reduction in total cells recruited on wild-type platelets (22 ± 5.6 PMNs/field versus 32 ± 5.5 wild-type PMNs, mean ± SEM, n = 3, P < .05 by ANOVA). The percentage of firmly adherent αM−/− PMNs was significantly reduced compared with wild-type PMNs (19 ± 10 PMNs/field versus 57 ± 14, n = 4, P < .05; Figure 4C), suggesting that, like their human counterparts, mouse PMNs require αMβ2 to resist shear and firmly adhere to platelets.
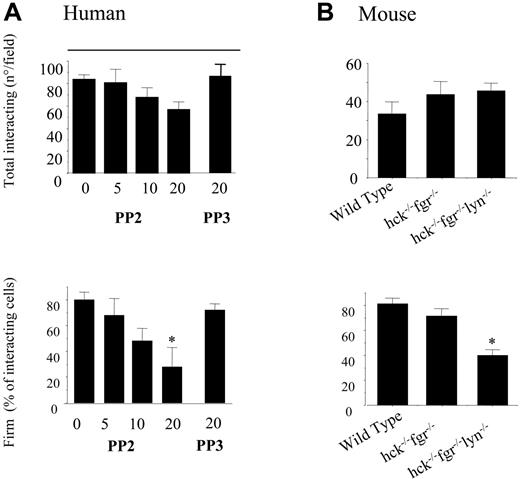
Analogous to blockade of αMβ2 integrin, the SFK inhibitor PP2 only slightly reduced the total number of PMNs (from 90 to 60 PMNs/field) recruited by the adherent platelets at 2 dynes/cm2, but dose-dependently reduced the fraction of firmly adherent PMNs at 5 dynes/cm2 (Figure 5A). The inactive control analog PP3 was without effect. Similarly to SFK blockade the Pyk2 inhibitor tyrphostin A9 dose-dependently reduced firm PMN adhesion to adherent platelets in flow (data not shown), supporting the functional role of Pyk2 as downstream effector of SFK.
SFKs are required for PMN firm adhesion to adherent platelets under conditions of physiologic flow. (A) Human PMNs, pretreated with different concentration of PP2, PP3, or vehicle (DMSO) for 10 minutes, were perfused across a platelet surface at a flow rate of 2 dynes/cm2 for 2 minutes. Then, the chamber was perfused with fresh media for an additional 2 minutes at 5 dynes/cm2, without interruption in the perfusion. The interaction of PMNs with adherent platelets was observed by contrast phase video microscopy with a 20×/0.40 NA objective. The total number of cells interacting at 2 minutes and the fractions of firmly adhered PMNs in the last 20 seconds of flow were measured using an ad hoc software for image analysis as described in “Materials and methods.” The results presented are means ± SEM from 5 different experiments performed using cells from different donors. *P < .05 for treatment versus control. (B) PMNs were isolated from bone marrow of wild-type, hck−/−fgr−/−, or hck−/−fgr−/−lyn−/− mice. Flow experiments were performed exactly as described for human cells. The results presented are mean ± SEM for 6 different experiments each performed in triplicate with cells pooled from 5 wild-type or knock-out mice. *P < .05 for wild-type versus hck−/−fgr−/−lyn−/− cells.
SFKs are required for PMN firm adhesion to adherent platelets under conditions of physiologic flow. (A) Human PMNs, pretreated with different concentration of PP2, PP3, or vehicle (DMSO) for 10 minutes, were perfused across a platelet surface at a flow rate of 2 dynes/cm2 for 2 minutes. Then, the chamber was perfused with fresh media for an additional 2 minutes at 5 dynes/cm2, without interruption in the perfusion. The interaction of PMNs with adherent platelets was observed by contrast phase video microscopy with a 20×/0.40 NA objective. The total number of cells interacting at 2 minutes and the fractions of firmly adhered PMNs in the last 20 seconds of flow were measured using an ad hoc software for image analysis as described in “Materials and methods.” The results presented are means ± SEM from 5 different experiments performed using cells from different donors. *P < .05 for treatment versus control. (B) PMNs were isolated from bone marrow of wild-type, hck−/−fgr−/−, or hck−/−fgr−/−lyn−/− mice. Flow experiments were performed exactly as described for human cells. The results presented are mean ± SEM for 6 different experiments each performed in triplicate with cells pooled from 5 wild-type or knock-out mice. *P < .05 for wild-type versus hck−/−fgr−/−lyn−/− cells.
To confirm that the effect of PP2 was specific for SFK activity, we investigated the ability of PMNs from hck−/−fgr−/− or hck−/−fgr−/−lyn−/− mice to adhere to wild-type platelets under flow. There was a slight but not statistically significant reduction of firm adhesion in PMNs isolated from hck−/−fgr−/− compared with wild-type PMNs (70% ± 7.8% versus 81% ± 4.6%) at 5 dynes/cm2, whereas, significantly fewer PMNs from triple knock-out hck−/−fgr−/−lyn−/− mice displayed firm adhesion at higher shear (40% ± 4.5%, P < .05 vs control by ANOVA) (Figure 5B). Thus, β2-integrin–dependent shear resistance and firm adhesion of PMNs tethered to platelets under physiologic flow require SFK activity.
SFK are required for PMN recruitment by activated platelets following arterial injury in mice
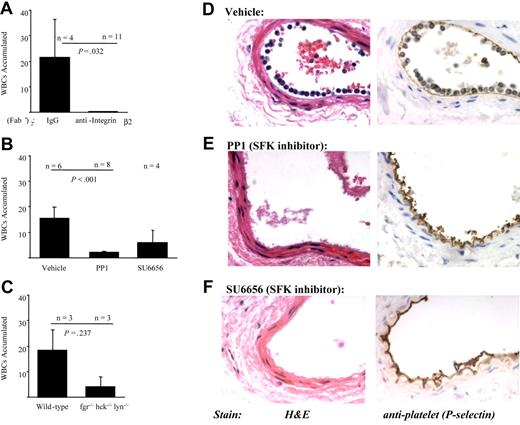
We have previously demonstrated that one hour after denudation injury of the femoral artery PMNs are recruited to the site of injury by adherent platelets in a P-selectin–dependent manner.5 To establish the role of β2 integrins in PMN accumulation in our model, wild-type mice were treated with an anti–β2-integrin mAb F(ab′)2 prior to injury. In comparison with mice treated with control F(ab′)2 fragments (n = 4), mice treated with anti–β2-integrin F(ab′)2 (n = 11) displayed significant reductions in average PMN accumulation (mean ± SD) at one hour after injury (21.5 ± 14 cells/field versus 0.25 ± 0.09; P = .032 by Student t test; Figure 6A).
β2 integrins and SFKs mediate PMN recruitment to adherent platelets at the sites of vascular injury in mice. Arterial injury was performed as described in “Materials and methods,” and the vessels were examined one hour after injury. (A) Wild-type mice were pretreated with control F(ab)′2 or anti–β2-integrin F(ab)′2 prior to performing injury. The results presented are the average number of WBCs that accumulated along the vessel (mean ± SEM) in 4 control and 11 anti-integrin–treated animals. (B) Wild-type mice (n = 6) were pretreated with vehicle, PP1 (1.5 mg/kg; n = 8), or SU6656 (0.015 mg; n = 4) prior to injury. The results presented are the average number of WBCs that accumulated along the vessel (mean ± SEM). (C) Arterial injury was performed in wild-type (n = 3) or hck−/−fgr−/−lyn−/− mice (n = 3). The results presented are the average number of WBCs that accumulated along the vessel (mean ± SEM). Representative images of vessel sections taken from a wild-type mouse treated with vehicle (D), PP1 (E), or SU6656 (F) one hour after injury. Sections were stained with hematoxylin and eosin (left panels) or processed for immunohistochemical analysis with a polyclonal antibody to P-selectin (right panels; brown staining).
β2 integrins and SFKs mediate PMN recruitment to adherent platelets at the sites of vascular injury in mice. Arterial injury was performed as described in “Materials and methods,” and the vessels were examined one hour after injury. (A) Wild-type mice were pretreated with control F(ab)′2 or anti–β2-integrin F(ab)′2 prior to performing injury. The results presented are the average number of WBCs that accumulated along the vessel (mean ± SEM) in 4 control and 11 anti-integrin–treated animals. (B) Wild-type mice (n = 6) were pretreated with vehicle, PP1 (1.5 mg/kg; n = 8), or SU6656 (0.015 mg; n = 4) prior to injury. The results presented are the average number of WBCs that accumulated along the vessel (mean ± SEM). (C) Arterial injury was performed in wild-type (n = 3) or hck−/−fgr−/−lyn−/− mice (n = 3). The results presented are the average number of WBCs that accumulated along the vessel (mean ± SEM). Representative images of vessel sections taken from a wild-type mouse treated with vehicle (D), PP1 (E), or SU6656 (F) one hour after injury. Sections were stained with hematoxylin and eosin (left panels) or processed for immunohistochemical analysis with a polyclonal antibody to P-selectin (right panels; brown staining).
The requirement for SFKs in PMN adhesion to adherent platelets was investigated first by pretreating wild-type mice with SFK inhibitors PP1 (n = 8), SU6656 (n = 4), or vehicle (n = 6) prior to endothelial denudation. Both PP1 and SU6656 significantly reduced the number of PMNs that accumulated along adherent platelets at the site of vascular injury by 85% and 60%, respectively (Figure 6B, E, and F). The SFK inhibitors did not appear to exert their effect by reducing platelet P-selectin expression, as judged by immunohistochemical analysis (Figure 6D-F). To confirm a role for SFKs, arterial injury was performed in hck−/−fgr−/−lyn−/− mice. In comparison with wild-type matched controls (n = 3), hck−/−fgr−/−lyn−/− mice (n = 3) had fewer PMNs that accumulated along the arteries at one hour after injury (Figure 6C). At 4 weeks after injury, there was less intimal formation in SFK-deficient mice (n = 3) than in wild-type mice (n = 7) (intimal areas 2970 ± 560 mm2 versus 11 470 ± 2580 mm2, respectively, P = .071).
Discussion
The results of the present study confirm the central role of αMβ2-integrin function in supporting firm PMN-platelet interactions in vitro and in vivo.6–8 Of importance, our findings establish that the myeloid SFK members Hck, Fgr, and Lyn are required to sustain αMβ2-dependent firm adhesion of PMNs to activated platelets under (patho)physiologic conditions and strongly implicate the focal adhesion kinase Pyk2 as an important downstream mediator of SFK activity.
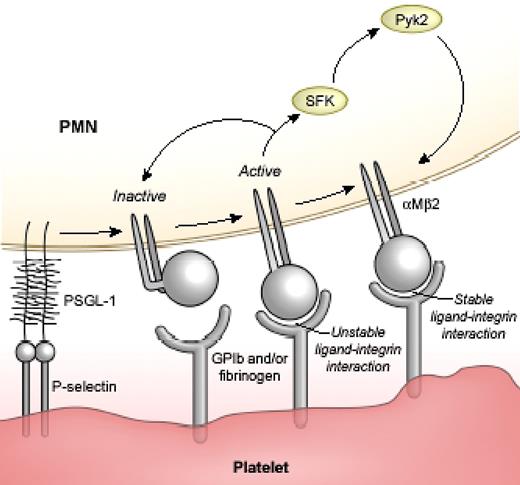
The role for SFK in promoting PMN-platelet interactions is supported by evidence in human and mouse cells in in vitro models and in an arterial injury model in mice. Pharmacologic inhibition and genetic targeting of Hck, Fgr, and Lyn attenuate platelet-PMN coaggregate formation in a mixed cell suspension assay, reduce shear-resistance interactions of PMNs with platelets in a flow assay, and block PMN accumulation along adherent platelets at site of vascular injury in mice. In contrast, the initial attachment of PMNs with activated platelets occurs in the absence of either SFK or αMβ2, suggesting SFK activity is not required for P-selectin–PSGL-1 interactions. Using confocal microscopy and 2 different monoclonal antibodies recognizing distinct activation-dependent epitopes of the β2 chain, we were able to show that platelet interactions are accompanied by promotion of a “fully-active” conformation of β2 integrin that is characterized by the assumption of extended conformation and changes in the β2-I domain. The fully active integrins localize specifically along cell contact sites between PMNs and platelets in an SFK-dependent manner. These results, together with our findings that SFK activation is downstream of β2 integrins,10,11,15 provide unequivocal evidence that initial integrin engagement generates SFK-dependent signals that are required to sustain a fully-active integrin state. Building upon our previous observations of integrins,10,11,15 we propose that platelet-PMN interactions are characterized by “initial” activation and ligand binding to αMβ2 followed by generation of an outside-in, SFK-mediated signal(s) that stabilizes firm, shear-resistance adhesion (Figure 7). This model is in agreement with a recent study by Giagulli et al21 that examined the role of SFK members in inside-out and outside-in regulation of β2-integrin function. Together, the data would suggest that SFKs are not involved in the chemoattractant or selectin-mediated inside-out signaling pathways that increased integrin affinity and/or clustering, but that SFKs are required for outside-in integrin signaling essential for sustained PMN adhesion.
Hypothetical scheme of a 3-step model of αMβ2 activity during PMN-platelet adhesion. Step 1: platelet-PMN interactions induce initial activation and ligand binding to αMβ2. Step 2: generation of an outside-in, SFK-Pyk2–mediated signal(s). Step 3: stabilization of integrin-ligand binding.
Hypothetical scheme of a 3-step model of αMβ2 activity during PMN-platelet adhesion. Step 1: platelet-PMN interactions induce initial activation and ligand binding to αMβ2. Step 2: generation of an outside-in, SFK-Pyk2–mediated signal(s). Step 3: stabilization of integrin-ligand binding.
The mechanism by which SFK activity supports firm PMN attachment to platelets is not clear. As PMNs roll over adherent, activated platelets or during rapid collisions between platelets and PMNs in mixed cell suspensions, it is conceivable that shear-resistant αMβ2 interactions with its platelet counterreceptors (glycoprotein Ib [GPIb] or fibrinogen or other molecules) occur in discrete membrane regions due to localization of an outside-in signal transduced by SFK. In the absence of the SFK signal, integrins may be unable to adopt a fully open/extended conformation, or, alternatively, may rapidly revert from a fully open/extended form to a low-affinity conformation and release ligand. There may also be an “intermediate” integrin activation state that is responsible for the initial integrin-ligand interaction, but we provide no direct evidence for the existence of an intermediate affinity state in our models. Previously, Ma et al12 demonstrated that P-selectin binding to PSGL-1 triggers ligand binding to β2 integrins by microclustering integrin receptors and thereby increasing their avidity in a static system. Microclustering of the integrin could represent the trigger that allows initial integrin-ligand binding. Shear forces, which are present in our models, may promote full conformational activation of the integrins. However, it is also possible that P-selectin alone,10–12 or in combination with platelet surface chemoattractant(s),22 signals via an as-yet-unidentified inside-out pathway to transiently shift αMβ2 to an affinity state capable of binding ligand. The finding that soluble P-selectin alone can trigger the binding of the β2-integrin activation reporter KIM127 under our experimental conditions suggests that P-selectin can directly trigger β2-integrin binding under our experimental conditions. This interpretation is in agreement with our previous studies10 and with a more recent study by Ma et al,12 but it is inconsistent with other observations22–25 suggesting that P-selectin binding to PSGL-1 has only a priming effect and that full PMN activation (including β2-integrin adhesion) requires an additional stimulus. The reasons for these discrepancies are unclear. However, in our experimental model PMNs are challenged with soluble P-selectin in the presence of stirring. This allows rapid and frequent collisions between cells, which may increase the frequency of efficient interactions between adhesive receptors and/or promote the exposure of additional agonists on the PMN surface that could cooperate with P-selectin to trigger full PMN activation.
A calcium-dependent pathway may possibly be involved in the initial activation of β2 integrins, because the initial interaction of platelets mobilizes calcium in PMNs in an integrin-independent manner, and preventing calcium mobilization interferes with the formation of PMN-platelet aggregates (V.E., unpublished data, January 2005). KIM127 binding occurs more rapidly and to a greater extent in PMNs stirred with activated but unfixed platelets then in PMNs exposed to activated and fixed platelets. It is possible that transcellular metabolism and/or other signaling systems between platelet-PMNs contribute to adhesion and are blocked by PFA fixation. A recent report provided evidence that, under flow conditions, intermediate-affinity binding of LFA-1 to ICAM-1 triggered by immobilized chemokines in lymphocytes is followed by rapid transition to a high-affinity state possibly induced by outside-in signals. Of interest, the same report demonstrated that this transition requires integrin association with the actin cytoskeleton,23 an event that may be mediated by SFK/Pyk2 pathways.20
With the use of PMNs from both αM−/− as well as from hck−/−fgr−/−lyn−/− mice, we establish that αMβ2 initiates an SFK-mediated outside-in signaling that triggers Pyk2 phosphorylation. Pharmacological blockade of Pyk2 activity correlates with a reduction in αMβ2-mediated interactions of PMNs with platelets, suggesting that Pyk2 may be a candidate downstream mediator of SFK signaling in this system. These observations are in agreement with other reports placing Pyk2 downstream of SFKs in integrin signaling in myeloid leukocytes.20 Pyk2 is an interesting candidate mediator of β2-integrin and SFK signaling. Others have implicated Pyk2 in several different leukocyte responses.20,24–29 Pyk2 may link SFK activity with cytoskeletal reorganization to promote or sustain αMβ2-mediated firm adhesion of PMNs to activated platelets. Additional approaches, such as genetic manipulation of Pyk2 expression in cultured cells or mice, will be required to establish a definite role for the kinase in sustaining PMN-platelet interactions. Moreover, other signaling proteins downstream of SFK may also be involved in this process. Of interest, recent data indicate that combined genetic deficiency of Vav-1 and Vav-3 results in a severe defect in the ability of PMNs to establish β2-integrin–dependent firm adhesion but does not affect β2-integrin affinity changes triggered by normal inside-out signaling.30
In vivo experiments demonstrated that both pharmacological inhibition and genetic manipulation of αMβ2 or SFK results in a significant impairment of PMN accumulation on adherent platelets at the site of vascular injury. These results indicate that the mechanistic model we propose based on our in vitro results may also occur under pathophysiologic conditions. Additionally, the early recruitment of PMNs by adherent platelets appears to contribute to subsequent development of intimal hyperplasia.9,5,14 Of note, SFKs regulate many PMN functions, including respiratory burst and oxygen radical formation,31 which may contribute to the arterial response to injury.32 Thus, blocking SFK signaling could potentially limit neointimal formation through a variety of mechanisms.
In conclusion, the new role for SFKs and possibly of their downstream mediator Pyk2 in promoting PMN recruitment by activated platelets at the sites of vascular injury, together with the known importance of these molecules as regulators of other leukocyte inflammatory functions, supports the contention that these signaling pathways may prove to be useful targets of novel pharmacologic approaches aimed at disrupting platelet-PMN interactions and their potential pathologic sequelae in atherosclerosis and restenosis.
Authorship
Contribution: V.E., L.T., and S.S.S. designed the experiments, analyzed data, and prepared the article; Z.P., A.P., S.M., G.D., N.M., L.F., and M.R. designed the experiments, conducted the research, and analyzed the data; G.B. and C.A.L. contributed vital new reagents and prepared the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Virgilio Evangelista, Laboratory of Vascular Biology and Pharmacology, Consorzio Mario Negri Sud, Via Nazionale 8/A, 66030 Santa Maria Imbaro, Italy; e-mail: evangelista@negrisud.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from Ministero della Sanità, Convenzione no. ICS060.2/RF99.74, Italian MIUR-DM L623/96-2002, and by Servier, Award for Cardiovascular Research (V.E.) and NIH grants HL070304, HL074219, HL080166 (S.S.S.), and P30 DK34987.
We gratefully thank Drs M. K. Robinson, D. Staunton, and C. Beals for valuable monoclonal antibodies. We thank F. Cinalli for help in preparing the article.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal