Abstract
CD4+CD25+ regulatory T cells (Tregs) suppress immune responses to alloantigens. The in vivo circulation and tissue localization of Tregs during an adaptive immune response remain unclear. We noninvasively tracked luciferase-expressing Tregs over time in an allogeneic bone marrow transplant model and demonstrated colocalization with effector T cells and initial expansion in secondary lymphoid organs before migration into inflamed tissues. Inflammation induced by irradiation and the allogeneic setting provided crucial stimuli for early Treg expansion and migration, leading to parallel reduction of effector T-cell proliferation in lymphoid organs and peripheral tissues. Treg transplants conferred long-term protection from systemic inflammatory challenge consistent with Treg in vivo survival. Suppression occurred during multiple phases of inflammation, but is optimal in the initial phase, providing protection from graft-versus-host disease while maintaining the graft-versus-tumor effect even at physiologic doses of Tregs due to their in vivo expansion, hence overcoming a major barrier to potential clinical applications of Tregs given their rarity.
Introduction
CD4+CD25+ regulatory T cells (Tregs) suppress immune responses in models of autoimmunity, tumor and microbial immunity, and solid- and hematopoietic-cell transplantation tolerance. Much attention has focused on understanding the mechanism by which Tregs exert their suppressor function and on defining their origin, generation, and phenotype.1 However, little is known about Treg migration, tissue localization, and sites of suppression in the adaptive immune response. In vitro, Tregs are anergic to antigenic stimulation.2,3 In vivo studies demonstrated different Treg dynamics, with homeostatic proliferation in immunodeficient mice4,5 and antigen-driven expansion and local accumulation in draining lymph nodes (LNs).6–9 By intravital microscopy, Tregs appear to exert their suppressor function by preventing stable contacts between dendritic cells (DCs) and naive T cells, which are required for T-cell priming and activation in LNs.10,11
In addition to localization to secondary lymphoid organs, Tregs have been identified within inflamed tissues, tumors, and infectious sites.12 Tregs deficient in homing molecules permit effective clearance in infection.13,14 In murine graft-versus-host disease (GvHD), Treg expression of CD62L15,16 or CCR517 was required for protection. These studies suggest that appropriate Treg localization is important for their suppressor function in vivo. In the current study, we noninvasively and longitudinally monitored the in vivo trafficking properties of Tregs in an allogeneic bone marrow transplant (BMT)/leukemia model and evaluated the functional consequences of their in vivo dynamics during an inflammatory reaction.
Materials and methods
Animals
FVB (H-2q, Thy 1.1) and Balb/c (H-2d, Thy 1.2) mice were from Charles River Laboratory (Wilmington, MA) and Jackson Laboratories (Jackson, MA). Luciferase-expressing (luc+) FVB mice are derived from transgenic founder line FVB-L2G85.18 Rag2−/− gamma chain (γC)−/− mice on the Balb/c background were obtained from I. Weissman, Stanford University. Mice were used between 8 to 16 weeks of age. Animal protocols were approved by the Institutional Animal Care and Use Committee at Stanford University.
Flow cytometric analysis
Antibodies were purchased from BD Pharmingen (San Diego, CA) and eBiosciences (San Diego, CA): CD4(RM4-5), CD8α (53-6.7), CD25(PC61), CD45/B220(RA3-6B2), CD62L(MEL14), H-2Kq(KH114), H-2Dq(KH117), H-2Dd (34-2-12), NK1.1(PK136), Thy1.1(H1S51), Thy1.2 (53-2.1), FoxP3(FJK-16), and appropriate isotype controls. Antibody-conjugated microbeads (CD4, CD8, and PE) were obtained from Miltenyi Biotec (Auburn, CA). Intranuclear staining of Foxp3 was performed using PE or FITC antimouse/rat Foxp3 Staining Set from eBiosciences.
Cell isolation
T-cell subsets were isolated from the spleen and the lymph nodes of FVB mice. For CD25+ cells, single-cell suspension was enriched with anti–CD25-PE– and anti-PE–conjugated magnetic beads with AutoMACS (Miltenyi Biotec). Enriched CD25+ population was stained with anti–CD4-APC and sorted on the MoFlo sorter (Cytomation, Fort Collins, CO) to a purity of 98% to 99% for CD4+CD25hi cells. CD4+ and CD8+ cells were isolated with CD4 and CD8 microbeads, respectively, using AutoMACS to achieve a purity of 94% to 97%. Bone marrow was harvested and single-cell suspension was prepared as previously described.19 T cells were depleted with CD4+ and CD8+ microbeads using LS columns or AutoMACS (Miltenyi Biotech), with more than 99% purity.
Transplant model to evaluate GvHD and graft-versus-tumor (GvT)
Wild-type Balb/c and syngeneic FVB transplant recipients were lethally irradiated with 8 Gy and 9 Gy TBI (200 Kv X-ray source), given in 2 split doses of 4 Gy and 4.5 Gy, respectively. Within 24 hours of irradiation, FVB donor cells were injected into recipient mice via the tail vein: T-cell–depleted bone marrow cells (TCD-BMs; 5 × 106) with or without conventional CD4+ and CD8+ T cells (Tcons; 1.0 × 106) and/or Tregs (1.0 × 106) were used for trafficking experiments; and Tcons (1.0 × 106) and/or Tregs (1 × 105 to 1 × 106) were given at different time points in other experiments as described under “Early infusion of lower Treg numbers is sufficient for GVHD suppression” and in Figures 6 and 7. Rag2−/−γC−/− recipients received similar doses of TCD-BMs, Tcons, and Tregs, as described in Figure 3, and did not undergo irradiation. In the tumor model, A20-luc/yfp B-cell leukemia (1 × 104) was administered intravenously on day 0 of transplantation. The luciferase- and ypf-expressing A20 tumor cell line in the Balb/c background was generated as previously described.20 Bioluminescence imaging methodology has been previously described.20 Imaging was performed using an IVIS200 charged coupled device (CCD) (Xenogen, Alameda, CA). Data were analyzed with Living Image software (Xenogen) and IgorProCarbon (Wavemetrics, Lake Oswego, OR).
Initial dose titration experiments were performed to determine the number of Tcons necessary to induce lethal GvHD. At a 1 × 106 cell number, FVB-derived Tcons caused significant GvHD in lethally-irradiated Balb/c recipients and led to a median survival of 40 to 45 days, a duration of time sufficient to evaluate the trafficking and survival of Tregs in vivo and to assess the role of Tregs during the various phases of GvHD. Tregs were coadministered at a 1:1 ratio with Tcons based on previous in vitro and in vivo studies,19,21 which showed that this dose ratio provided the greatest protection from GvHD. At a 1:1 ratio, Tregs similarly protected recipients from GvHD induced by higher doses of Tcons (ie, 1.2-1.5 × 106). However, the lower dose of 1 × 106 was chosen based on the rarity of Tregs.
Animals that underwent transplantation were given antibiotic water (sulfamethoxazole-trimethropim; Schein Pharmaceutical, Corona, CA) for the first 30 days. Animals were monitored and weighed daily for the first 30 days, and 2 to 3 times per week thereafter. Clinical evidence of GvHD was evaluated and scored as previously described.22 A20 residual disease was assessed on single-cell suspension of bone marrow and spleen using flow cytometry based on yfp expression.
Real-time quantitative RT-PCR assay
Total RNA was extracted from sorted CD4+CD25hi, CD4+CD25lo, CD4+CD25− cells with the RNAeasy Mini-kit (QIAGEN, Valencia, CA). cDNA was generated as a template for real-time reverse-transcription–polymerase chain reaction (RT-PCR) using iScript cDNA kit (Biorad Labs, Hercules, CA). Expression of FoxP3 was measured using the following primers: reverse, 5′-GTCGATCATGGCTGGGTTGT-3′ and forward, 5′ GGCCCTTCTCCAGGACAGA-3′; and an internal TaqMan probe: 5-/56-FAM/ACTTCATGCATCAGCTCTCCACTGTGGAT/3BHQ-1/-3′. Data are expressed as normalized Foxp3 expression, which was obtained by dividing the relative quantity of Foxp3 for each sample by the relative quantity of mouse cyclophilin or 18S rRNA for the same sample.
Histopathology
Tissues were collected 8 days after transplantation and fixed in 10% formalin. Paraffin sections of 4- to 5-μm thickness were stained with hematoxylin and eosin using standard methods. Stained tissue sections were evaluated by Nikon microscope (Eclipse 300; Nikon, Melville, NY). Standard magnification was 200×/0.45 NA and 400×/0.06 NA. Photos were obtained with a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI).
Statistical analysis
The log-rank test was used to compare groups in Kaplan-Meier survival analysis. For comparison of absolute cell counts between experimental groups, the natural logarithm of each measure was used, comparing the geometric means. An analysis of variance was first performed to demonstrate statistically significant differences among experimental groups at the .05 level. The 2-tailed unpaired t test was subsequently used to compare geometric means of each pair, with their nominal P values reported. To adjust for multiple comparisons using the same control in each of the experiments, the Dunnett test was used. For groups demonstrated to be significantly different from the control group, the 2-tailed unpaired t test was then used, and their nominal P values are reported. For comparison of 2 groups with measures equal to 0, the rank sum (or Mann-Whitney) test was used.
Results
Early proliferation and localization of Tregs in vivo
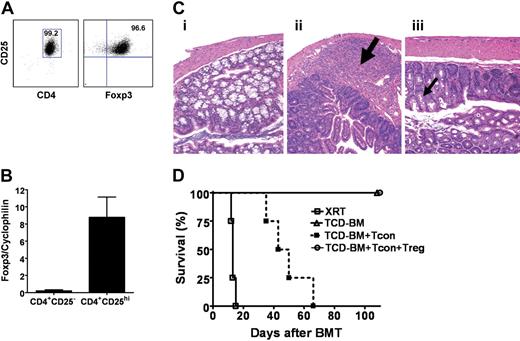
We purified CD4+CD25hi (Tregs) (> 99%) from transgenic donor mice (FVB, H2q) that constitutively expressed luciferase (luc+)18 and showed expression of Foxp3 by quantitative RT-PCR and flow cytometry (> 96%) (Figure 1A-B). Luc+ transgenic splenocytes are functionally comparable with their wt counterparts.23 In a major-MHC mismatched BMT model, Balb/c (H2d) recipients were lethally irradiated and reconstituted with 5 × 106 FVB TCD-BMs; these mice were healthy and survived without GvHD (Figure 1C-D). To induce acute GvHD, 1.0 × 106 Tcons were coinfused with TCD-BMs on day 0 of BM transplantation. The median survival of these animals was 40 to 45 days, permitting longitudinal trafficking of T cells in vivo. Coinfusion of luc+ Tregs at a 1:1 ratio with wt Tcons reduced GvHD incidence, severity, and mortality (Figure 1C-D), in parallel with a significant reduction of Tcon proliferation in both lymphoid and nonlymphoid tissues as measured by whole body bioluminescence imaging (BLI) (data not shown).
Characterization of the phenotype and function of Tregs. (A) CD4+CD25hi cells from either wild-type FVB or luciferase-transgenic FVB mice were sorted to purities of more than 99%, with more than 96% expressing Foxp3 on FACS analysis. (B) By quantitative RT-PCR, sorted CD4+CD25hi cells had significantly greater Foxp3 expression compared with CD4+CD25− cells (P = .03). Error bars represent standard error. (C) Histopathologic findings in the colon of lethally-irradiated recipients 8 days after transplantation of T-cell–depleted bone marrow alone (i), with Tcons (physiologic ratio of CD4+ and CD8+ T cells) (ii), or with Tcons and CD4+CD25hi (iii). Goblet-cell (thin black arrow) depletion, lymphocytic infiltration (thick black arrow), and mucosal disruption in colon of animals with Tcons but without CD4+CD25hi. (D) Lethal GvHD induced by FVB Tcons (▪) in Balb/c recipients was inhibited with the cotransfer of FVB Tregs (○) at 1:1 dose ratio (n = 4; P = .007). Recipients receiving only TCD-BMs (▵; n = 4) survived without evidence of GvHD. Data are representative of 3 or more experiments.
Characterization of the phenotype and function of Tregs. (A) CD4+CD25hi cells from either wild-type FVB or luciferase-transgenic FVB mice were sorted to purities of more than 99%, with more than 96% expressing Foxp3 on FACS analysis. (B) By quantitative RT-PCR, sorted CD4+CD25hi cells had significantly greater Foxp3 expression compared with CD4+CD25− cells (P = .03). Error bars represent standard error. (C) Histopathologic findings in the colon of lethally-irradiated recipients 8 days after transplantation of T-cell–depleted bone marrow alone (i), with Tcons (physiologic ratio of CD4+ and CD8+ T cells) (ii), or with Tcons and CD4+CD25hi (iii). Goblet-cell (thin black arrow) depletion, lymphocytic infiltration (thick black arrow), and mucosal disruption in colon of animals with Tcons but without CD4+CD25hi. (D) Lethal GvHD induced by FVB Tcons (▪) in Balb/c recipients was inhibited with the cotransfer of FVB Tregs (○) at 1:1 dose ratio (n = 4; P = .007). Recipients receiving only TCD-BMs (▵; n = 4) survived without evidence of GvHD. Data are representative of 3 or more experiments.
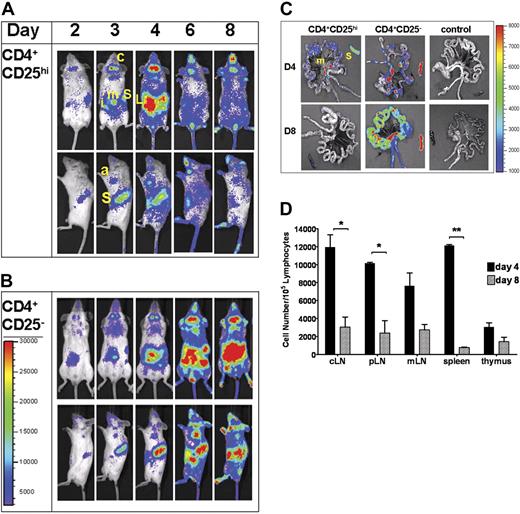
Treg trafficking was evaluated in this BMT model. Luc+ Tregs and wt Tcons were cotransferred at a 1:1 ratio of 1.0 × 106 cells/animal. BLI revealed dramatic early expansion of Tregs in secondary lymphoid organs (Figure 2A). Within the first 24 to 48 hours after transfer, donor luc+ Tregs localized to cervical and peripheral LNs and the spleen. Signal intensity significantly increased and peaked on day 4, consistent with Treg migration to and proliferation within these secondary lymphoid organs. Donor luc+ CD4+CD25− T cells had important similarities and differences in early localization and proliferation when transplanted into allogeneic BMT recipients (Figure 2B). Like Tregs, robust expansion of Tcons occurred in lymphoid organs followed by infiltration of peripheral tissues on day 4. However, unlike Tregs, CD4+CD25− T cells continued to persist and proliferate in lymphoid organs, while simultaneously increasing in number and localization to peripheral tissues such as the skin, liver, and gastrointestinal tract, all targets of GvHD (Figure 2A-C), corresponding to the clinical development of GvHD. Concurrent transfer of wt Tregs significantly reduced the expansion of alloreactive luc+ CD4+CD25− T cells in both lymphoid and nonlymphoid tissues without affecting their homing pattern (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article).
Early proliferation and localization of Tregs. Trafficking of allogeneic luciferase (luc+)–expressing CD4+CD25hi (Tregs) (A) or CD4+CD25− (B) cells at early time points following their adoptive cotransfer with donor wild-type CD4+CD25− T cells and TCD-BMs (A) or TCD-BMs alone (B) into lethally-irradiated Balb/c hosts. Animals were imaged daily for the first week, then every other day thereafter. Images shown were chosen on specific days that indicate change in the trafficking pattern or signal intensity. Data show ventral and left lateral images of a single animal, representative of at least 3 animals per group per experiment. Data are representative of 3 independent experiments. S indicates spleen; c, cervical lymph node; i, inguinal LN; m, mesenteric LN; a, axillary LN; T, thymus; and L, liver. (C) Day-4 and day-8 ex vivo imaging of gut and spleen in animals that received luc+ CD4+CD25hi or CD4+CD25− cells. S indicates spleen; m, mesenteric LN. Control tissues (far right) are from animals without luc+ cells. Color bar represents signal intensity code over whole-body surface or organs in photons per second. (D) FACS analysis of absolute numbers of donor (H2q) CD4+Foxp3+ cells in lymphoid tissues following transplantation showed significant reduction of cells from day 4 to day 8 in secondary lymphoid organs (*P < .05; **P < .001). One of 2 independent experiments with similar results is shown. Color bars represent signal intensity scale over whole body: (A-B) 3000 to 30 000; (C) 1000 to 8000. Error bars represent standard error of mean value.
Early proliferation and localization of Tregs. Trafficking of allogeneic luciferase (luc+)–expressing CD4+CD25hi (Tregs) (A) or CD4+CD25− (B) cells at early time points following their adoptive cotransfer with donor wild-type CD4+CD25− T cells and TCD-BMs (A) or TCD-BMs alone (B) into lethally-irradiated Balb/c hosts. Animals were imaged daily for the first week, then every other day thereafter. Images shown were chosen on specific days that indicate change in the trafficking pattern or signal intensity. Data show ventral and left lateral images of a single animal, representative of at least 3 animals per group per experiment. Data are representative of 3 independent experiments. S indicates spleen; c, cervical lymph node; i, inguinal LN; m, mesenteric LN; a, axillary LN; T, thymus; and L, liver. (C) Day-4 and day-8 ex vivo imaging of gut and spleen in animals that received luc+ CD4+CD25hi or CD4+CD25− cells. S indicates spleen; m, mesenteric LN. Control tissues (far right) are from animals without luc+ cells. Color bar represents signal intensity code over whole-body surface or organs in photons per second. (D) FACS analysis of absolute numbers of donor (H2q) CD4+Foxp3+ cells in lymphoid tissues following transplantation showed significant reduction of cells from day 4 to day 8 in secondary lymphoid organs (*P < .05; **P < .001). One of 2 independent experiments with similar results is shown. Color bars represent signal intensity scale over whole body: (A-B) 3000 to 30 000; (C) 1000 to 8000. Error bars represent standard error of mean value.
The early abdominal signal, corresponding to Treg localization and proliferation in the mesenteric LNs as assessed by ex vivo imaging of the gut (Figure 2C), was detected within 48 hours after transplantation and peaked on day 4. Treg infiltration of the liver and the gut was detected on day 4 (Figure 2A,C) followed by skin infiltration—as indicated by signals from the ears, paws, and tail, between days 5 and 6. However, dramatic differences in infiltration of the gut parenchyma were observed on day 8 between CD4+CD25hi and CD4+CD25− T cells (Figure 2C). Increased peripheral tissue signals coincided with decreased signal intensity in lymphoid organs, consistent with Treg migration out of lymphoid tissues following initial proliferation. Reduction of CD4+Foxp3+ cells in representative lymphoid organs on day 8 compared with day 4 corroborated BLI observations of Treg movement out of lymphoid organs (Figure 2D).
Allogeneic environment and tissue damage provide stimuli for Treg proliferation
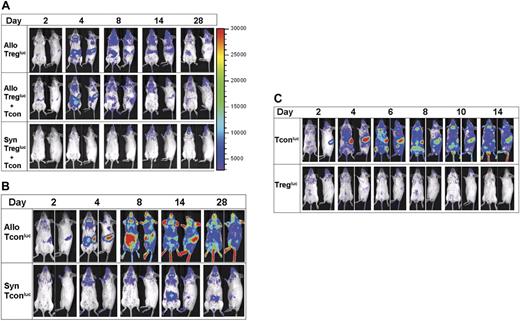
To explore stimuli required for Treg proliferation, we evaluated the role of the allogeneic environment by transferring FVB luc+ Tregs into syngeneic recipients. Unlike the allogeneic setting where Tregs expand in the presence or absence of wt Tcons (Figure 3A), adoptively transferred luc+ Tregs were not detected in secondary lymphoid organs of syngeneic BMT recipients by BLI (Figure 3A), and signal intensity was not significantly different from background levels in control animals without luc+ T cells (data not shown). Host lymphopenia typically develops within 7 to 10 days following total body irradiation (V.H.N., unpublished data, March 2005), providing a niche for homeostatic peripheral proliferation of adoptively transferred T cells. In our model, there was no evidence of homeostatic expansion of syngeneic Tregs due to lymphopenia for up to 4 weeks following transplantation (Figure 3A). Syngeneic luc+ Tcons had significantly different in vivo dynamics. Expansion was evident but moderate and delayed by up to 4 days after transfer, detected first in the cervical LNs and subsequently in other LNs. Proliferation was not detected in the spleen, unlike their allogeneic Tcon counterparts (Figure 3B), nor in the bone marrow compartments, as seen in lymphopenic models. Our findings indicate that the allogeneic environment alone or with conditioning, is critical for early Treg expansion and localization in secondary lymphoid organs.
Allogeneic environment required for Treg proliferation and localization. Adoptive transfer of FVB luc+ Tregs with or without cotransfer of Tcons at a 1:1 cell ratio (A) or Tcons (B) into lethally-irradiated allogeneic Balb/c or syngeneic FVB hosts. Representative bioluminescent images that indicate changes in trafficking or signal intensity up to 4 weeks following transplantation are shown. Treg proliferation and localization were not observed or detected in syngeneic hosts. No significant differences in homing or signal intensity were noted for Treg proliferation in the presence or absence of Tcons. Delayed and moderate expansion of Tcons in lymph nodes was detected in syngeneic hosts. (C) Rag2−/−γC−/− unirradiated hosts received allogeneic luc+ Tregs or Tcons at transplantation. Images of a single animal are shown, which is representative of at least 3 animals per group per experiment. All experiments were performed at least twice. Color bars represent signal intensity scale over whole body: 3000 to 30 000.
Allogeneic environment required for Treg proliferation and localization. Adoptive transfer of FVB luc+ Tregs with or without cotransfer of Tcons at a 1:1 cell ratio (A) or Tcons (B) into lethally-irradiated allogeneic Balb/c or syngeneic FVB hosts. Representative bioluminescent images that indicate changes in trafficking or signal intensity up to 4 weeks following transplantation are shown. Treg proliferation and localization were not observed or detected in syngeneic hosts. No significant differences in homing or signal intensity were noted for Treg proliferation in the presence or absence of Tcons. Delayed and moderate expansion of Tcons in lymph nodes was detected in syngeneic hosts. (C) Rag2−/−γC−/− unirradiated hosts received allogeneic luc+ Tregs or Tcons at transplantation. Images of a single animal are shown, which is representative of at least 3 animals per group per experiment. All experiments were performed at least twice. Color bars represent signal intensity scale over whole body: 3000 to 30 000.
Conditioning with lethal irradiation causes significant tissue injury, resulting in the activation of host cells, release of proinflammatory cytokines,24 and an increase in the expression of adhesion, costimulatory, and MHC proteins.25,26 To assess the role of conditioning in Treg in vivo dynamics in the allogeneic setting, we transferred luc+ Treg or luc+ Tcon control into unirradiated Balb/c Rag2−/−gamma-chain (γC)−/− recipients, which lack T, B, and natural killer (NK) cells. After transfer, robust Tcon proliferation was observed; however, Treg expansion was weakly detectable in Rag−/−γC−/− recipients, without specific localization to lymphoid or nonlymphoid tissues (Figure 3C). In Rag−/−γC−/− recipients that were irradiated, adoptively transferred Tregs proliferated in secondary lymphoid tissues initially prior to localization to peripheral tissues such as the skin (data not shown). These findings suggest that damaged host tissue resulting from conditioning with irradiation likely provided stimulation for host APC activation, which, together with alloantigens, promotes Treg expansion in vivo.
Long-term survival of Tregs in vivo correlates with persistent protection
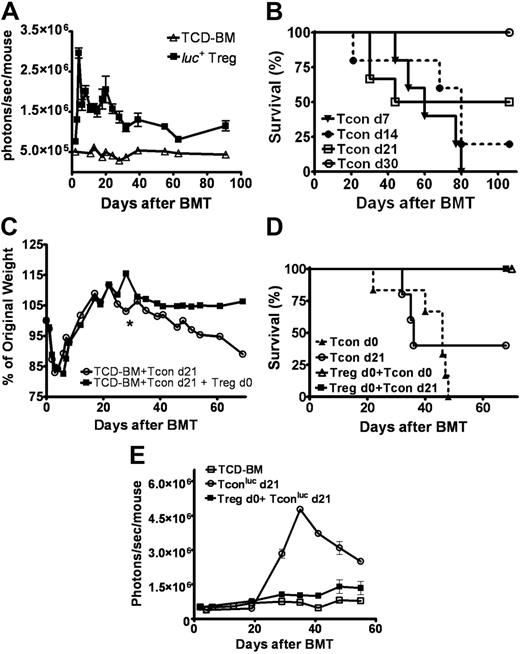
Transplant recipients of luc+ Tregs had a persistent and stable bioluminescent signal in secondary lymphoid organs and the skin (Figure S2) lasting up to 90 days after adoptive transfer (Figure 4A). We asked whether the protracted presence of the same Treg subset in vivo could protect recipients from delayed induction of GvHD. Based on findings that GvHD can be induced in animals receiving Tcons up to 21 days (Figure 4B), luc+ Tcons were adoptively transferred at 1, 2, or 3 weeks after BM transplantation into Balb/c hosts receiving Tregs on day 0. Recipients of Tregs on day 0 were protected from GvHD (Figure 4C), leading to a 100% survival for all 3 time points evaluated (Figure 4D). In contrast, animals not infused with Tregs on day 0 and challenged with Tcons up to 3 weeks following BM transplantation had a significant decreased survival due to GvHD (Figure 4D). BLI of luc+ Tcons in animals receiving Tregs on day 0 showed a significant signal reduction consistent with decreased Tcon proliferation, supporting our findings that Tregs survive long term in vivo and maintain their suppressor function (Figure 4E).
Prolonged survival of Tregs in vivo correlates with persistent protection. (A) Bioluminescent imaging of donor allogeneic luc+ Tregs in lethally irradiated Balb/c host (▪, n = 4) showed persistent and stable signal over a 3-month period following transplantation compared with control animals that received only TCD-BMs and no luc+ cells (▵, n = 4) (P < .001, d91). Data are representative of 2 separate experiments. (B) Lethally-irradiated Balb/c recipients were challenged with Tcons at various time points to induce GvHD (d7 ▾, n = 5; d14 •, n = 5; d21 □, n = 6; d30 ○, n = 6). By day 30, no evidence of GvHD was noted. Data represent 1 of 2 independent experiments. (C-D) When transplant recipients received Tregs on day 0, they were protected from GvHD with the infusion of Tcons on days 7, 14, and 21 (data shown for day 21). Average weight change (indicator of GvHD; *2 animals remain alive after day 39) (C) and survival (D) shown for animals that underwent transplantation with (▪, n = 6) or without transferred Tregs on day 0 (○, n = 5) and challenged with Tcons on day 21 (P < .05). Animals that received Tcons with (▵, n = 6) or without (▴, n = 6) Tregs on day 0 were controls (P < .001). Data were combined from 2 independent experiments. (E) Protection from delayed GvHD induction was associated with the reduction of Tcon proliferation by Tregs infused on day 0 of transplantation (▪, n = 3) compared with animals that received only Tcons on day 21 (○, n = 5) (P = .001, day 29; P < .001, day 35). Data are representative of 2 independent experiments. Error bars represent standard error of the mean.
Prolonged survival of Tregs in vivo correlates with persistent protection. (A) Bioluminescent imaging of donor allogeneic luc+ Tregs in lethally irradiated Balb/c host (▪, n = 4) showed persistent and stable signal over a 3-month period following transplantation compared with control animals that received only TCD-BMs and no luc+ cells (▵, n = 4) (P < .001, d91). Data are representative of 2 separate experiments. (B) Lethally-irradiated Balb/c recipients were challenged with Tcons at various time points to induce GvHD (d7 ▾, n = 5; d14 •, n = 5; d21 □, n = 6; d30 ○, n = 6). By day 30, no evidence of GvHD was noted. Data represent 1 of 2 independent experiments. (C-D) When transplant recipients received Tregs on day 0, they were protected from GvHD with the infusion of Tcons on days 7, 14, and 21 (data shown for day 21). Average weight change (indicator of GvHD; *2 animals remain alive after day 39) (C) and survival (D) shown for animals that underwent transplantation with (▪, n = 6) or without transferred Tregs on day 0 (○, n = 5) and challenged with Tcons on day 21 (P < .05). Animals that received Tcons with (▵, n = 6) or without (▴, n = 6) Tregs on day 0 were controls (P < .001). Data were combined from 2 independent experiments. (E) Protection from delayed GvHD induction was associated with the reduction of Tcon proliferation by Tregs infused on day 0 of transplantation (▪, n = 3) compared with animals that received only Tcons on day 21 (○, n = 5) (P = .001, day 29; P < .001, day 35). Data are representative of 2 independent experiments. Error bars represent standard error of the mean.
Tregs impact various phases of inflammation
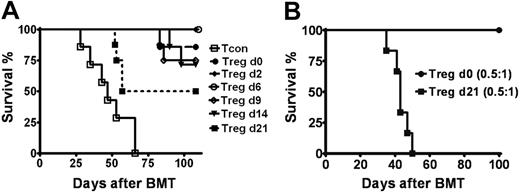
Murine studies suggest that GvHD proceeds through several overlapping phases.27 To assess during which phase(s) of inflammation Tregs mediate protection from GvHD, we transferred Tregs at various time points following BM transplantation into Balb/c recipients receiving luc+ Tcons on day 0. There was a significant reduction in GvHD-related mortality when Tregs and Tcons were given concurrently on day 0 of BM transplantation, compared with animals not receiving Tregs (Figure 1D). During the first week following BM transplantation, tissue damage from conditioning with irradiation and the afferent phase of GvHD involving Tcon activation and early proliferation occur. When administered during this period, Tregs protected nearly all transplant recipients from GvHD (Figure 5A). With increasing Tcon expansion and the onset of effector responses, Treg infusion on day 9 or day 14 led to a moderate decrease of ongoing Tcon proliferation (data not shown). Mortality from GvHD was 25% and 28% in animals that received Tregs at day 9 and day 14, respectively (Figure 5A). Death occurred after day 80 following BM transplantation due to a less severe course of GvHD, compared with a median survival of 47 days in animals receiving no Tregs. By 3 weeks after BM transplantation, the effector phase amplified and most recipients developed clinical signs of GvHD. The addition of Tregs during this period still reduced the morbidity and mortality of GvHD (P = .024), however at a significantly lower rate (Figure 5A). The observed benefit of Treg transfer during the course of acute GvHD is dose dependent and may be correlated with the absolute numbers of Tcons present. In early phases of inflammation, lower Treg numbers were required to protect from GvHD (Figure 5B). However, when clinical GvHD developed in parallel with an increasing BLI signal representing increasing Tcon numbers (data not shown), a higher number of Tregs was required to provide protection (Figure 5B).
Tregs impact multiple phases of GvHD. (A) Survival is shown for Balb/c hosts that received Tregs at various time points at or after transplantation of Tcons (cell ratio of 1:1) and TCD-BMs on day 0 (P < .001 for Tcons vs Tcons + Tregs on d2, d6, d9, or d14; P < .05 for Tcons vs Tcons + Tregs on d21; n ≥ 7). Data are combined from 2 independent experiments. (B) Survival of animals that received donor Tcons on day 0 for GvHD induction and Tregs on day 0 (•, n = 7) versus day 21 (▪, n = 6) at cell ratio of 0.5:1 with Tcons (P < .001). Survival data showed protection by Tregs is dose dependent at later phases of GvHD. Data are combined from 2 independent experiments.
Tregs impact multiple phases of GvHD. (A) Survival is shown for Balb/c hosts that received Tregs at various time points at or after transplantation of Tcons (cell ratio of 1:1) and TCD-BMs on day 0 (P < .001 for Tcons vs Tcons + Tregs on d2, d6, d9, or d14; P < .05 for Tcons vs Tcons + Tregs on d21; n ≥ 7). Data are combined from 2 independent experiments. (B) Survival of animals that received donor Tcons on day 0 for GvHD induction and Tregs on day 0 (•, n = 7) versus day 21 (▪, n = 6) at cell ratio of 0.5:1 with Tcons (P < .001). Survival data showed protection by Tregs is dose dependent at later phases of GvHD. Data are combined from 2 independent experiments.
Early infusion of lower Treg numbers is sufficient for GvHD suppression
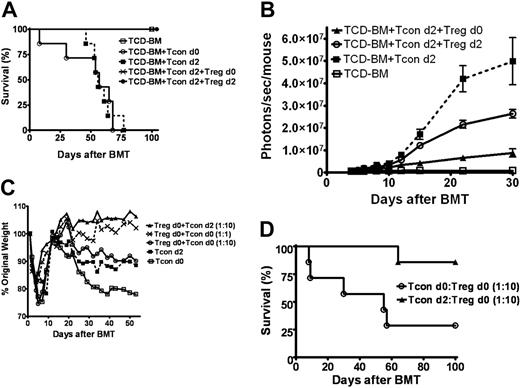
The finding that Tregs proliferated in an allogeneic environment without the requirement of donor Tcons led to the hypothesis that adoptive transfer of lower Treg numbers prior to GvHD induction may provide effective suppression. First, we evaluated the impact of early Treg infusion at a 1:1 dose ratio with Tcons. GvHD was lethal when luc+ Tcons (1 × 106) were transferred on day 0 or day 2 following BM transplantation (Figure 6A). When Tregs were infused on day 0 or concurrently with Tcons on day 2, signal intensity was decreased in all animals (Figure 6B). However, the reduction was significantly greater when Tregs were given prior to GvHD induction. Studies in which Tregs were transferred up to 10 days prior to the infusion of Tcons showed similar results (data not shown). We next evaluated the impact of early infusion of significantly lower Treg numbers on GvHD induction. When Tregs were given 2 days prior to Tcon transfer, a 10-fold dose reduction in Treg numbers was sufficient to reduce Tcon proliferation and protect from GvHD (Figure 6C-D).
Early infusion of lower Treg numbers is sufficient for GvHD control and suppresses Tcon proliferation. (A) Induction of GVHD was complete and lethal when allogeneic FVB donor Tcons were infused on day 0 (○) or 2 (▪) following transplantation of TCD-BMs into lethally-irradiated Balb/c hosts (○ versus ▪, NS) compared with complete survival of animals that received Tregs on day 0 (X) or 2 (•) (▪ versus X or •, P < .001) (combined data of 2 independent experiments, n = 7). (B) When Tregs were infused on day 0 or concurrently with luc+ Tcons on day 2 at a 1:1 ratio, a reduction of Tcon proliferation was observed and was more significant with earlier infusion of Tregs on day 0 (▴, n = 4) compared with day 2 (○, n = 4) (▪ versus ▴, P = .001; ▪ versus ○, P = .03). Animals that received a transplant with only TCD-BMs (□, n = 4) were controls for background bioluminescence. Data represent 2 independent experiments. Dose ratio titrations of Tregs/Tcons of 1:1, 1:2 (data not shown), and 1:10 were evaluated (C-D). A 10-fold dose reduction protected transplant recipients from GvHD as demonstrated by average weight change (C) and GvHD-related survival (D) (P = .02 for ▴ versus ○, n = 7). Data were combined from 2 independent experiments. Error bars represent standard error of the mean.
Early infusion of lower Treg numbers is sufficient for GvHD control and suppresses Tcon proliferation. (A) Induction of GVHD was complete and lethal when allogeneic FVB donor Tcons were infused on day 0 (○) or 2 (▪) following transplantation of TCD-BMs into lethally-irradiated Balb/c hosts (○ versus ▪, NS) compared with complete survival of animals that received Tregs on day 0 (X) or 2 (•) (▪ versus X or •, P < .001) (combined data of 2 independent experiments, n = 7). (B) When Tregs were infused on day 0 or concurrently with luc+ Tcons on day 2 at a 1:1 ratio, a reduction of Tcon proliferation was observed and was more significant with earlier infusion of Tregs on day 0 (▴, n = 4) compared with day 2 (○, n = 4) (▪ versus ▴, P = .001; ▪ versus ○, P = .03). Animals that received a transplant with only TCD-BMs (□, n = 4) were controls for background bioluminescence. Data represent 2 independent experiments. Dose ratio titrations of Tregs/Tcons of 1:1, 1:2 (data not shown), and 1:10 were evaluated (C-D). A 10-fold dose reduction protected transplant recipients from GvHD as demonstrated by average weight change (C) and GvHD-related survival (D) (P = .02 for ▴ versus ○, n = 7). Data were combined from 2 independent experiments. Error bars represent standard error of the mean.
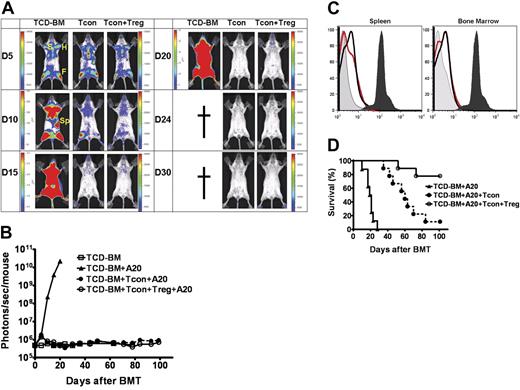
A potential concern regarding rapid Treg expansion in our model, particularly when injected prior to GvHD induction, was the significant reduction in Tcon numbers and their functionality. Prior studies from our lab provided evidence for a threshold effect whereby a small but sufficient number of Tcons was crucial to exert a GvT effect rather than a more robust alloreactive response from large numbers of Tcons that are required to induce a systemic disease such as acute GvHD.21 To evaluate the GvT effect with early infusion of Tregs, we transferred 1 × 105 Tregs from wt FVB and 1 × 104luc+/yfp A20 leukemia cells intravenously into lethally-irradiated Balb/c hosts on day 0 of BM transplantation. Tcons, at a 10:1 dose ratio with Tregs, were transferred 2 days following transplantation. Tumor engraftment and expansion, assessed by BLI of luc+ A20, were confirmed in recipients prior to Tcon infusion (data not shown). In transplant recipients that received TCD-BMs and luc+ A20, rapid leukemic-cell proliferation and death were observed at a median time of 19 days. Animals that received Tcons on day 2 cleared the leukemia, but later died from GvHD. The infusion of Tregs on day 0 of BM transplantation protected animals from GvHD while permitting Tcons to clear the leukemia (Figure 7A-B,D). Fluorescence-activated cell sorting (FACS) analysis for yfp+-expressing A20 cells in the bone marrow and spleen of surviving animals showed no residual disease or relapse of leukemia at least 120 days after BM transplantation (Figure 7D).
Earlier infusion of Tregs does not abrogate Tcon-mediated GvT effect. Lethally-irradiated Balb/c hosts received TCD-BMs and luc/yfp A20 leukemia cells, with or without, 1 × 105 Tregs from wt FVB, on day 0 followed by infusion of 1 × 106 Tcons 2 days later. Animals that received TCD-BMs and A20 cells alone served as controls. (A) Tumor engraftment is noted in bone marrow compartments (F indicates femur; H, humerus; S, sternum) and spleen (Sp) in all groups. Tumor progression is observed in animals that received TCD-BMs alone, while animals that received Tcons with or without Tregs cleared tumor by day 15. (B) Tumor clearance was stable, with no evidence of relapse with prolonged follow up by BLI at day 100 (□ versus • or □ versus ○, NS). Color bars in panel A represent signal intensity scale over whole body: (B) 3000 to 30 000 for all images of Tcons + A20 and Tcons + Tregs + A20, and day-5 image of TCD-BMs + A20; and 3 × 104 to 3 × 105, 3 × 105 to 3 × 106, and 3 × 106 to 3 × 107 for days 10, 15, and 20 images of TCD-BMs + A20 and (C) FACS analysis for yfp-expressing A20 cells in the bone marrow and splenic compartment 120 days following transplantation (dark gray fill is A20 cell positive control; light gray fill is BM negative control; black line is Tregs + Tcons + A20; red line is Tcons + A20). (D) Survival corresponded with BLI findings. Animals that received only TCD-BMs died from progressive tumor growth (n = 8); animals that also received Tcons (n = 9) cleared the tumor but died from progressive GvHD (▴ versus •, P < .001). Transplant recipients that received earlier infusion of low-dose Tregs (n = 9) were protected from leukemia (▴ versus ○, P < .001) and had significantly improved survival from GvHD (○ versus •, P = .004). Data were combined from 3 independent experiments.
Earlier infusion of Tregs does not abrogate Tcon-mediated GvT effect. Lethally-irradiated Balb/c hosts received TCD-BMs and luc/yfp A20 leukemia cells, with or without, 1 × 105 Tregs from wt FVB, on day 0 followed by infusion of 1 × 106 Tcons 2 days later. Animals that received TCD-BMs and A20 cells alone served as controls. (A) Tumor engraftment is noted in bone marrow compartments (F indicates femur; H, humerus; S, sternum) and spleen (Sp) in all groups. Tumor progression is observed in animals that received TCD-BMs alone, while animals that received Tcons with or without Tregs cleared tumor by day 15. (B) Tumor clearance was stable, with no evidence of relapse with prolonged follow up by BLI at day 100 (□ versus • or □ versus ○, NS). Color bars in panel A represent signal intensity scale over whole body: (B) 3000 to 30 000 for all images of Tcons + A20 and Tcons + Tregs + A20, and day-5 image of TCD-BMs + A20; and 3 × 104 to 3 × 105, 3 × 105 to 3 × 106, and 3 × 106 to 3 × 107 for days 10, 15, and 20 images of TCD-BMs + A20 and (C) FACS analysis for yfp-expressing A20 cells in the bone marrow and splenic compartment 120 days following transplantation (dark gray fill is A20 cell positive control; light gray fill is BM negative control; black line is Tregs + Tcons + A20; red line is Tcons + A20). (D) Survival corresponded with BLI findings. Animals that received only TCD-BMs died from progressive tumor growth (n = 8); animals that also received Tcons (n = 9) cleared the tumor but died from progressive GvHD (▴ versus •, P < .001). Transplant recipients that received earlier infusion of low-dose Tregs (n = 9) were protected from leukemia (▴ versus ○, P < .001) and had significantly improved survival from GvHD (○ versus •, P = .004). Data were combined from 3 independent experiments.
Discussion
Analyses of Treg survival and localization previously relied on CFSE or BrDU labeling and flow cytometry, respectively, in selected tissues at specific time points. These studies provided snapshots of in vivo Treg dynamics. With intravital microscopy, recent studies elegantly showed the movement of Tregs within antigen-draining lymph nodes in autoimmune models, providing insights into the cellular interactions among Tregs, naive T cells, and DCs in the local antigenic environment.10,11 However, the timing and sequence of homing events leading to Treg localization to secondary lymphoid organs and/or peripheral tissues and their spatial distribution during an immune response remained unclear.
Here, we noninvasively and longitudinally monitored whole-body in vivo dynamics of luc+ Tregs in an allogeneic and inflammatory setting to determine their proliferative capacity and trafficking pattern and evaluated the functional consequences of timed addition of Tregs relative to Tcons in the induction and progression of GvHD. Our results showed early robust expansion of Tregs in secondary lymphoid organs with sequential migration and localization to peripheral tissues. Alloreactive CD4+CD25− T cells had a similar pattern of early expansion and localization; however, proliferation continued to increase in both lymphoid tissues and GvHD target tissues in parallel with the development of signs of acute GvHD. Our findings suggest that Tregs and Tcons are first primed in secondary lymphoid tissues prior to exerting their suppressor and effector function, respectively. Earlier studies showed that Treg expression of CD62L, a homing marker for migration into secondary lymphoid tissues, was required for protection from GvHD lethality15,16 ; however, it was not determined whether this protective effect was due to the requisite priming of Tregs and/or their suppressor activity in lymphoid tissues. In the presence of Tregs, we found that the initial proliferation of Tcons in secondary lymphoid tissues was significantly reduced, indicating that in addition to being primed and activated in lymphoid tissues, Tregs also exerted suppressor function there. Studies in nonobese diabetic (NOD) mice similarly suggested the importance of Treg-mediated suppression of diabetogenic T cells in pancreatic draining lymph nodes.11,28 Recent findings provided an alternative or additional role of Treg suppression of the diabetogenic T cells only in the insulitic lesion.29 We found Tregs in GvHD target tissues following their early expansion in secondary lymphoid tissues, suggesting that they have effector function in nonlymphoid tissues as well. In mice that received donor Tregs, the Tcon proliferation was significantly decreased in peripheral tissues such as the liver, skin, and gut in parallel with findings of peripheral Tregs at these sites. While these studies do not directly prove active Treg-mediated suppression in peripheral tissue, they suggest an important colocalization of Tregs and Tcons at sites of inflammation. Studies in which Tregs are prevented from either entry into or exit from lymphoid organs or from migrating into effector sites during the development of GvHD are required to elucidate the requisite sites of Treg priming, activation, and suppressor function.
Recent studies have shown differential migration and in vivo suppressive capacity of subsets of Tregs based on the expression of specific homing molecules.12 Expression of molecules such as αEβ714,30 and E/P-selectin31 define subsets of Tregs that migrate to the gut and the skin, respectively, with different suppressive potency. In our model, a heterogenous population of naive Tregs was sorted and adoptively transferred. We observed a pattern of sequential migration of Tregs from secondary lymphoid organs into peripheral tissues, including the skin and the gut. However it is unclear whether Tregs found in the skin or the gut following adoptive transfer represent distinct populations of Tregs that have intrinsic skin or gut tropism or that have acquired specialized homing markers during their priming in secondary lymphoid organs. Future studies will evaluate the temporal expression of homing molecules on Tregs in various tissues following transplantation to better define Treg subsets, and by adoptive transfer of these subsets of Tregs or Tregs deficient in a single or combination of homing molecules to assess their impact on trafficking and clinical outcomes.
Studies of TCR-transgenic Tregs demonstrated localization and proliferation only in draining lymph nodes bearing the antigen and not in irrelevant lymph nodes.28,32 In our allogeneic BMT recipients, Tregs were distributed throughout lymphoid tissues early after transfer including the spleen and the major lymph node regions, a pattern consistent with the systemic pathology of GvHD. Differences between Tcon and Treg expansion in syngeneic or unirradiated allogeneic Rag2−/−γC−/− hosts suggest an important role of conditioning with irradiation alone or in concert with the allogeneic environment, in providing distinct signals for Tcon versus Treg activation, proliferation, and localization. Donor Tcons, which are alloreactive and were presumed to provide stimulatory signals, did not affect the early expansion or localization of Tregs in our allogeneic BMT model, indicating that the allogeneic environment, with host APCs, and the proinflammatory condition induced by irradiation sufficiently provided the required signals such as costimulatory molecules, including CD30L expression41 and IL2, respectively. However, in unirradiated allogeneic Rag2−/−γC−/− hosts, Tregs expanded and localized in secondary lymphoid tissues when Tcons were cotransferred or with exogenous IL2 administration, suggesting that Tcons provided requisite stimulatory signals for Treg expansion, such as IL2 (V.H.N., unpublished data, July 2006), that are inducible by irradiation in the allogeneic setting. Further studies are required to better define other chemokines and cytokines, such as IL15 or IL7,33,34 which may be involved in the expansion, maintenance, and survival of Tregs during an allogeneic immune response.
The robust early expansion of Tregs was followed by a sustained population of functional Tregs that provided extended protection from an inflammatory challenge of alloreactive effector T cells for up to 3 weeks following BM transplantation. Our studies were limited to the first 3 weeks following transplantation, as induction of GvHD was not possible beyond this period due to the development of donor tolerance. The relative stability of Treg signal intensity up to 3 months following BM transplantation likely represented a quiescent survival of Tregs that become functionally active upon encountering inflammatory signals. Previous studies reported the persistence of CFSE-labeled Tregs in vivo in a syngeneic host, but it was not determined whether these Tregs were anergic or were able to provide continued protection in vivo.6
Colocalization of Tregs with Tcons in both lymphoid and nonlymphoid tissues and the reduction of Tcon proliferation at these sites suggest that Tregs likely prevented early proliferation of effector T cells via interaction with DCs in priming sites as recently reported10,11 and/or by inhibiting ongoing expansion of effector T cells in the periphery by an as-yet-unknown mechanism, perhaps involving TGFβ.35–37 Improved survival in recipients of donor Tregs transferred during various phases of GvHD indicated the ability of Tregs to mediate an inflammatory process at multiple levels, including preventing the onset of disease and mediating evolving, and to a lesser extent established, GvHD. Earlier infusion of Tregs led to greater reduction in Tcon proliferation and better survival. This effect, however, is dose dependent, requiring higher doses of Tregs to reverse established inflammation. The potential role of Treg-mediated suppression at all phases of GvHD, with dose dependency in more advanced disease, suggests multiple mechanisms of Treg-mediated suppression that likely involve DCs,38 NK cells,39 B cells,40 and effector T cells,21 and that the balance between Treg and Tcon numbers determines whether a controlled or pathogenic immune response ensues. In the preventative setting, we showed that early administration of Tregs provided the greatest protection from GvHD due to their ability to proliferate in vivo in the absence of Tcon stimuli. Of importance, we determined that a nearly physiologic ratio of Tregs and Tcons (1:10) was sufficient to protect allogeneic BMT recipients from GvHD without abrogating the critical graft-versus-tumor effect if Tregs were administered 2 days prior to Tcons, thereby overcoming a major barrier to the potential clinical application of Tregs given their rarity.
Authorship
Contribution: V.H.N. designed and performed research, analyzed data, and wrote the paper; R.Z., D.L.D., and D.S.C. helped perform research; A.B. contributed vital reagents; C.H.C. contributed vital new reagents and analytic tools and reviewed the data for publication; R.S.N. provided overall research advice and guidance.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert S. Negrin, Center for Clinical Science Research, 269 W Campus Dr, Rm 2205, Division of Bone Marrow Transplantation, Stanford University School of Medicine, Stanford, CA 94305; e-mail: negrs@stanford.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by the National Institutes of Health: R01 CA0800065 and P01 HL075462 (R.S.N.), K08 AI060888 (V.H.N.) and R24 CA92862 (C.H.C.); the American Society of Clinical Oncology Young Investigator Award (V.H.N.); and Dr Mildred-Scheel-Stiftung, Germany (R.Z.).
We thank Leah Schaefer, Neja Talreja, Janelle Olson, Jeanette Baker, and Mobin Karimi for technical assistance; Yuan Cao for contributing new reagents; and Ruby Wong for statistical support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal