Abstract
Blood group O and the cysteine allele of the Y/C1584 change in von Willebrand factor (VWF) are enriched in type 1 VWD, but neither causes disease. We investigated the effect of C1584, alone and in combination with the ABO blood group, on the level and properties of plasma VWF. A cohort of 5052 blood donors was recruited: 50 donors were heterozygous for Y/C1584 and 5002 were homozygous for Y/Y1584. Mean VWF antigen (VWF:Ag) for heterozygotes (82 ± 35 IUdL−1) was significantly lower than for homozygotes (111 ± 37 IUdL−1) (P < .001). For each ABO blood group, VWF:Ag was decreased among Y/C1584 heterozygotes compared with Y/Y1584 homozygotes; a larger decrease was observed for group O. Among donors with VWF:Ag levels of 50 IUdL−1 or lower, Y/C1584 heterozygosity was markedly enriched (18%) compared with the entire cohort (1.5%). Blood group O was enriched to a lesser extent (2.4%), but Y/C1584 in conjunction with group O was strikingly enriched (34.8%). VWF collagen binding activity (VWF:CB) and ristocetin cofactor activity (VWF:RCo) were significantly lower for Y/C1584 heterozygotes than for Y/Y1584 homozygotes, and a qualitative difference in Y/C1584 plasma VWF multimer profile was observed compared with that for Y/Y1584 VWF. The data support a multifactorial basis for low VWF levels in some individuals.
Introduction
Von Willebrand factor (VWF) is essential for normal hemostasis. It carries and protects coagulation factor VIII in the circulation, and it recruits platelets to the site of clot formation during primary hemostasis. VWF cross-links platelets with exposed collagen at a site of vessel damage, and, together with fibrinogen, it cross-links platelets during subsequent platelet aggregation. The efficacy of VWF in primary hemostasis depends on its level and function.
The mechanisms that control plasma VWF level are not understood. Approximately 70% of variability is explained by genetic factors,1 of which the ABO blood group is a major influence.2 The highest levels are associated with group AB and the lowest with group O.3 Genetic factors responsible for the remaining variability are unknown. The VWF gene does not appear to be a major factor,4 though certain single-nucleotide polymorphisms (SNPs) located in the gene promoter correlate with minor differences in plasma VWF levels.5,6 Environmental factors are important determinants of VWF level. Increased levels are associated with age,3 inflammatory disease, stress, and exercise.7
Elucidating the mechanisms that control plasma VWF level is important in understanding the pathogenesis of bleeding and thrombotic diseases. Low VWF levels are associated with bleeding and lead to the hemorrhagic disorder von Willebrand disease (VWD).8 Three subtypes of VWD are defined according to a quantitative deficiency (types 1 and 3) or qualitative deficiency (type 2). Type 1 VWD is the commonest form and is characterized by a partial quantitative deficiency of VWF.8 In contrast, increased VWF levels are associated with risk for ischemic heart disease and myocardial infarction.9
In addition to VWF level, VWF function is important in the context of bleeding. VWF circulates as a series of polymers (multimers) of differing lengths; the longest multimers are the most biologically active.10,11 Multimer length is regulated, at least in part, by the metalloprotease ADAMTS13 (a disintegrin and metalloprotease with thrombospondin repeats) that proteolyzes the Y1605-M1606 peptide bond in the VWF A2 domain.12-15 ADAMTS13 has been intensively investigated in recent years after the discovery that its deficiency underlies thrombotic thrombocytopenic purpura (TTP).15 Although insufficient proteolysis of VWF predisposes to TTP, excessive proteolysis by ADAMTS13 gives rise to bleeding, as seen in some patients with VWD subtype 2A.16-18
Recently, VWF proteolysis has also been implicated as a possible mechanism for bleeding in some type 1 VWD patients. A cohort of unrelated Canadian type 1 VWD patients was screened for a possible VWF gene founder haplotype, and the G allele of the SNP 4751A/G was found to be enriched among patients compared with controls.19 This SNP encodes the amino acid variation Y/C1584 (G = Cys, A = Tyr) in VWF. Independently, VWF heterozygous for Y/C1584 was shown to be more susceptible to proteolysis by ADAMTS13 than homozygous Y/Y1584 VWF, an observation first made in a family with type 1 VWD.20
Subsequent studies have corroborated the enrichment of C1584 in type 1 VWD21,22 and have shown a strict correlation between heterozygosity for Y/C1584 and enhanced VWF proteolysis by ADAMTS13.21 However, C1584 is not, on its own, causative of type 1 VWD. In families in which the variant is present, members who had no bleeding history and normal VWF levels were heterozygous for Y/C1584.21 This observation led to the proposal that C1584 is a risk factor for bleeding and must be coinherited with other predisposing factors for a bleeding diathesis to result.21,23 In 2 cohort studies, more than 90% of patients with type 1 VWD who were heterozygous for Y/C1584 had blood group O19,21 (in contrast, 70% of type 1 VWD patients had blood group O3 ). The reason for this association is unknown.
It is also unknown what effect the variant C1584 has on the level and properties of circulating VWF. Furthermore, an accurate estimate of the population frequency of C1584 has not been reported. In the present study, an accurate estimate of the frequency of C1584 in the local population was obtained by genotyping 5052 blood donors for the 4751A/G SNP. Subsequently, the in vivo level of VWF in the subset of heterozygous Y/C1584 donors was investigated, and relevant properties of VWF from these donors—multimer profile, collagen binding activity (VWF:CB), ristocetin cofactor activity (VWF:RCo), and susceptibility to proteolysis—were determined:. The data show that the C1584 variant changes the properties of VWF and, especially in combination with blood group O, is associated with decreased VWF level.
Materials and methods
Collection of 5052 blood samples from blood donors in South Wales
Blood samples were collected as part of routine blood donation from donors attending Welsh Blood Service (WBS) sessions. The ethnic distribution for this locality is 96% white. Donors gave written, informed consent. The study was approved by the South East Wales Local Research Ethics Committee and the Cardiff and Vale NHS Trust Research and Development Office. Approval did not allow access to clinical information; therefore, it was not possible to obtain clinical details for any blood donor. Women known to be pregnant were excluded from donating blood. All subjects were lying down and rested at the time of blood sampling. Blood (5-10 mL) was collected into sodium citrate (0.105 molL−1) and was kept chilled until it reached the laboratory for separation (typically less than 3 hours).
Sex, date of birth, and ABO blood group were recorded for each donor. The latter was determined at the WBS with an automated blood analyzer (PK7200; Olympus UK, London, United Kingdom).
Preparation of paired samples of plasma plus DNA
Citrated whole blood samples were centrifuged at 3000g for 10 minutes, and the plasma layer was decanted and stored at −70°C in 1-mL aliquots. The buffy coat was decanted and stored frozen (−70°C) until DNA extraction. DNA was extracted using a rapid alkali plus heat lysis procedure.24 Thus, from each blood sample, a set of paired plasma plus DNA samples was obtained.
PCR amplification
Amplification reactions contained DNA (1 μL), dNTP (100 μmolL−1 each), Tris-HCl, pH 8.0 (10 mM−1), MgCl2 (1.5 mmolL−1), Taq DNA polymerase (1 U; Applied Biosystems, Warwickshire, United Kingdom), and primers (0.5 μmolL−1) in a final volume of 25 μL. PCR consisted of 42 cycles at 95°C for 2 minutes followed by 67°C for 2 minutes using a 2720 DNA Thermal Cycler (Applied Biosystems). The upstream primer was 5′-GCG AGA GAT CCG CTA CCA GGG C-3′, and the downstream primer was 5′-ACC ATG TAG ACC AGG TTG GGC G-3′.25
Analysis of the 4751A/G genotype
Genotyping was performed using induced heteroduplex formation with a heteroduplex generator26 (details available on request). Heteroduplexes were analyzed on an ABI3100 Genescanner (Applied Biosystems) with POP4C polymer at 30°C under the standard electrophoretic conditions specified by the supplier.
VWF:Ag
VWF antigen (VWF:Ag) was determined with the STA-LIATEST VWF assay (Diagnostica Stago, Asnieres, France) on a Multichannel Discrete Analyser B3.0 (Biomerieux UK, Basingstoke, United Kingdom). VWF:Ag values greater than 250 IUdL−1 were confirmed with ELISA.27
VWF:CB and VWF:RCo
VWF multimer analysis
VWF proteolysis
Cryoprecipitate was prepared from 500 μL plasma, resuspended in Tris-HCl, pH 8.0 (5 mmolL−1), and assayed for VWF:Ag with the use of ELISA.27 For all incubations, an equal quantity of cryoprecipitated VWF (24 IUdL−1 in reaction) was incubated with an equal volume of cryodepleted plasma as a source of ADAMTS13 in the presence of urea (1.5 molL−1), barium ions (3 mmolL−1), and Tris-HCl, pH 8.0 (mmolL−1), as previously described.20,28 VWF:CB was determined at 0 hours and after 2 hours' incubation at 37°C. Proteolysis was expressed as percentage of loss in VWF:CB (decrease in VWF:CB between 0 hours and 2 hours expressed as a percentage of the VWF:CB at 0 hours).
Statistical analysis
Data were analyzed with the use of Microsoft Excel. Unless otherwise stated, significance levels were calculated with the Student t test.
Results
Frequency of Y/C1584: entire cohort
Heteroduplex analysis reproducibly distinguished the genotypes at the 4751A/G locus. Allele frequencies f(4751A) = 0.995 and f(4751G) = 0.005 (n = 10 104 VWF genes) were obtained, implying that f(Y1584) = 0.995 and f(C1584) = 0.005.
Fifty of the 5052 (0.99%) donors were heterozygous, and these composed the Y/C1584 subgroup in subsequent studies. No donors homozygous for C1584 were detected. Consistent with Hardy-Weinberg equilibrium, the prevalence of heterozygosity for Y/C1584 among men (1.1%) was similar to that among women (0.9%).
Phenotypic data: entire cohort
ABO blood group.
Among the 5052 donors, the frequencies of the ABO blood groups were consistent with those reported previously for a large white cohort (Table 1).3 Frequencies were equivalent in men and women (Table 1).
ABO blood group frequencies and VWF:Ag levels among 5052 blood donors
| ABO blood group . | Cohort . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All donors . | Men . | Women . | |||||||
| No. . | % . | VWF:Ag, IU/dL−1 . | No. . | % . | VWF:Ag, IU/dL−1 . | No. . | % . | VWF:Ag, IU/dL−1 . | |
| O | 2330 | 46 | 94 ± 29 | 1131 | 47 | 98 ± 30 | 1199 | 46 | 91 ± 28 |
| A | 2089 | 41 | 123 ± 37 | 983 | 40 | 126 ± 39 | 1106 | 42 | 121 ± 36 |
| B | 452 | 9 | 130 ± 37 | 233 | 10 | 129 ± 34 | 219 | 8 | 131 ± 40 |
| AB | 181 | 4 | 137 ± 43 | 77 | 3 | 139 ± 35 | 104 | 4 | 135 ± 48 |
| All | 5052 | 100 | 111 ± 37 | 2424 | 100 | 114 ± 37* | 2628 | 100 | 109 ± 37* |
| ABO blood group . | Cohort . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All donors . | Men . | Women . | |||||||
| No. . | % . | VWF:Ag, IU/dL−1 . | No. . | % . | VWF:Ag, IU/dL−1 . | No. . | % . | VWF:Ag, IU/dL−1 . | |
| O | 2330 | 46 | 94 ± 29 | 1131 | 47 | 98 ± 30 | 1199 | 46 | 91 ± 28 |
| A | 2089 | 41 | 123 ± 37 | 983 | 40 | 126 ± 39 | 1106 | 42 | 121 ± 36 |
| B | 452 | 9 | 130 ± 37 | 233 | 10 | 129 ± 34 | 219 | 8 | 131 ± 40 |
| AB | 181 | 4 | 137 ± 43 | 77 | 3 | 139 ± 35 | 104 | 4 | 135 ± 48 |
| All | 5052 | 100 | 111 ± 37 | 2424 | 100 | 114 ± 37* | 2628 | 100 | 109 ± 37* |
VWF:Ag values are expressed as mean ± SD.
VWF:Ag increased according to ABO blood group in the order O < A < B < AB, as previously observed.3 This was evident in men and women. Mean VWF:Ag was statistically significantly higher for men than for women (*P < .001).
VWF:Ag.
The distribution of VWF:Ag was skewed slightly to higher values, and this was corrected by logarithmic transformation. There was a negligible difference between statistical values when comparisons were performed using VWF:Ag or lnVWF:Ag; therefore, VWF:Ag was used for all calculations.
VWF:Ag increased according to ABO blood group in the order O < A < B < AB (P < .001, ANOVA; Table 1), as previously shown.3
Mean VWF:Ag differed significantly between men and women (P < .001; Table 1); however, the absolute difference (approximately 114 IUdL−1 for men vs 109 IUdL−1 for women) was unlikely to be clinically significant (Table 1). A potential (but not the only possible) explanation for the statistical result is that it might have reflected a minor effect of sex that became evident as a result of the analysis of a large number of individuals. As observed in previous studies,3 VWF:Ag showed a progressive increase with age in men and women (data not shown).
Phenotypic data: Y/Y1584 and Y/C1584 donors
ABO blood group.
ABO blood group frequencies were essentially similar to those of the entire cohort, with minor differences for groups A and B among Y/C1584 donors that may be explained by the relatively small numbers involved (Table 2). The distribution of ABO blood groups was comparable for homozygous or heterozygous men and women (data not shown).
ABO blood group frequencies and VWF:Ag level for Y/Y1584 and Y/C1584 donors
| ABO blood group . | Y/Y1584 . | Y/C1584 . | ||||
|---|---|---|---|---|---|---|
| No. . | % . | VWF:Ag IU/dL−1 . | No. . | % . | VWF:Ag IU/dL−1 . | |
| O | 2307 | 46 | 95 ± 29* | 23 | 46 | 58 ± 14* |
| A | 2072 | 41 | 124 ± 37† | 17 | 34 | 98 ± 34† |
| B | 444 | 9 | 131 ± 37 | 8 | 16 | 108 ± 38 |
| AB | 179 | 4 | 137 ± 43 | 2 | 4 | 119 ± 20 |
| All | 5002 | 100 | 111 ± 37‡ | 50 | 100 | 82 ± 35‡ |
| ABO blood group . | Y/Y1584 . | Y/C1584 . | ||||
|---|---|---|---|---|---|---|
| No. . | % . | VWF:Ag IU/dL−1 . | No. . | % . | VWF:Ag IU/dL−1 . | |
| O | 2307 | 46 | 95 ± 29* | 23 | 46 | 58 ± 14* |
| A | 2072 | 41 | 124 ± 37† | 17 | 34 | 98 ± 34† |
| B | 444 | 9 | 131 ± 37 | 8 | 16 | 108 ± 38 |
| AB | 179 | 4 | 137 ± 43 | 2 | 4 | 119 ± 20 |
| All | 5002 | 100 | 111 ± 37‡ | 50 | 100 | 82 ± 35‡ |
VWF:Ag values are expressed as mean ± SD.
For both phenotypes, VWF:Ag increased according to ABO blood group in the order O < A < B < AB. Mean VWF:Ag was significantly lower in group O and group A heterozygotes than in the respective homozygotes, and the overall mean for heterozygotes was significantly lower than for homozygotes.
P < .001.
P = .006.
P < .001.
VWF:Ag.
Mean VWF:Ag level was significantly lower for Y/C1584 heterozygotes (82 ± 35 IUdL−1) than for Y/Y1584 homozygotes (111 ± 37 IUdL−1; P < .001) (Table 2). For each ABO blood group, mean VWF:Ag level was lower among Y/C1584 donors compared with Y/Y1584 donors. This was statistically significant for blood group O (P < .001) and blood group A (P = .006). Values for blood groups B and AB were insufficient for statistical comparison. The absolute decrease for group O (37%) was statistically greater than for group A (26%) (Table 2) (P = .017, ANOVA). This should, however, be interpreted with caution because of the small number of group O and group A Y/C1584 donors.
VWF:Ag was significantly different for Y/Y1584 men (114 ±37 IUdL−1) compared with women (109 ± 37 IUdL−1) (P < .001), but this was unlikely to have been clinically significant, as noted for the entire cohort. There was no statistical difference in mean VWF:Ag between heterozygous Y/C1584 men (89 ± 39 IUdL−1) and women (73.7 ± 28 IUdL−1; P = .12). As observed for the entire cohort, VWF:Ag increased with age in the Y/Y1584 group and the Y/C1584 group (data not shown).
VWF:CB and VWF:RCo.
These parameters were determined for the 50 Y/C1584 plasmas and for 50 Y/Y1584 plasmas matched for the ABO blood group with the heterozygous plasmas. Mean VWF:CB values were 124% ± 41% (Y/Y1584) and 83% ± 36% (Y/C1584) (P < .001). Mean VWF:RCo values were 110% ± 43% (Y/Y1584) and 73% ± 40% (Y/C1584) (P < .001).
The mean ratio of VWF:CB to VWF:Ag was 1.16 ± 0.29 and 1.04 ± 0.20 for the Y/Y1584 and Y/C1584 groups, respectively (P = .02). The mean ratio of VWF:RCo to VWF:Ag was 1.0 ± 0.2 for homozygous plasmas and 0.9 ± 0.2 for heterozygous plasmas (P = .01). VWF:CB and VWF:RCo data, therefore, demonstrated a significant difference between Y/C1584 VWF and Y/Y1584 VWF. Decreased functionality was observed for heterozygous VWF.
VWF multimer analysis.
The multimer profile was investigated for the 50 Y/C1584 plasmas and 50 homozygous Y/Y1584 plasmas matched for ABO blood group with the heterozygotes. For all plasmas, a normal multimer profile was obtained. A subtle but highly reproducible difference in the triplet structure, representing a decrease in the intensity of the upper satellite band, was observed for all Y/C1584 plasmas compared with Y/Y1584 plasmas, and this was evident in densitometric scans (Figure 1).
Multimer analysis of VWF from Y/Y1584 and Y/C1584 donors. (A) 1.4% (wt/vol) agarose gel. (B) 2.0% (wt/vol) agarose gel. Lanes 1 to 4 in each panel contain plasma VWF from 2 donors of each phenotype. (left) Multimer gel. (right) Densitometric scans of all lanes of the gel. A subtle but highly reproducible decrease in the upper satellite triplet band in heterozygous VWF was visible on the multimer gel and in the densitometric scans (arrows). This difference may extend to higher multimers but was beyond the resolution of the gel. The scale at the base of each scan corresponds to color intensity: 0 = black; 300 = white.
Multimer analysis of VWF from Y/Y1584 and Y/C1584 donors. (A) 1.4% (wt/vol) agarose gel. (B) 2.0% (wt/vol) agarose gel. Lanes 1 to 4 in each panel contain plasma VWF from 2 donors of each phenotype. (left) Multimer gel. (right) Densitometric scans of all lanes of the gel. A subtle but highly reproducible decrease in the upper satellite triplet band in heterozygous VWF was visible on the multimer gel and in the densitometric scans (arrows). This difference may extend to higher multimers but was beyond the resolution of the gel. The scale at the base of each scan corresponds to color intensity: 0 = black; 300 = white.
VWF proteolysis by ADAMTS13.
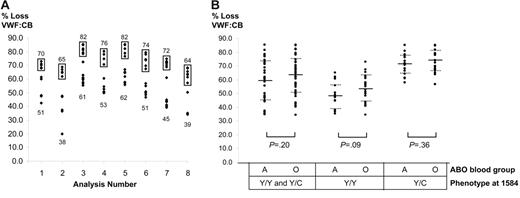
Proteolysis was compared for the 50 Y/C1584 plasmas and 50 Y/Y1584 plasmas matched for ABO blood group with the heterozygous plasmas. One hundred samples were randomly divided into 8 sets, and for each set the VWF:CB at 0 hours and 2 hours was measured in the same microtiter plate. Within each set, plasma VWF from Y/C1584 donors showed increased proteolysis compared with Y/Y1584 VWF, and no overlap in the extent of proteolysis was observed between the phenotypes (Figure 2A). To allow comparison across all 8 analyses, the mean proteolysis for Y/Y1584 VWF was adjusted to 100% and the mean proteolysis for Y/C1584 VWF was adjusted by the same proportion. This normalized the Y/Y1584 VWF means to 100% and allowed comparison with the adjusted Y/C1584 VWF means. Proteolysis for Y/C1584 VWF was significantly greater (average, 49%) than that for Y/Y1584 VWF (P < .001).
ADAMTS13 proteolysis of ABO-matched Y/Y1584 and Y/C1584 VWF. (A) Y/Y1584 VWF (n = 50) and Y/C1584 VWF (n = 50) were randomly divided into 8 sets. Proteolysis was then measured for each set (“Analysis Number”). Data points for Y/C1584 VWF are boxed, whereas those for Y/Y1584 VWF are not. The mean for Y/C data points is above the box, and the mean for Y/Y data points is below the data points. Within each analysis, no overlap occurred in proteolysis between Y/Y1584 VWF and Y/C1584 VWF. (B) Proteolysis data for the 8 sets of analyses in panel A were combined to allow inspection of the effect of ABO blood group. Proteolysis of group O and group A VWF was not statistically different for Y/Y1584 and Y/C1584 phenotypes together or individually, but it was marginally higher for group O in each comparison, and this approached significance for Y/Y1584 VWF. In both panels, proteolysis is measured as the percentage of loss in VWF:CB.
ADAMTS13 proteolysis of ABO-matched Y/Y1584 and Y/C1584 VWF. (A) Y/Y1584 VWF (n = 50) and Y/C1584 VWF (n = 50) were randomly divided into 8 sets. Proteolysis was then measured for each set (“Analysis Number”). Data points for Y/C1584 VWF are boxed, whereas those for Y/Y1584 VWF are not. The mean for Y/C data points is above the box, and the mean for Y/Y data points is below the data points. Within each analysis, no overlap occurred in proteolysis between Y/Y1584 VWF and Y/C1584 VWF. (B) Proteolysis data for the 8 sets of analyses in panel A were combined to allow inspection of the effect of ABO blood group. Proteolysis of group O and group A VWF was not statistically different for Y/Y1584 and Y/C1584 phenotypes together or individually, but it was marginally higher for group O in each comparison, and this approached significance for Y/Y1584 VWF. In both panels, proteolysis is measured as the percentage of loss in VWF:CB.
To substantiate that proteolysis was ADAMTS13 mediated, multimer analysis was performed on proteolysis reactions at 0 hours and 2 hours for 6 Y/Y1584 and 6 Y/C1584 samples (Figure S1, available on the Blood website; click on the Supplemental Figure link at the top of the online article). The result corroborated the VWF:CB data; increased proteolysis was evident for Y/C1584 VWF compared with Y/Y1584 VWF, as previously demonstrated.20,21 That this was attributable to ADAMTS13 was indicated by the following: proteolysis was accompanied by an increase in the triplet structure, a feature of ADAMTS13-mediated proteolysis.13 No smearing or spurious bands appeared after proteolysis, indicating no nonspecific degradation; the kinetics of proteolysis were compatible with those for ADAMTS13 in the in vitro reaction conditions used (data not shown).
Previous data have demonstrated an influence of the ABO blood group on VWF proteolysis by ADAMTS13.30,31 Therefore, we sought to assess proteolysis according to the ABO blood group and the 1584 phenotype. Data from the 8 sets of analyses were combined to allow statistical comparisons to be made (Figure 2B), but these were, at best, approximations due to variations from analysis to analysis (Figure 2A). The data indicated that the effect of blood group O and C1584 was not additive; indeed, the latter might have swamped the former (Figure 2B). Compared with previous data that showed a subtle but significant increase in proteolysis for VWF of blood group O compared with blood group A,30,31 the present data do not show a statistically significant difference for any of the comparisons. In each case, however, proteolysis for group O was greater than for group A, and this approached significance for Y/Y1584 VWF (P = .09) (Figure 2B). There are several potential explanations for the smaller effect of the ABO blood group in the present data, among them fundamental differences in the previous and present studies, such as the use of a purified high-molecular–weight fraction of VWF in the previous studies30,31 and the use of cryoprecipitated VWF from single donors in the present study or the combining of data from 8 separate sets of proteolysis experiments in the present study, as noted.
VWF:Ag lower than 50 IUdL−1
A VWF level lower than 50 IUdL−1 is often taken as an arbitrary cut-off when considering a diagnosis of VWD. Within the entire cohort, 1.5% of donors had a VWF level 50 IUdL−1 or lower. This increased to 2.4% among group O donors, 18.0% among Y/C1584 donors, and 34.8% among Y/C1584 heterozygotes of group O (Table 3). These data demonstrated enrichment for Y/C1584 among donors with a low VWF level; this enrichment was greater than that of blood group O. The data further indicate that the combination of blood group O and Y/C1584 heterozygosity was increased to approximately the product of each (34.8% vs 2.4% and 18.0%), suggesting a synergistic rather than an additive interaction between blood group O and Y/C1584 in influencing VWF level. Thus, group O and C1584 individually, and especially together, are associated with low VWF levels and appear to interact synergistically.
ABO blood group and 1584 phenotype in blood donors with VWF:Ag less than or equal to 50 IUdL−1
| Phenotype . | No. donors . | Donors with VWF:Ag at or below50 IUdL−1, % . |
|---|---|---|
| All | 5052 | 1.5 |
| Group O | 2330 | 2.4 |
| Y/C1584 | 50 | 18.0 |
| Group O and Y/C1584 | 23 | 34.8 |
| Group non-O | 2722 | 0.7 |
| Y/Y1584 | 5002 | 1.3 |
| Group O and Y/Y1584 | 2307 | 2.1 |
| Group non-O and Y/C1584 | 27 | 3.7 |
| Group non-O and Y/Y1584 | 2695 | 0.6 |
| Phenotype . | No. donors . | Donors with VWF:Ag at or below50 IUdL−1, % . |
|---|---|---|
| All | 5052 | 1.5 |
| Group O | 2330 | 2.4 |
| Y/C1584 | 50 | 18.0 |
| Group O and Y/C1584 | 23 | 34.8 |
| Group non-O | 2722 | 0.7 |
| Y/Y1584 | 5002 | 1.3 |
| Group O and Y/Y1584 | 2307 | 2.1 |
| Group non-O and Y/C1584 | 27 | 3.7 |
| Group non-O and Y/Y1584 | 2695 | 0.6 |
Within this low VWF group there was a higher proportion of donors with blood group O and Y/C1584, particularly both together. The data suggest a synergistic effect of blood group O and C1584 on VWF level.
Discussion
The data provide an accurate estimate of the frequency of C1584 in the local population that correlates with previous preliminary estimates.19,20 A frequency of heterozygosity of 1% was obtained, giving the allele frequency f(C1584) = 0.005. Based on this allele frequency, approximately 1 in 10 000 individuals would be expected to be homozygous for C1584.
The data demonstrate that the mean VWF level for Y/C1584 heterozygotes was significantly lower than for Y/Y1584 homozygotes. This observation was also applicable for group O Y/C1584 heterozygotes compared with group O Y/Y1584 homozygotes and for group A Y/C1584 heterozygotes compared with group A Y/Y1584 homozygotes. The decrease in the mean VWF level observed for group O Y/C1584 heterozygotes was statistically significantly greater than that observed for group A Y/C1584 heterozygotes, perhaps reflecting an effect of group O and C1584 together that is greater than the effect of either alone; comparison of a larger number of individuals is necessary to confirm or refute this, but it is consistent with the observations made in donors with VWF values lower than 50 IUdL−1.
The decreased level of VWF associated with C1584 may, in part, be related to the finding that, when expressed in homozygous state in eukaryotic cell culture, C1584 caused marked intracellular retention of VWF.19 Statistically nonsignificant intracellular retention was observed when C1584 was expressed in heterozygous form. Thus, among blood donors, the decreased mean VWF level for heterozygotes might have reflected intracellular retention in vivo, and this might have become apparent as a result of the analysis of a large number of heterozygotes.
When individuals with VWF:Ag levels lower than 50 IUdL−1 were studied, there was an enrichment for blood group O, a much greater enrichment for Y/C1584 heterozygosity, and an enrichment for both combined that was approximately equal to the product of each alone, suggesting a synergistic interaction. These data suggest that coinheritance of blood group O and C1584 markedly increases the likelihood of low VWF level and may partially explain the observation, made in 2 independent studies of type 1 VWD, that more than 90% of patients with C1584 had blood group O.19,21 (In comparison, approximately 70% of patients with type 1 VWD overall have blood group O.3 ) Clinical data were not available for the blood donors with low VWF levels; therefore, it was not possible to ascertain whether this correlated with a greater bleeding tendency. However, an association between low VWF level, whether caused by VWD or not, and increased tendency to bleed have been reported.32
It has been suggested that the chance coincidence of bleeding symptoms (which are common) and decreased VWF level potentially lead to false-positive diagnoses of type 1 VWD.33 Our data strongly support this argument. Coinheritance of blood group O and C1584 (present in 0.5% of our population) was associated with a markedly increased likelihood of finding a VWF level in a range that could lead to a diagnosis of VWD if a patient by chance had a bleeding history.
In addition to an effect on VWF level, heterozygosity for Y/C1584 was associated with a functional change in VWF, evidenced by a significant decrease in VWF:CB and VWF:RCo for Y/C1584 VWF compared with Y/Y1584 VWF. The decrease may be explained by a direct effect of the C1584 variant on VWF:CB and VWF:RCo, such as through a structural change in VWF. An alternative possibility is that a decrease in the HMW component of circulating VWF in individuals with Y/C1584 may arise from increased susceptibility of VWF to proteolysis. VWF heterozygous for Y/C1584 shows enhanced proteolysis,20 as observed for each heterozygous VWF in this study. A decrease in the high-molecular–weight fraction was not, however, detected on multimer analysis, either indicating that it does not occur or that it is below the limit of detection of this analytical technique. VWF of blood group O has been shown to be proteolyzed more rapidly than non-O VWF,30,31 and the ratio of VWF:CB to VWF:Ag is significantly lower in blood group O than in non-group O.34 Despite these data, the multimer profile in blood group O is normal.
Multimer analysis did, however, indicate a reproducible and consistent difference in the triplet structure of heterozygous Y/C1584 VWF: a relative decrease in the upper satellite triplet band. This result is difficult to explain, but it is entirely consistent with previously published profiles for VWF multimers in patients with type 2A VWD in whom VWF shows markedly increased susceptibility to proteolysis. A relative decrease in the upper satellite band is observed in some patients, giving the multimer profile the appearance of doublets rather than triplets.35 The result clearly indicates that heterozygous Y/C1584 plasma VWF differs in structure from homozygous Y/Y1584 VWF, but whether this difference is of functional significance is unclear.
Whether a direct relationship exists between VWF level and VWF proteolysis has yet to be determined. The two may be mutually exclusive. In a study of VWF clearance in mice, there appeared to be no preferential loss of any multimer size from the circulation, suggesting equivalent rates of clearance.36 Thus, VWF proteolysis may not be a necessary prerequisite for removal from the circulation. Our finding of synergistic enrichment for blood group O and Y/C1584 heterozygosity in patients with low VWF level, with no additional enhancement in proteolysis for VWF that included blood group O and Y/C1584 heterozygosity, suggested that proteolysis and level may not be directly related.
In conclusion, our data indicate that the level and function of circulating VWF are decreased in Y/C1584 heterozygotes compared with Y/Y1584 heterozygotes. Additionally, the data demonstrate a significant synergistic enrichment for Y/C1584 heterozygosity and blood group O in individuals with low VWF levels (lower than 50 IUdL−1). These findings, together with the subtle changes in VWF multimers and the in vitro evidence of retention of the C1584 allele,19 provide strong circumstantial and biologically plausible associations with decreased VWF level and function. The data for Y/C1584 heterozygosity in combination with blood group O indicate that in some individuals, a low VWF level may be a complex attribute reflecting, in part, genetic factors outside the VWF gene. A revised classification of VWD, in which the requirement for a VWF gene mutation has been removed from the diagnostic criteria, permits a formal diagnosis of type 1 VWD.8
The data support the possibility of C1584 as a VWD mutation showing incomplete penetrance that may be influenced by ABO blood group. The association of heterozygosity for Y/C1584 with decreased VWF level, decreased VWF function, and increased susceptibility to proteolysis all help explain the observed enrichment for the C1584 variant in type 1 VWD19,21,22 and provide a pathogenic basis for this enrichment.
Authorship
Contribution: J.A.D. collected samples and data, interpreted data, and wrote the manuscript. P.W.C. designed the study, collected samples, interpreted data, and wrote the manuscript. L.S.H. collected data. D.J.B. designed and organized the research, collected samples, collected and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
J.A.D. and P.W.C. contributed equally to this study.
Correspondence: Derrick J Bowen, Department of Haematology, School of Medicine, Cardiff University, Heath Park, Cardiff, CF14 4XN, United Kingdom; e-mail: bowendj1@cardiff.ac.uk.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was funded by British Heart Foundation project grant PG/04/091/1746 and was supported by National Health Service Research and Development through the University Hospital of Wales.
We thank the Welsh Blood Service, in particular Dr Keith Wilson, Elizabeth Meech, Sian Gorst, and Teams 1, 2, 3, and 4, without whose help sample collection would not have been possible. We also thank Mr Alan Eldridge for his considerable help with the organization and collection of samples, Marie-Ann Doward, Delyth Davies, Gwyneth Jennings, Anna O'Grady, Heather Ralis, and Steve Bowley for their help with sample collection and sample processing, all members of the Coagulation Laboratory who helped with sample processing, Dr John Giddings for kindly providing staff and resources, and Dr Robert K. Hills for statistical advice.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal