Abstract
Activated phagocytes express considerable amounts of MRP8 and MRP14, 2 calcium-binding S100 proteins forming stable heterodimers that are specifically secreted at inflammatory sites in many diseases. We previously reported that treatment of human microvascular endothelial cells with purified MRP8/MRP14 leads to loss of endothelial cell contacts. In this study, we demonstrate that MRP8/MRP14 complexes furthermore trigger cell death of endothelial cells after the onset of cell detachment. Morphologic analysis of dying endothelial cells revealed characteristic features of both apoptosis and necrosis. Furthermore, MRP8/MRP14 induced apoptotic caspase-9 and caspase-3 activation, DNA fragmentation, and membrane phosphatidylserine exposure in target cells. These events were independent of death receptor signaling and in part controlled by a mitochondrial pathway. Consistently, overexpression of antiapoptotic Bcl-2 abrogated caspase activation and externalization of phosphatidylserine; however, MRP8/MRP14 still induced plasma membrane damage and even DNA fragmentation. Thus, our results demonstrate that MRP8/MRP14 triggers cell death via caspase-dependent as well as -independent mechanisms. Excessive release of cytotoxic MRP8/MRP14 by activated phagocytes might therefore present an important molecular pathomechanism contributing to endothelial damage during vasculitis and other inflammatory diseases.
Introduction
Myeloid-related protein 8 (MRP8, S100A8) and MRP14 (S100A9) belong to a large family of calcium-binding proteins of the S100 family. They form stable heterodimers that are expressed by circulating neutrophils and monocytes representing the first cells to invade inflammatory lesions. MRP8/MRP14 complexes account for up to 40% of total cytosolic protein in neutrophils.1–3 After contact with extracellular matrix proteins or inflamed endothelium, activated phagocytes secrete MRP8/MRP14 at the sites of inflammation.4,5 In distinct inflammatory conditions, including rheumatoid arthritis, allograft rejections, and inflammatory bowel and lung diseases, the protein complex can be detected in high amounts in serum and exudates.6–12
The hypothesis of a direct pathogenic role of MRP8/MRP14 is promoted by a recently identified inflammatory disorder with the hallmark of an extraordinarily high abundance of MRP8/MRP14.13 MRP8/MRP14 is deposited on endothelial cells (ECs) and was shown to play a role for leukocyte recruitment.14,15 Until today, however, no classical receptor of MRP8/MRP14 on ECs could be identified, although binding to heparan sulfate proteoglycans or carboxylated N-glycans could be demonstrated.15 Previously, we could show that MRP8/MRP14 directly induces a thrombogenic and inflammatory response in human microvascular endothelial cells (HMECs). This response was caused by induction of proinflammatory chemokines and adhesion molecules as well as by a loss of cell-cell contacts impairing the endothelial integrity.16 Furthermore, MRP8/MRP14 inhibits cell growth and induces cytotoxicity in various human tumor cells, myoblasts, and fibroblasts.12,17–19 However, it is unclear whether MRP8/MRP14 also exerts cytotoxic effects in ECs.
In the present study, we therefore assessed whether MRP8/MRP14 causes EC death and tracked the involved cell death pathways. Our results show that MRP8/MRP14 induces EC death with features of both apoptosis and necrosis. However, although inhibition of caspases or overexpression of Bcl-2 prevented several apoptotic features, MRP8/MRP14-treated cells still revealed necrotic alterations. Endothelial disintegration strictly preceded cell death and is presumably the responsible trigger for the death-inducing effect of MRP8/MRP14. Our results therefore suggest that MRP8/MRP14 can trigger both caspase-dependent and -independent cell death mechanisms. This cytotoxic effect might play a role in several inflammatory diseases associated with endothelial dysfunction and pathologic accumulation of MRP8/MRP14.
Materials and methods
Reagents
MRP8 and MRP14 were purified from human granulocytes isolated from buffy coats as previously described.20,21 Briefly, granulocytes were sonicated, and most of the proteins were precipitated with 70% ammonium sulfate. The main fraction of MRP8 and MRP14 remained in the supernatant. After dialysis, anion exchange chromatography was performed using Mono Q sepharose (Pharmacia, GE Healthcare, Freiburg, Germany) and a linear salt from 0 to 1 M NaCl. The main fraction of MRP8 and MRP14 was eluted at NaCl concentrations of approximately 175 mM. The identity of MRP8 and MRP14 was ascertained by SDS polyacrylamide gel electrophoresis, amino acid sequencing, and immunostaining with specific antisera against the 2 proteins. The purity of the MRP8/MRP14 complex was more than 98%. MRP8/MRP14-containing stock solutions (15.2 mg/mL) were essentially free of endotoxin as tested by a limulus lysate assay (E-TOXATE reagent kit; Sigma, Deisenhofen, Germany). Staurosporine (STS) was obtained from Alexis (Lausen, Switzerland). Actinomycin D (ActD), zinc sulfate, propidium iodide (PI), and cycloheximide were purchased from Sigma. The irreversible broad-spectrum caspase inhibitor zVAD-fmk (N-benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone) and human recombinant tumor necrosis factor α (TNFα) were obtained from Bachem (Heidelberg, Germany) and R&D Systems (Wiesbaden, Germany), respectively.
Cell culture
HMEC-1 cells were kindly provided by Dr F. Candal (Atlanta, GA) and maintained as described at 37°C in 3% CO2 in MCDB131 medium (Biochrom, Berlin, Germany) supplemented with 10% fetal calf serum (FCS; PAA Laboratories, Cölbe, Germany), 10 mM l-glutamine, 50 μg/mL (≥ 30 U/mL) gentamicin (Cytogen, Berlin, Germany), 10 ng/mL epidermal growth factor (Boehringer, Mannheim, Germany), and 1 μg/mL hydrocortisone (Sigma).16,22 Jurkat cell lines were cultured in RPMI-1640 medium supplemented with 10% FCS, 2 mM l-glutamine, 100 U/mL of each penicillin, and 100 μg/mL streptomycin. Cells were grown at 37°C in a 5% CO2 atmosphere and maintained in log phase. Jurkat cells overexpressing Bcl-2 have been described previously.23 FADD- and caspase-8–deficient Jurkat cells were kindly provided by Dr J. Blenis (Boston, MA).24,25 In all experiments the HMEC monolayer and Jurkat cells were either left untreated or stimulated with MRP8/MRP14 (200 μg/mL/8.3 nM), STS (200 nM), or ActD (1 μg/mL) for the indicated time periods. In some experiments, cells were incubated with 1 μM cycloheximide for 30 minutes before the addition of 20 ng/mL TNFα.
RNA isolation and microarray analysis
For microarray analyses, HMEC monolayers were stimulated with MRP8/MRP14 for 6 hours. Isolated total RNA (5 μg) obtained from HMECs in 4 independent experiments were processed for microarray hybridization (Human Genome U95A Array; Affymetrix, Santa Clara, CA) as described previously.16,26 Data analysis was performed using either the MAS 5.0 software (Affymetrix) selecting only genes with a change P value of less than .05 and mean log ratio changes of at least 0.7, or the Expressionist Suite software package (GeneData, Martinsried, Germany) retaining only genes with significant fold changes relying on t test–based P values of less than .05. Details for data analysis have been described previously.16,26 The complete datasets are deposited in the Gene Expression Omnibus database (accession no. GSE5474)27 .
Electron microscopy
For ultrastructural examination by transmission electron microscopy, HMECs were plated in 8-well chambers (LabTec Chamber Slide System; Nalge Nunc International, Rochester, NY) and grown to confluence. Cells were stimulated for 24 and 48 hours with MRP8/MRP14 and processed using a standard protocol.28 Briefly, cultured cells were washed in phosphate-buffered saline (PBS), centrifuged for 5 minutes at 200g, and fixed in 2% glutaraldehyde/0.1 M sodium cacodylate buffer (pH 7.4) at room temperature for 1 hour. Cells were washed twice in PBS and postfixed in 2% OsO4/PBS at room temperature for 1 hour, dehydrated in ethanol, and embedded in Epon Serva, Heidelberg, Germany. Thin sections were contrasted with uranylacetate and lead citrate and examined on a transmission electron microscope (Philips, Eindhoven, the Netherlands). Images were collected on an EM300 Kodak electron microscope and printed on Kodabromide photographic paper.
Determination of cell death by flow cytometry
One of the early features of apoptosis is the loss of cell membrane asymmetry and the externalization of phosphatidylserine,29 which was measured by flow cytometric staining with FITC-conjugated annexin-V and PI according to the manufacturer's protocol (BD Pharmingen, Heidelberg, Germany). Cell death was also assessed by staining of cells with PI (2 μg/mL) and subsequent flow cytometric measurement of dye uptake. PI does not permeate intact membranes of living cells, and therefore stains only dead or dying cells. The formation of fragmented DNA in apoptotic nuclei was assessed by the method of Nicoletti et al.30 In brief, apoptotic nuclei were prepared by lysis in hypotonic buffer (0.1% sodium citrate, 0.1% Triton X-100), stained with PI (50 μg/mL), and analyzed by flow cytometry. Nuclei exhibiting a lower fluorescence (sub-G1) than normal diploid cells were considered as apoptotic. All flow cytometric analyses were performed using a FACSCalibur and CellQuest software (both from Becton Dickinson, Heidelberg, Germany).
Immunoblotting
Cellular lysates were prepared on ice for 30 minutes with lysis buffer containing 350 mM NaCl, 0.5 mM EDTA, 0.1 mM EGTA, 20 mM HEPES (pH 7.9), 1 mM MgCl2, 20% glycerol, and 1% NP-40 supplemented with protease inhibitor cocktail (Roche, Penzberg, Germany). Samples were adjusted to equal protein concentrations, separated on SDS-polyacrylamide gels, and subjected to immunoblotting with a monoclonal mouse anti–Bcl-2 antibody (clone 11870; R&D Systems). Blots were then incubated with secondary antibodies conjugated to horseradish peroxidase (Dako, Glostrup, Denmark) followed by enhanced chemiluminescence staining (Amersham, Uppsala, Sweden).
Caspase activity assays
Cells were lysed in lysis buffer (20 mM HEPES [pH 7.4], 84 mM KCl, 10 mM MgCl2, 0.2 mM EDTA, 0.2 mM EGTA, 1 mM DTT, and 0.5% NP-40 supplemented with protease inhibitors) on ice for 15 minutes and then centrifuged at 12000g at 4°C for 20 minutes. Caspase activity was determined by incubating cell lysates (20 μg protein as determined by the Bradford assay) at 37°C with 50 μM of the fluorogenic caspase-8 substrate Ac-IETD-AMC (N-acetyl-Ile-Glu-Thr-Asp-aminomethylcoumarin; Alexis), caspase-3 substrate Ac-DEVD-AMC (N-acetyl-Asp-Glu-Val-Asp-aminomethylcoumarin), or caspase-9 substrate Ac-LEHD-AMC (N-acetyl-Leu-Glu-His-Asp-aminomethylcoumarin; both from Bachem) in 200 μL buffer containing 50 mM HEPES (pH 7.3), 100 mM NaCl, 10% sucrose, 0.1% CHAPS, and 10 mM DTT. The release of AMC was measured by fluorometry (HTS 7000; Perkin Elmer, Boston, MA) using an excitation and emission wavelength of 360 nm and 465 nm, respectively. The activities were expressed as fluorescence increase (delta F) per microgram of protein.
TER
Transendothelial resistance (TER) was analyzed as described.16,31 HMEC monolayers were treated overnight with 200 μg/mL MRP8/MRP14. For caspase inhibition, 40 μM or 100 μM zVAD-fmk were added 1 hour prior to MRP8/MRP14 treatment. Electrical resistance of monolayer was recorded by an EVOM voltohmmeter (EVOL, Aston, Herts, United Kingdom) and expressed as Ω per square centimeter.
Data analysis
For statistical analyses we used the SPSS software version 11.0.1 (SPSS, Chicago, IL) in each case comparing 2 independent parameters. We applied the Mann-Whitney test and considered P values less than .05 as significant.
Results
Apoptosis-related genes are differentially expressed in HMECs after treatment with MRP8/MRP14
In 4 independent experiments, HMECs were grown to 90% confluence and incubated for 6 hours with 200 μg/mL MRP8/MRP14 or were left untreated. Total RNA was isolated and processed for expression profiling using oligonucleotide microarrays covering about 12 000 human full-length transcripts. Only genes with a significant (P < .05) change of mRNA expression of at least 1.7-fold were considered. Thereby, 16 genes were identified as differentially expressed, which are involved in either apoptotic pathways or cell cycle and growth control (Table 1). Only 3 gene transcripts were found to be up-regulated. These encoded the proapoptotic transcription factor p53 and its target genes Bax and Bak that are considered as the gatekeepers of mitochondrial cytochrome c release. The 13 down-regulated genes comprised mainly antiapoptotic (apoptosis inhibitor 5, c-FLIP, and calpastatin) and cell-cycle regulatory genes (Table 1).
Apoptosis- and cell-cycle-related genes differentially regulated by MRP8/MRP14 in HMECs
| Gene . | Gene symbol . |
|---|---|
| Genes up-regulated by MRP8/MRP14 | |
| Bak (Bcl-2—antagonist/killer 1) | BAK1 |
| Bax (Bcl-2—associated X protein) | BAX |
| Transcription factor p53 | TP53 |
| Genes down-regulated by MRP8/MRP14 | |
| ERCC5 (excision repair protein complementation group 5) | ERCC5 |
| NIMA (never in mitosis gene A)–related kinase 3 | NEK3 |
| MAD2 (mitotic arrest deficient 2)–like 1 | MAD2L1 |
| Cell division cycle 2 (G1 to S and G2 to M) | CDC2 |
| Apoptosis inhibitor 5 | API5 |
| NIMA-related kinase 4 | NEK4 |
| Stromal antigen 2 | STAG2 |
| c-FLIP (CASP8 and FADD-like apoptosis regulator) | CFLAR |
| Cell growth regulator with ring finger domain 1 | CGRRF1 |
| Beta-amyloid binding protein | BBP |
| Postmeiotic segregation increased 1 | PMS1 |
| Calpastatin | CAST |
| Retinoblastoma 1 | RB1 |
| Gene . | Gene symbol . |
|---|---|
| Genes up-regulated by MRP8/MRP14 | |
| Bak (Bcl-2—antagonist/killer 1) | BAK1 |
| Bax (Bcl-2—associated X protein) | BAX |
| Transcription factor p53 | TP53 |
| Genes down-regulated by MRP8/MRP14 | |
| ERCC5 (excision repair protein complementation group 5) | ERCC5 |
| NIMA (never in mitosis gene A)–related kinase 3 | NEK3 |
| MAD2 (mitotic arrest deficient 2)–like 1 | MAD2L1 |
| Cell division cycle 2 (G1 to S and G2 to M) | CDC2 |
| Apoptosis inhibitor 5 | API5 |
| NIMA-related kinase 4 | NEK4 |
| Stromal antigen 2 | STAG2 |
| c-FLIP (CASP8 and FADD-like apoptosis regulator) | CFLAR |
| Cell growth regulator with ring finger domain 1 | CGRRF1 |
| Beta-amyloid binding protein | BBP |
| Postmeiotic segregation increased 1 | PMS1 |
| Calpastatin | CAST |
| Retinoblastoma 1 | RB1 |
MRP8/MRP14 induces morphologic signs of apoptosis and necrosis in ECs
Since MRP8/MRP14 modulates apoptotic gene expression in HMECs, we investigated its effect on morphologic alterations of cell death. To this end, HMECs were treated for 24 and 48 hours with MRP8/MRP14, and subsequently analyzed by electron microscopy. Figure 1A demonstrates that MRP8/MRP14 induced typical signs of apoptosis such as chromatin condensation after 24 hours. Furthermore, margination of the chromatin and, less frequently, a complete fragmentation of the nuclei were observed. However, after prolonged exposure to MRP8/MRP14 (48 hours), cells showed additional morphologic alterations. For instance, the cytoplasm revealed extensive vacuolization more indicative of alternate death mechanisms such as necrosis (Figure 1B). Moreover, long-term–treated cells appeared in part exclusively necrotic and contained degenerated, swollen mitochondria, amorphous dense bodies, and plasma membrane rupture (Figure 1C).
Ultrastructural morphology of MRP8/MRP14-treated HMECs. Confluent HMEC monolayers treated for 24 hours (A) and 48 hours (B-C) with MRP8/MRP14 were processed for transmission electron microscopy. (A) Ultrastructural analysis reveals signs of apoptosis, such as condensed chromatin (arrows) after 24 hours of treatment. (B) After 48 hours of incubation with MRP8/MRP14, HMECs show alterations of apoptosis (eg, condensed mitochondria [arrows], shrunken nuclei [N], and concomitant necrotic features, such as cytoplasmic vacuolization [V]). (C) Advanced necrotic cell with a pycnotic nucleus (N), swollen mitochondria with amorphous dense bodies (M), and disrupted plasma membrane (arrows). Magnifications, ×2650.
Ultrastructural morphology of MRP8/MRP14-treated HMECs. Confluent HMEC monolayers treated for 24 hours (A) and 48 hours (B-C) with MRP8/MRP14 were processed for transmission electron microscopy. (A) Ultrastructural analysis reveals signs of apoptosis, such as condensed chromatin (arrows) after 24 hours of treatment. (B) After 48 hours of incubation with MRP8/MRP14, HMECs show alterations of apoptosis (eg, condensed mitochondria [arrows], shrunken nuclei [N], and concomitant necrotic features, such as cytoplasmic vacuolization [V]). (C) Advanced necrotic cell with a pycnotic nucleus (N), swollen mitochondria with amorphous dense bodies (M), and disrupted plasma membrane (arrows). Magnifications, ×2650.
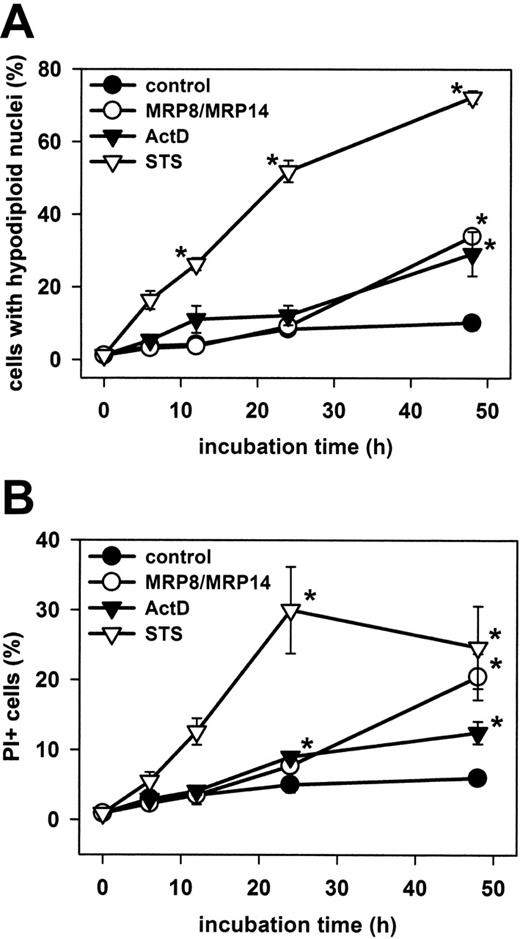
Kinetics of MRP8/MRP14-induced EC death
To investigate the kinetics of MRP8/MRP14-induced EC death, we treated HMECs with MRP8/MRP14 for different time periods and assessed different alterations of cell death by flow cytometry. We first measured late apoptotic DNA fragmentation (Figure 2A). Incubation of HMECs with ActD and STS was used as a positive control. MPR8/MRP14 induced significant apoptosis (34% ± 1.9% SEM) in HMECs after 48 hours of incubation, compared with the control (10% ± 2.0% SEM). Apoptosis was in a similar range as that observed after incubation with ActD (29% ± 6.1% SEM), but was clearly delayed when compared with STS that already induced apoptosis after 6 hours. Similar effects were observed when apoptosis was assessed by annexin-V staining, which detects phosphatidylserine exposure in early stages of apoptosis (data not shown). We further determined EC death by the uptake of PI, which labels necrotic or postapoptotic cells (Figure 2B). The kinetics of PI uptake largely coincided with the formation of hypodiploid DNA and became detectable not before 24 hours of MRP8/MRP14 treatment.
Kinetic of apoptosis and necrosis in HMECs. HMEC monolayers were treated for 6, 12, 24, and 48 h with 200 μg/mL MRP8/MRP14, 1 μg/mL ActD, 200 nM STS, or were left untreated (control). The percentages of late apoptotic cells with hypodiploid cell nuclei (A) and necrotic PI-positive cells (B) were determined by flow cytometry. The results show the mean ± SEM of 6 independent experiments. *Significant differences between treated and untreated cells (P < .05).
Kinetic of apoptosis and necrosis in HMECs. HMEC monolayers were treated for 6, 12, 24, and 48 h with 200 μg/mL MRP8/MRP14, 1 μg/mL ActD, 200 nM STS, or were left untreated (control). The percentages of late apoptotic cells with hypodiploid cell nuclei (A) and necrotic PI-positive cells (B) were determined by flow cytometry. The results show the mean ± SEM of 6 independent experiments. *Significant differences between treated and untreated cells (P < .05).
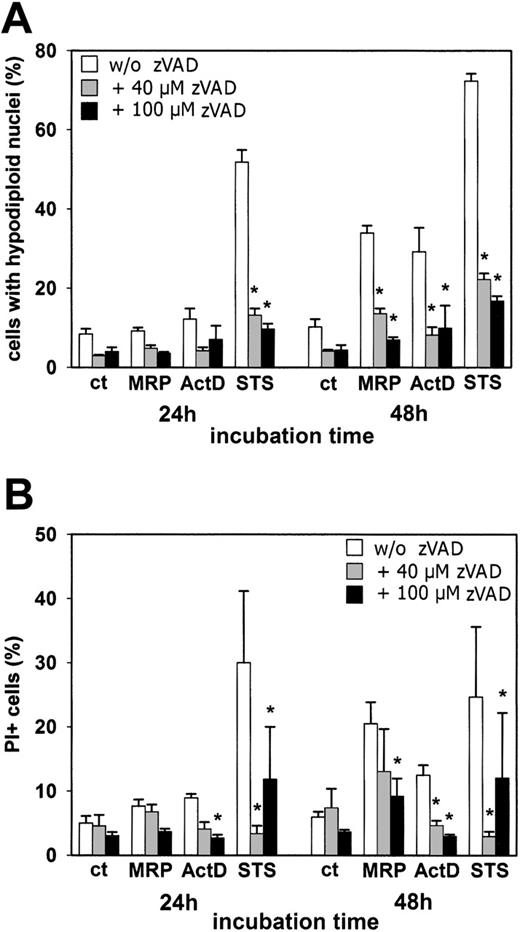
To investigate the functional contribution of caspase activation to MRP8/MRP14-induced cell death, we used the broad-spectrum caspase inhibitor zVAD-fmk. The results obtained by measuring DNA fragmentation (Figure 3A) and annexin-V staining (data not shown) were comparable. As expected, apoptosis induced after 24 hours of incubation with STS was strongly inhibited by the addition of 40 μM and 100 μM zVAD-fmk, resulting in a reduction of apoptotic cells by 78% and 84%, respectively. Importantly, apoptosis (ie, DNA fragmentation), induced by 48 hours of treatment with MRP8/MRP14 or ActD, was also strongly impaired by the caspase inhibitor, resulting in a more than 60% inhibition of cell death (Figure 3A). Interestingly, however, zVAD-fmk was less potent in inhibiting necrotic alterations induced by MRP8/MRP14, as assessed by the measurement of PI uptake (Figure 3B). Although 40 μM zVAD-fmk significantly inhibited ActD- and STS-induced secondary necrosis, only the addition of 100 μM zVAD-fmk was able to partially reduce MRP8/MRP14-induced PI uptake.
Effect of caspase inhibition on MRP8/MRP14-induced apoptotic and necrotic alterations. HMEC monolayers were left either untreated (ct) or were stimulated with 200 μg/mL MRP8/MRP14 (MRP), 1 μg/mL ActD, or 200 nM STS in the absence (□) or presence of 40 μM (⊡) or 100 μM zVAD-fmk (▪). After 24 and 48 hours, cells were analyzed for the percentages of hypodiploid cell nuclei (A) and PI-positive cells (B) by flow cytometry. The results represent means ± SEM from 6 independent experiments. *Significant inhibition by zVAD-fmk (P < .05).
Effect of caspase inhibition on MRP8/MRP14-induced apoptotic and necrotic alterations. HMEC monolayers were left either untreated (ct) or were stimulated with 200 μg/mL MRP8/MRP14 (MRP), 1 μg/mL ActD, or 200 nM STS in the absence (□) or presence of 40 μM (⊡) or 100 μM zVAD-fmk (▪). After 24 and 48 hours, cells were analyzed for the percentages of hypodiploid cell nuclei (A) and PI-positive cells (B) by flow cytometry. The results represent means ± SEM from 6 independent experiments. *Significant inhibition by zVAD-fmk (P < .05).
Loss of cell-cell contacts precedes MRP8/MRP14-induced death of ECs
Previously, we have shown that MRP8/MRP14 impairs the endothelial monolayer integrity by down-regulating cell junction proteins.16 We therefore investigated whether the proapoptotic effect of MRP8/MRP14 is causative for the decrease of cell junction proteins or represents rather a consequence of disturbed endothelial integrity. To this end, we analyzed the TER of HMECs after treatment with MRP8/MRP14 in the presence or absence of the caspase inhibitor zVAD-fmk. Whereas first signs of apoptotic cell death were not detectable prior to 24 hours of MRP8/MRP14 treatment (Figure 2), a decrease in TER was already measurable after 6 hours (Figure 4A). Moreover, the caspase inhibitor zVAD-fmk could not inhibit the reduction of TER induced by MRP8/MRP14 (Figure 4B), indicating that the loss of EC contacts was not mediated by the engagement of caspases.
Effects of MRP8/MRP14 on the endothelial integrity. (A) HMEC monolayers were left untreated (⊡) or treated with 200 μg/mL MRP8/MRP14 (▪). After the indicated time periods, TER was determined as described in “Materials and methods.” (B) HMEC monolayers were left untreated or treated for 16 hours (⊡) and 24 hours (▪) with 200 μg/mL MRP8/MRP14 in the presence of the caspase inhibitor zVAD-fmk (40 μM and 100 μM) and analyzed for TER. *Significant decrease of the TER by following MRP8/MRP14 treatment (P < .05). Error bars indicate standard deviations in 3 independent experiments.
Effects of MRP8/MRP14 on the endothelial integrity. (A) HMEC monolayers were left untreated (⊡) or treated with 200 μg/mL MRP8/MRP14 (▪). After the indicated time periods, TER was determined as described in “Materials and methods.” (B) HMEC monolayers were left untreated or treated for 16 hours (⊡) and 24 hours (▪) with 200 μg/mL MRP8/MRP14 in the presence of the caspase inhibitor zVAD-fmk (40 μM and 100 μM) and analyzed for TER. *Significant decrease of the TER by following MRP8/MRP14 treatment (P < .05). Error bars indicate standard deviations in 3 independent experiments.
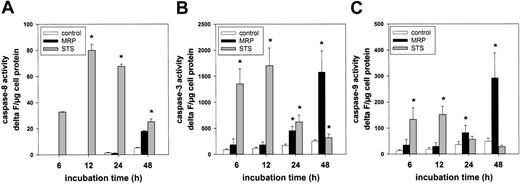
Caspase activation induced by MRP8/MRP14 in ECs
Since the previous results indicated an inhibition of the apoptotic effect of MRP8/MRP14 by the caspase inhibitor zVAD-fmk, we tried to identify the specific caspases involved in MRP8/MRP14-induced apoptosis. Using caspase-specific peptide substrates, we analyzed the respective caspase activities in MRP8/MRP14-treated HMECs after different time intervals. Judging from the results of these assays, MRP8/MRP14 did not induce an activation of caspase-8 in ECs (Figure 5A). This finding furthermore argues against an involvement of death receptors in MRP8/MRP14-mediated apoptosis. Caspase-9 is an essential component of the mitochondrial pathway of apoptosis. Once activated, caspase-9 can directly cleave and activate the effector caspase-3. Proteolytic activities of caspase-9 and caspase-3 were readily measurable in HMECs after 24 hours of MRP8/MRP14 stimulation and increased upon prolonged treatment (Figure 5B-C). Consistent with previous results, treatment with STS led to significant caspase-3 and caspase-9 activation already after 6 hours (Figure 5B-C).
Induction of caspase-3 and caspase-9 activity by MRP8/MRP14. HMECs were treated for the indicated time points with either the medium control (□), 200 μg/mL MRP8/MRP14 (▪), or 200 nM STS (⊡). Cell lysates were then prepared and incubated with the caspase-8 substrate Ac-IETD-AMC (A), the caspase-3 substrate Ac-DEVD-AMC (B), and the caspase-9 substrate Ac-LEHD-AMC (C). The fluorometric determination of AMC release was expressed as fluorescence increase (delta F) per microgram of cell protein. *Significant differences compared with the control cells of the respective time period (n = 5; P < .05). Error bars indicate the standard error of the mean.
Induction of caspase-3 and caspase-9 activity by MRP8/MRP14. HMECs were treated for the indicated time points with either the medium control (□), 200 μg/mL MRP8/MRP14 (▪), or 200 nM STS (⊡). Cell lysates were then prepared and incubated with the caspase-8 substrate Ac-IETD-AMC (A), the caspase-3 substrate Ac-DEVD-AMC (B), and the caspase-9 substrate Ac-LEHD-AMC (C). The fluorometric determination of AMC release was expressed as fluorescence increase (delta F) per microgram of cell protein. *Significant differences compared with the control cells of the respective time period (n = 5; P < .05). Error bars indicate the standard error of the mean.
MRP8/MRP14-induced cell death is independent of the death receptor–mediated apoptotic pathway
Apoptosis can be triggered by activation of either the intrinsic mitochondrial or the extrinsic death receptor pathway. To investigate the contribution of these pathways to the induction of apoptosis by MRP8/MRP14 in more detail, we took advantage of different Jurkat T-cell lines either lacking or overexpressing key components of these pathways. In wild-type Jurkat cells, MRP8/MRP14 induced cell death similarly as observed in ECs with both signs of apoptosis (ie, DNA fragmentation; Figure 6A) and necrosis (ie, PI uptake; Figure 6B). Moreover, MRP8/MRP14 also activated caspase-3 and caspase-9 in those cells, but not caspase-8 (data not shown).
The death receptor pathway is not involved in MRP8/MRP14-induced cell death. The time course of apoptosis (A) and necrosis (B) was analyzed in Jurkat cells that were stimulated with MRP8/MRP14 or left untreated similarly to HMECs (Figure 2). *Significant differences between treated and untreated cells (n = 5; P < .05). (C-D) Wild-type (wt), caspase-8–deficient, and FADD-deficient Jurkat cells were left untreated (□) or were incubated for 24 hours (C) and 48 hours (D) with MRP8/MRP14 (▪) or 20 ng/mL TNFα and 1 mM cycloheximide (⊡) and analyzed for the percentage of apoptotic cells with hypodiploid nuclei. The data represent the means ± SEM from 4 independent experiments. *Significantly increased amounts of apoptotic cells compared with control cells (P < .05).
The death receptor pathway is not involved in MRP8/MRP14-induced cell death. The time course of apoptosis (A) and necrosis (B) was analyzed in Jurkat cells that were stimulated with MRP8/MRP14 or left untreated similarly to HMECs (Figure 2). *Significant differences between treated and untreated cells (n = 5; P < .05). (C-D) Wild-type (wt), caspase-8–deficient, and FADD-deficient Jurkat cells were left untreated (□) or were incubated for 24 hours (C) and 48 hours (D) with MRP8/MRP14 (▪) or 20 ng/mL TNFα and 1 mM cycloheximide (⊡) and analyzed for the percentage of apoptotic cells with hypodiploid nuclei. The data represent the means ± SEM from 4 independent experiments. *Significantly increased amounts of apoptotic cells compared with control cells (P < .05).
To investigate the influence of death receptor signaling on MRP8/MRP14-mediated cell death, we used Jurkat T cells deficient in either caspase-8 or FADD expression. Caspase-8 is the main initiator activated upon death receptor–induced apoptosis. FADD is essential for the recruitment of caspase-8 into death receptor complexes, finally resulting in activation of this protease. Importantly, Jurkat T cells deficient in either caspase-8 or FADD expression were similarly or even more sensitive to MRP8/MRP14-induced cell death than the wild-type control (Figure 6C-D). In contrast, apoptosis induced by the death receptor ligand TNFα in combination with cycloheximide was significantly reduced in both the caspase-8– and FADD-deficient Jurkat cells (Figure 6C-D). In unison with the results of the caspase activity measurements, this confirms an independence of MRP8/MRP14-induced cell death from the death receptor pathway.
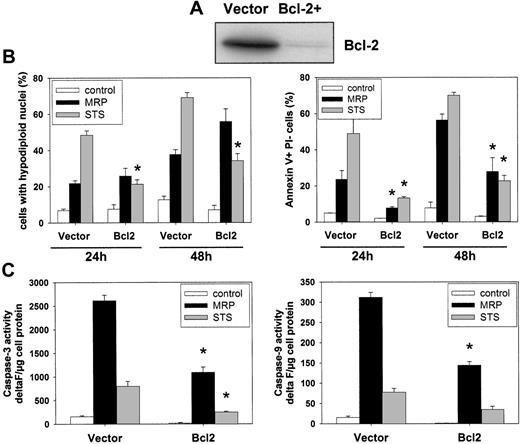
MRP8/MRP14-induced cell death is not entirely dependent on the intrinsic mitochondrial apoptotic pathway
The mitochondrial pathway of apoptosis is controlled by pro- and antiapoptotic Bcl-2 proteins that regulate Apaf-1–dependent caspase-9 activation.32,33 To investigate the contribution of the intrinsic mitochondrial pathway to MRP8/MRP14-induced cell death, we used Jurkat T cells stably overexpressing antiapoptotic Bcl-2 and compared them with Jurkat cells transfected with the empty vector alone (Figure 7A). Whereas STS-induced DNA fragmentation was decisively reduced by Bcl-2 overexpression compared with vector controls, remarkably, Bcl-2 overexpression did not decrease the MRP8/MRP14-induced percentage of cells with fragmented DNA (Figure 7B; left panel). However, applying annexin-V staining as readout, MRP8/MRP14-induced apoptosis was as strongly inhibited by Bcl-2 overexpression, as was STS-induced cell death (Figure 7B; right panel). Furthermore, Bcl-2 overexpression led to a significant reduction of MRP8/MRP14-induced activation of caspase-3 and caspase-9 (Figure 7C). These results therefore demonstrate that MRP8/MRP14-induced DNA fragmentation is largely independent from the mitochondria-mediated apoptotic pathway, whereas other apoptotic features, including caspase activation and phosphatidylserine exposure, are more clearly dependent on the activation of this pathway. MRP8/MRP14 therefore induces cell death substantially, but not exclusively via a Bcl-2–dependent mitochondrial pathway.
Effect of Bcl-2 on MRP8/MRP14-induced cell death. (A) Status of Bcl-2 expression. Cell lysates were prepared from Jurkat cells stably overexpressing either Bcl-2 or the empty vector control and subjected to immunoblotting with anti–Bcl-2 antibodies. (B) Effect of Bcl-2 on MRP8/MRP14-induced DNA fragmentation and phosphatidylserine exposure. Jurkat cells overexpressing Bcl-2 or the empty vector control were treated for 24 or 48 hours with MRP8/MRP14 (▪) or STS (⊡), or left untreated (□). Subsequently, DNA fragmentation was determined by flow cytometric measurement of hypodiploid DNA (left panel). Exposure of phosphatidylserine exposure was measured by flow cytometric detection of annexin-V–positive/PI-negative cells (right panel). (C) Effect of Bcl-2 on caspase activation. Jurkat cells overexpressing Bcl-2 or the empty vector control were treated for 24 hours with MRP8/MRP14 (▪) or STS (⊡), or left untreated (□). Caspase-3–like (left panel) and caspase-9–like (right panel) activities were measured using the fluorimetric substrates Ac-DEVD-AMC and Ac-LEHD-AMC, respectively. Caspase activity is indicated as delta F per microgram of protein. The data represent the means ± SEM from 4 independent experiments. Note that the relatively weak caspase activation in the STS-treated samples is due to the fact that caspase activation is maximal within 12 hours after STS treatment and declines thereafter. *Significant decreases of the apoptotic and caspase-activating effects of MRP8/MRP14 and STS by Bcl-2 overexpression compared with empty vector control cells (P < .05).
Effect of Bcl-2 on MRP8/MRP14-induced cell death. (A) Status of Bcl-2 expression. Cell lysates were prepared from Jurkat cells stably overexpressing either Bcl-2 or the empty vector control and subjected to immunoblotting with anti–Bcl-2 antibodies. (B) Effect of Bcl-2 on MRP8/MRP14-induced DNA fragmentation and phosphatidylserine exposure. Jurkat cells overexpressing Bcl-2 or the empty vector control were treated for 24 or 48 hours with MRP8/MRP14 (▪) or STS (⊡), or left untreated (□). Subsequently, DNA fragmentation was determined by flow cytometric measurement of hypodiploid DNA (left panel). Exposure of phosphatidylserine exposure was measured by flow cytometric detection of annexin-V–positive/PI-negative cells (right panel). (C) Effect of Bcl-2 on caspase activation. Jurkat cells overexpressing Bcl-2 or the empty vector control were treated for 24 hours with MRP8/MRP14 (▪) or STS (⊡), or left untreated (□). Caspase-3–like (left panel) and caspase-9–like (right panel) activities were measured using the fluorimetric substrates Ac-DEVD-AMC and Ac-LEHD-AMC, respectively. Caspase activity is indicated as delta F per microgram of protein. The data represent the means ± SEM from 4 independent experiments. Note that the relatively weak caspase activation in the STS-treated samples is due to the fact that caspase activation is maximal within 12 hours after STS treatment and declines thereafter. *Significant decreases of the apoptotic and caspase-activating effects of MRP8/MRP14 and STS by Bcl-2 overexpression compared with empty vector control cells (P < .05).
Discussion
Neutrophils and monocytes are vital components of the innate immune system endowed with multiple bactericidal functions and immune response–launching abilities. Among others, they accumulate a considerable amount of 2 calcium-binding proteins, MRP8 and MRP14, in their cytosol that are specifically secreted as protein complexes after contact of phagocytes with extracellular matrix proteins or inflamed endothelium.4,5 In the last 2 years, several gene expression analyses have been published showing that MRP8 and MRP14 belong to the most strongly up-regulated genes in numerous inflammatory diseases.63–67 Our group found elevated serum levels and the accumulation of the complex in inflammatory fluids in distinct inflammatory conditions such as rheumatoid arthritis, allograft rejections, inflammatory bowel and lung diseases, and vasculitis.6,8,9,11,12,34,35 Especially in systemic vasculitis (Kawasaki disease), expression of both proteins is closely associated with disease activity and response to therapy.16,34 However, the central functional role of these S100 proteins has not been identified yet.
In a recent study, we demonstrated that MRP8/MRP14 exerts prothrombotic and proinflammatory activity and leads to the loss of cell junction proteins in endothelial cells.16 Thus, we hypothesized that MRP8/MRP14 might also damage the endothelium by induction of cell death. Indeed, it has been recently proposed that MRP8/MRP14 might suppress cell proliferation or induce cell death in several tumor or normal cells.12,17–19 The molecular mechanisms of this effect are entirely unclear. In this work, we demonstrate that MRP8/MRP14 exerts potent cytotoxic activities by inducing both apoptotic and necrotic alterations in human ECs.
Induction of apoptosis is generally achieved by 2 major signaling routes, the death receptor–mediated and the mitochondrial death pathway, both of which depend on the formation of large multiprotein complexes.36,37 Apoptosis mediated by death receptors is initiated upon ligand binding, which results in activation of caspase-8 at the death-inducing signaling complex.38 The mitochondrial pathway that is triggered by a variety of stress conditions is essentially controlled by pro- and antiapoptotic members of the Bcl-2 family. Activation of this pathway triggers mitochondrial outer membrane permeabilization (MOMP), which in turn leads to the release of cytochrome c and other proapoptotic factors, including Smac/DIABLO, endonuclease G, apoptosis-inducing factor (AIF), and OMI/HtrA2.31,35,39–41 In the cytosol, cytochrome c binds to Apaf-1 and forms, together with caspase-9, the apoptosome, which leads to caspase-9 activation. MOMP and the subsequent loss of the mitochondrial membrane potential may also be involved in caspase-independent death pathways.32,40,42–44
In this study, we demonstrate that concentrations of MRP8/MRP14 similar to those found in inflammatory exudates in vivo cause not only apoptosis, but also necrosis of HMECs. Both apoptosis and necrosis occurred after 24 to 48 hours of MRP8/MRP14 treatment, whereas the protein kinase inhibitor STS, a classic proapoptotic stimulus, induced typical apoptotic changes in ECs within a few hours. The apoptotic alterations induced by MRP8/MRP14 were accompanied by activation of caspase-3 and caspase-9, and were consequently blocked by the general caspase inhibitor zVAD-fmk. In contrast, necrotic alterations induced by MRP8/MRP14 were only inhibited by high concentrations of zVAD-fmk, suggesting that MRP8/MRP14-induced necrosis is not simply secondary to caspase-mediated apoptosis. Inhibition of necrosis by high concentrations of zVAD-fmk might be unspecific and involve other proteases as previously reported.45 Our results therefore imply that MRP8/MRP14 trigger both a caspase-dependent and -independent mechanism of cell death.
Using Jurkat cells deficient for specific, important regulators of apoptosis, we further tried to delineate the pathways involved in MRP8/MRP14-induced cell death. Apoptosis induction was not significantly impaired in caspase-8– or FADD-deficient cells, indicating that death receptors are not required for MRP8/MRP14-induced cell death. In contrast, overexpression of Bcl-2 prevented the induction of caspase activation as well as phosphatidlyserine exposure, suggesting that MRP8/MRP14 triggers these alterations via the classic mitochondrial pathway.
Surprising, however, was our finding that Bcl-2 overexpression could not prevent DNA fragmentation induced by MRP8/MRP14, indicating that this event is probably independent of the caspase-activated DNase. A failure of Bcl-2 to prevent DNA fragmentation has also been reported in other experimental systems.46 AIF or endonuclease G that are both released from mitochondria as well as lysosomal nucleases have been shown to cause chromatin condensation in the absence of caspase-3 activation.39,42,43,47–50 Whether MRP8/MRP14-induced DNA fragmentation in the presence of high levels of Bcl-2 involves lysosomal nucleases, AIF, or endonuclease G remains to be shown. Nevertheless, these findings suggest that inhibition not only of the death receptor but also of the classic mitochondrial cell death pathway is insufficient to prevent MRP8/MRP14-induced cell death.
Several studies have shown that apoptosis is accompanied by the loss of cell junction proteins, several of which are indeed caspase substrates.51,52 Our results, however, suggest that the impairment of endothelial integrity induced by MRP8/MRP14 is not a consequence of apoptosis and caspase activation, but may be rather a trigger of the cell death process. The loosening of inter-EC contacts occurred at least 18 hours before caspase activation and other signs of apoptosis or necrosis were observed. This is in accordance with data from the murine system where the deficiency of cell-contact proteins like vascular endothelial (VE)–cadherin–53 or PECAM-1–54 induced EC apoptosis via the mitochondrial pathway. Recently, in mouse hepatocytes occludin was shown to mediate survival signals via the mitogen-activated protein kinase (MAPK) and Akt pathway thereby inihibiting apoptosis and claudin-2 expression.55 Likewise, in human ECs, VE-cadherin antibodies56 or targeted VE-cadherin gene silencing57 are able to induce apoptosis. In a recent work, VE-cadherin clustering was made responsible for the expression of the growth arrest–specific 1 integral membrane protein, which acts antiapoptotically on ECs.58 Thus, the impairment of endothelial integrity and a decreased expression of cell junction proteins in response to MRP8/MRP14 might lead to a loss of survival signals, which subsequently triggers cell death by anoikis.
MRP8 and MRP14 are not only calcium-binding proteins, but also zinc-binding proteins;59,60 however, there are conflicting data regarding the role of zinc depletion for MRP8/MRP14-induced cell death. It has been described that the cytotoxic activity of MRP8/MRP14 could be suppressed by the addition of zinc ions in several cell systems.18,19,61 Also, Ghavami et al showed that MRP8/MRP14 treatment of colon carcinoma cells leads to activation of caspase-3 and caspase-9, which was partially reversed by the addition of zinc.17 However, sole extracellular zinc depletion was a weak activator of caspase-3 and caspase-9 in these cells compared with MRP8/MRP14.17 Accordingly, MRP8/MRP14-induced apoptosis in mouse mammary carcinoma cells is not mediated by zinc depletion.62 Moreover, we observed that incubation of the HMEC monolayer with culture medium that had been dialysed against high concentrations of MRP8/MRP14 in order to deplete for the zinc-binding capacity of MRP8/MRP14 caused no decrease of the TER (data not shown). Instead, light microscopy revealed microcrystalline deposits on MRP8/MRP14-treated endothelial monolayers that correlate in size and quantity with the amount of added zinc ions. We therefore suggest that these inconsistent antagonistic effects of zinc are rather an artificial phenomenon that is due to chelation and loss of biological activity of MRP8/MRP14 by zinc.
In conclusion, our results show that an early effect of MRP8/MRP14 is EC disintegration, while cell death occurs clearly later and might be initiated by the loss of cell junction proteins. MRP8/MRP14 can induce alterations indicative of both apoptotic and necrotic cell death that are only partially prevented by Bcl-2 and involve both caspase-dependent and -independent pathways. These molecular mechanisms might be the principal mode of how MRP8/MRP14 induces endothelial dysfunction. Because high amounts of MRP8/MRP14 are released by activated phagocytes in the course of inflammation,6,8,9,11,12,34,35 MRP8/MRP14-induced endothelial damage is likely to represent an important pathomechanism involved in vasculitis and other inflammatory diseases associated with endothelial dysfunction and accumulation of MRP8/MRP14.
Authorship
Author contributions: D.V. and K.B. contributed equally to the work; D.V. designed and performed research, collected and analyzed data, and wrote the paper; K.B. performed research and collected and analyzed data; T.V. contributed vital new reagents; U.F. performed research and contributed vital new reagents; C.S. collected and analyzed data; K.S.-O. contributed vital new reagents and revised the written paper; and J.R. designed research, analyzed data, and revised the written paper.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Johannes Roth, Institute of Experimental Dermatology, University Hospital Münster, Röntgenstr. 21, 48149 Münster, Germany; e-mail: rothj@uni-muenster.de.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Supported by the Deutsche Forschungsgemeinschaft (grant SFB295/A16 to J.R.) and the Interdisciplinary Clinical Research Centre of the University of Münster (grant Fö2/005/06 to D.V.).

![Figure 1. Ultrastructural morphology of MRP8/MRP14-treated HMECs. Confluent HMEC monolayers treated for 24 hours (A) and 48 hours (B-C) with MRP8/MRP14 were processed for transmission electron microscopy. (A) Ultrastructural analysis reveals signs of apoptosis, such as condensed chromatin (arrows) after 24 hours of treatment. (B) After 48 hours of incubation with MRP8/MRP14, HMECs show alterations of apoptosis (eg, condensed mitochondria [arrows], shrunken nuclei [N], and concomitant necrotic features, such as cytoplasmic vacuolization [V]). (C) Advanced necrotic cell with a pycnotic nucleus (N), swollen mitochondria with amorphous dense bodies (M), and disrupted plasma membrane (arrows). Magnifications, ×2650.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/6/10.1182_blood-2006-08-040444/4/m_zh80060709730001.jpeg?Expires=1769080337&Signature=GC3bJyv1cQ8kfjnuVnAiBMAiuJTwdMJ2RUquxEJPt4RQmcNGeuYFk2ZSI6GHMOOJvxs7mKDPBAi18QwgbLvcuRQH9wngdE5eZUAJ2UhlHthUngbl8oAMI4CTrK9ziXdzysrwniuSS662UG8d4pU8BS~hQq7SzJzQbPH00VgY01WfOFmkoB-DXsY1uN2G4MIZr-JmAOXS~TxXwM8MpuGQ3qQ34q4rW-rlRgemnHRh7UCdaWj2QSzRi98gdyLeu3pZAJJ~So6~LfymBSr-ortlTKrsDnRDf0FHAOfZN-8aJUg~Nq~XZC-ozTh59FuwKT1XYDTQnk271i8NEpLtiP1-MQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal