Abstract
Circulating endothelial cells (CECs) have been detected in a variety of vascular disorders, but their interactions with healthy endothelium remain unknown. The aim of this study was to evaluate the response of human endothelial cells (ECs) to apoptotic or necrotic ECs in an in vitro model and to delineate pathogenetic pathways. Here we show that incubation of the human microvascular endothelial cell line (HMEC-1) with apoptotic ECs resulted in increased expression of chemokines and enhanced binding of leukocytes to HMEC-1 cells, whereas exposure of HMEC-1 cells to necrotic ECs caused no changes in leukocyte-binding affinity. Both apoptotic and necrotic cells were bound and engulfed by HMEC-1 cells and primary human umbilical vein endothelial cells (HUVECs). We therefore suggest that exposures to apoptotic and necrotic ECs induce different patterns of chemokine synthesis and leukocyte adhesion in healthy ECs. These data indicate that CECs are not only markers of vascular damage but may induce proinflammatory signals in the endothelium.
Introduction
Apoptosis is essential to maintain homeostasis in multicellular organisms and apoptotic cells are rapidly and effectively cleared by professional phagocytes before they undergo secondary necrosis and release their noxious cytoplasmic content into the environment.1 Ineffective clearance of apoptotic cells contributes to disease pathogenesis.2,3 This may be true especially for autoimmune diseases such as systemic lupus erythematosus (SLE) because apoptotic cells are thought to be a potential source of autoantigens and disturbed clearance of apoptotic corpses may initiate and drive autoimmunity.4-6 This concept of acquired autoimmunity has also been confirmed in several animal models that showed disturbed engulfment of apoptotic cells.7-10
Vascular endothelial cells (ECs) serve as a crucial barrier between tissues and the circulation. They secrete a variety of substances, regulate coagulation, and participate in the immune response.11 Under physiologic conditions circulating ECs (CECs) are almost not traceable, whereas in a variety of vascular diseases such as myocardial infarction, small-vessel vasculitis, transplantation, or cancer high numbers of CECs are detectable in the peripheral circulation.12-17 Consequentially, accumulation of CECs may affect the homeostasis of the vessel wall by interfering with the EC layer both in the vicinity of endothelial lesions or even distant from the site of injury.
Clearance of dying cells or cellular debris has been mostly ascribed to professional phagocytes, for example, antigen-presenting macrophages, neutrophils, or dendritic cells, but it is conceivable that other cell types such as epithelial cells or ECs may also partake in this process.18,19 ECs are not professional phagocytes although specific subpopulations, such as liver ECs or cells from high endothelial venules, are able to phagocytose apoptotic cells.20,21 Phagocytosis of circulating endothelial debris by healthy endothelium thus appears to be an intriguing concept.
The role of endothelial- or platelet-derived microparticles in the circulation is the subject of several recently published studies but few, if any, data, shed light on the impact of apoptotic endothelial corpses on the adjacent endothelium.22-31 The aim of the present study was to establish an in vitro model to study the interaction of apoptotic or necrotic ECs with a healthy EC layer. We here demonstrate that microvascular ECs, when exposed to apoptotic cells, react with the release of proinflammatory chemotactic cytokines and that this response triggers enhanced adhesion of primary neutrophils and macrophages.
Patients, materials, and methods
The study was approved by the Hannover Medical School Ethics Committee and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients included in this study.
EC culture
Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical cords by exposure of the vein to chymotrypsin for 30 minutes at 37°C. Cells were collected and cultured in EC medium (ECM+, Promocell, Heidelberg, Germany) and gentamicin. After 24 hours HUVECs were intensively washed with phosphate-buffered saline (PBS), subcultured in ECM+ medium, and used up to passage 4. The human microvascular endothelial cell line HMEC-1 was obtained from the Centers for Disease Control and Prevention (Atlanta, GA) and maintained in MCDB-131 medium supplemented with 10% fetal calf serum (FCS), gentamicin, 10 mM l-glutamine, 10 ng/mL endothelial growth factor (EGF), and 1 μg/mL hydrocortisone (BD Biosciences, Heidelberg, Germany).
Isolation of neutrophils
Blood was obtained from healthy volunteers. Granulocytes were isolated by density gradient centrifugation over isotonic Biocoll (Biochrom, Berlin, Germany). After hypertonic lysis of the erythrocytes with ice-cold lysis buffer (155 mM NH4Cl, 10 mM NaHCO3, and 0.5 mM EDTA, pH 7.4), the granulocytes (with a neutrophil content of 95%) were washed in PBS, resuspended in Hanks balanced salt solution (HBSS), and labeled with 5-chloromethylfluorescein diacetate (CMFDA; Invitrogen, Karlsruhe, Germany) for 30 minutes.
Enrichment of CECs
CECs were isolated as described previously.14 Briefly, 7 mL EDTA-blood was incubated with Dynabeads (Invitrogen) coated with anti–human CD146 (Biocytex, Marseille, France) and CECs were isolated using a magnetic device (Invitrogen). From each sample an aliquot was stained with FITC-coupled UEA-1 and examined under a fluorescence microscope.
Induction of apoptosis or necrosis
HMEC-1 cells were exposed to UV light at a wavelength of 254 nm at various doses. Generation of reactive oxygen species (ROS) was measured by dihydroxyethidium (DHE) staining, cell viability was assessed by measuring internal ATP concentration, and integrity of the cell membrane was confirmed by measuring lactate dehydrogenase (LDH) in the supernatant using the Celltiter-Glo luminescence and the Cytotox 96 cytotoxicity assay according to the manufacturer's instructions (Promega, Mannheim, Germany). Induction of apoptosis was confirmed by determining activity of caspase-3 and caspase-7 using the luminescence based Caspase-Glo 3/7 assay and fragmented nuclear DNA was stained with the Dead-End fluorometric TUNEL system according to the manufacturer's protocols (Promega). The percentage of apoptotic cells was assessed by fluorescence-activated cell sorting (FACS) analysis with FITC-labeled activated caspase ligand CaspACE FITC-VAD-FMK (Promega).
As a second approach to induce apoptosis, HMEC-1 cells were exposed to TNF-α (3 nM; BD Biosciences) and camptothecin (CPT, 0.15 μM; Sigma-Aldrich, München, Germany) for 24 hours. At these concentrations ECs were positive for active caspases-3 and -7 and showed positive staining for FITC-VAD-FMK.
To obtain necrotic-cell lysates HMEC-1 cells were resuspended in PBS and subjected to repeated freeze-thaw cycles at −80°C.
Coincubation of HMEC-1 cells and apoptotic or necrotic ECs
HMEC-1 cells were plated in 100 mm2 dishes at 4 × 105 cells/mL in MCDB-131 full medium. After 4 hours the medium was replaced with PBS and cells were exposed to UV light. PBS was again replaced with MCDB-131 full medium and HMEC-1 cells were incubated for another 16 hours. In some experiments TNF-α/CPT-treated HMEC-1 cells were used as controls for apoptotic cells. Lysed necrotic cells were obtained as described (see “Induction of apoptosis or necrosis”). The irradiated cells were harvested, washed in PBS, and coincubated with untreated HMEC-1 cells for different time points. The HMEC-1 cell layer was then washed 5 times with PBS, harvested by trypsination, and resuspended in RNA lysis buffer (Qiagen, Hilden, Germany). For the measurement of protein content supernatant of the cocultures was collected, cleared from cell debris by centrifugation at 13 000g, aliquoted, and stored at −80°C until the enzyme-linked immunosorbent assay (ELISA) was performed.
Transwell experiments
HMEC-1 cells were seeded onto collagen-coated membranes in transwell chambers (12 mm diameter, 3 μm pore size; Corning, Schiphol-Rijk, The Netherlands) at 1 × 106 cells/mL. In the meantime HMEC-1 cells were seeded in 6-well plates at a density of 1 × 106 cells/mL for 4 hours. Apoptosis or necrosis was induced as described (see “Induction of apoptosis or necrosis”). After an incubation time of 16 hours the transwell chambers with the untreated HMEC-1 cells were inserted into the 6-well plates containing the damaged cells and both were coincubated for different time points. In a second set of experiments both untreated and damaged cells were coincubated for 3 and 6 hours directly after induction of apoptosis or necrosis. HMEC-1 cells were removed from the membrane and subjected to RNA extraction and real-time, quantitative polymerase chain reaction (qPCR) analysis.
RNA isolation, quantification, and real-time qPCR
To obtain total RNA we used the RNeasy miniprep system in combination with an on-column DNase digest according to the manufacturer's protocol (Qiagen). Quality of the RNA was determined using a Bioanalyzer and the RNA 6000 Nano LabChip (Agilent Technologies, Waldbronn, Germany). For real-time qPCR, 2 μg total RNA was subjected to reverse transcription using a mix of random hexamers and oligo(dT)12-15 oligonucleotides (Stratagene, Amsterdam, The Netherlands) and MMLV RNase H− point mutant reverse transcriptase (Promega). qPCR was performed on a SDS 7700 system (Applied Biosystems, Darmstadt, Germany) with Rox dye as internal control (Invitrogen), FastStart Taq polymerase (Roche Diagnostics, Penzberg, Germany) and gene-specific primers in combination with SYBR-Green chemistry (Invitrogen) or with Fam-Tamra labeled TaqMan probes (BioTez, Berlin, Germany). Data were analyzed using Q-gene software.32 Primers were designed with Primer Express 2.0 software (Applied Biosystems). Sequence information of the oligonucleotides used for real-time qPCR is supplied in Table S1 (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
ELISA
Conditioned medium was collected from HMEC-1 cells before and after coincubation with apoptotic or necrotic HMEC-1 cells, centrifuged to remove particulates, and frozen at −80°C. IL-8 and MCP1 protein content was measured using the IL-8 and the MCP1 Quantikine kit according to the manufacturer's protocol (R&D Systems, Wiesbaden, Germany).
Leukocyte adhesion assay
ECs were seeded in 96-well plates at 8 × 104 cells/well. After adherence the cells were exposed to apoptotic or necrotic HMEC-1 cells (1 × 105 cells/well) or were left untreated. After 4 hours the supernatant was cleared of cells and cellular debris by centrifugation at 1000g. CMFDA-labeled neutrophils or macrophages were added to the ECs at a concentration of 5 × 104 cells/well. After 30 minutes the supernatant containing unbound cells was removed, ECs were washed 5 times with ice-cold PBS, fixed with 4% paraformaldehyde (PFA) in PBS, and embedded with Vectashield (Vector Laboratories, Burlingame, CA). The number of adherent neutrophils and macrophages was counted in 6 visual fields per well and averaged.
Adhesion of neutrophils to HMEC-1 cells exposed to human CECs
HMEC-1 cells were seeded on collagen-coated coverslips (Sigma-Aldrich) at a concentration of 1 × 105 cells/well. After reaching confluence HMEC-1 cells were exposed to CECs from patients with acute anti–neutrophil cytoplasmic antibody (ANCA)–associated vasculitis or from healthy controls. As control experiments the HMEC-1 cells were incubated with the same number of CD146-coated Dynabeads or left untreated. After 3 hours the supernatant was cleared from Dynabeads and cell debris and CMFDA-stained neutrophils were added to the cells at a concentration of 1 × 105 cells/well. After 30 minutes the supernatant was removed and the coverslips were washed 5 times with ice-cold PBS, fixed with methanol at −20°C for 15 minutes, and examined under a fluorescence microscope.
Phagocytosis of apoptotic or necrotic HMEC-1 cells
HMEC-1 cells were seeded on collagen-coated glass coverslips (12 mm in diameter) at a density of 4 × 105 cells. For some experiments HMEC-1 cells were labeled with the Chloromethylbenzamido Celltracker CM-Dil (Invitrogen) for 30 minutes. When the cells reached confluence they were incubated with equal numbers of CMFDA-labeled apoptotic or necrotic HMEC-1 cells or with FITC-UEA-1–stained cell fragments for different time points. Nonphagocytosed cells and fragments were then removed by intensive washing with ice-cold PBS and the cells were fixed in 4% PFA and embedded with Vectashield. Images of the cells were taken using an Axioplan 2 microscope equipped with an Axiocam MRm camera and a 40×/0.75 numerical aperture Plan Neofluar objective lens (Zeiss, Göttingen, Germany). Images were captured using Axiovision 4.3 software (Zeiss). Confocal images were taken using a Leica DM IRB microscope (Leica, Heidelberg, Germany) with a TCS SP3 AOBS scan head and equipped with argon and krypton laser beams. Micrographs were obtained using a 63×/1.4 numerical aperture HCX PL Apo objective lens. All images were processed with Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA).
Statistical analysis
Results were expressed as the mean ± SD of at least 3 independent experiments. When human donors were used, cells were from different donors. Results were analyzed for statistical significance using the 2-tailed Mann-Whitney U test.
Results
Dose-dependent induction of apoptosis or necrosis in HMEC-1 cells by UV light
UV irradiation was chosen to induce apoptosis in HMEC-1 cells to avoid effects of chemical compounds on the recipient cells in subsequent coculture experiments. Exposure of HMEC-1 cells to UV light induces ROS, leading to a dose-dependent pattern of reversible cell damage, apoptosis, and finally necrosis.
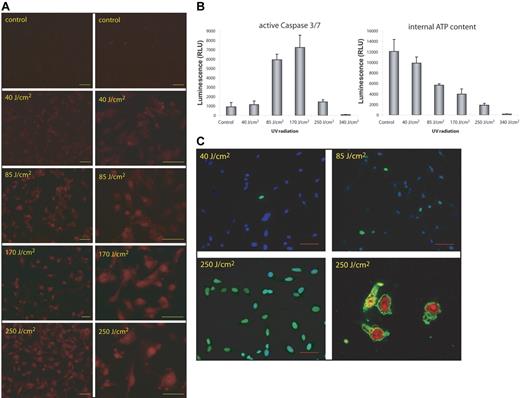
Exposure of HMEC-1 cells to low doses of UV light (40 J/cm2) led to markedly elevated levels of intracellular ROS (Figure 1A) and decreased intracellular ATP content but low levels of active caspases-3 and -7 and only a few cells with fragmented nuclear DNA were observed, suggesting absence of apoptosis (Figure 1B-C). Increasing doses of UV irradiation (85-170 J/cm2) led to a further decline of intracellular ATP, enhanced ROS generation, and marked activation of caspases-3 and -7. TUNEL staining demonstrated degradation of nuclear DNA and FACS analysis of the active caspase ligand FITC-VAD-FMK demonstrated positivity in 66% ± 8.4% (n = 3, P < .05 at 85 J/cm2) and 75% ± 3.5% (n = 3, P < .05 at 170 J/cm2), whereas in untreated cells only 1.7% ± 3.5% (n = 3, P < .05) were positive for FITC-VAD-FMK. Light microscopy of the cells showed condensed cytoplasm, blebbing of apoptotic bodies, and shrinkage of the nucleus, in keeping with apoptosis (data not shown). Higher doses of UV light (≥ 250 J/cm2) led to a dramatic decline of intracellular ATP and caspase activity fell to levels below those of untreated cells, indicating necrotic-cell death (Figure 1B-C). Necrosis was corroborated by release of LDH and propidium iodide staining (data not shown). These data gave us confidence that UV light at doses between 85 and 170 J/cm2 induced apoptosis, whereas higher doses led to necrosis.
UV light induces ROS generation and apoptosis in HMEC-1 cells. (A) Exposure to different doses of UV light enhances generation of ROS as determined by DHE staining. (B) UV light induces a dose-dependent activation of caspase-3 and caspase-7 and a decrease in internal ATP content. Both parameters were measured 3 hours after irradiation. Data are given as mean ± SD of 3 independent experiments. (C) Increasing amount of UV light triggers fragmentation of nuclear DNA as demonstrated by TUNEL staining 24 hours after irradiation. Necrosis occurs at high doses of UV light as seen by annexin V/propidium iodide-staining in the lower right micrograph. Bars represent 50 μm.
UV light induces ROS generation and apoptosis in HMEC-1 cells. (A) Exposure to different doses of UV light enhances generation of ROS as determined by DHE staining. (B) UV light induces a dose-dependent activation of caspase-3 and caspase-7 and a decrease in internal ATP content. Both parameters were measured 3 hours after irradiation. Data are given as mean ± SD of 3 independent experiments. (C) Increasing amount of UV light triggers fragmentation of nuclear DNA as demonstrated by TUNEL staining 24 hours after irradiation. Necrosis occurs at high doses of UV light as seen by annexin V/propidium iodide-staining in the lower right micrograph. Bars represent 50 μm.
Exposure of apoptotic or necrotic HMEC-1 cells to untreated ECs
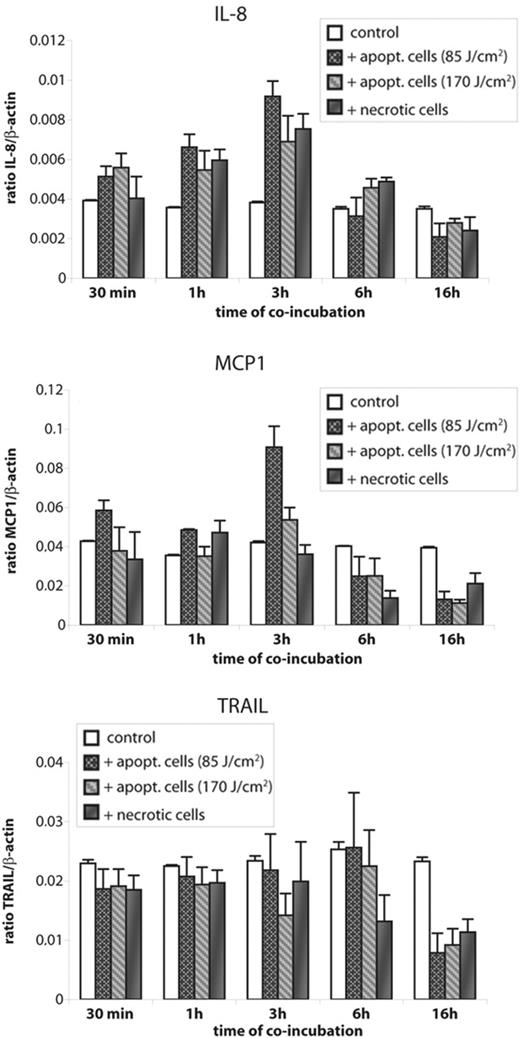
HMEC-1 cells were exposed to UV light at different doses and incubated overnight to obtain apoptotic or necrotic cells. Necrotic HMEC-1 material was also obtained by lysis of HMEC-1 cells during repeated freeze/thaw cycles. Exposure of apoptotic or necrotic HMEC-1 cells to untreated HMEC-1 cells did not induce any distinct changes in interleukin 6 (IL-6), interleukin-10 (IL 10), vascular endothelial growth factor (VEGF), or intracellular adhesion molecule-1 (ICAM-1) transcript at any time points (data not shown). In contrast, mRNA levels of IL-8 were clearly increased in HMEC-1 cells exposed for 3 hours to apoptotic or necrotic cells (≥ 2-fold increase, n = 5). Longer exposure times did not lead to further increase of IL-8 transcript and 16 hours of coincubation led to IL-8 levels below those of untreated HMEC-1 cells (Figure 2).
HMEC-1 cells exposed to apoptotic or necrotic ECs show altered expression of IL-8, MCP1, and TRAIL transcripts. HMEC-1 cells were incubated with apoptotic or necrotic ECs for different time periods and mRNA level of IL-8, MCP1, and TRAIL were determined by real-time qPCR. Data are given as mean ± SD of 5 independent experiments.
HMEC-1 cells exposed to apoptotic or necrotic ECs show altered expression of IL-8, MCP1, and TRAIL transcripts. HMEC-1 cells were incubated with apoptotic or necrotic ECs for different time periods and mRNA level of IL-8, MCP1, and TRAIL were determined by real-time qPCR. Data are given as mean ± SD of 5 independent experiments.
MCP1 transcript levels peaked (≥ 2-fold up-regulation compared to untreated HMEC-1 cells, n = 5) after 3 hours of exposure to apoptotic cells. Longer coincubation of HMEC-1 cells and apoptotic cells for up to 16 hours resulted in a decrease of MCP1 mRNA to almost half the amount seen in untreated HMEC-1 cells. In contrast to IL-8, coincubation with necrotic cells did not induce an increase of MCP1 expression in HMEC-1 cells (Figure 2). TRAIL expression in HMEC-1 cells did not change during the first 6 hours of coincubation with apoptotic or necrotic ECs. After 16 hours, however, TRAIL mRNA levels declined to less than half of those in untreated HMEC-1 cells. There was no difference between apoptotic and necrotic cells (Figure 2).
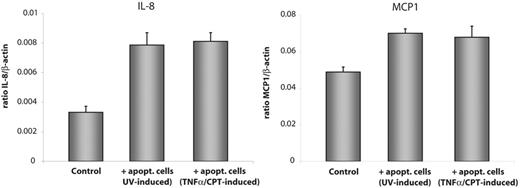
To prove that enhanced expression of IL-8 and MCP1 transcript was indeed mediated by the apoptotic conditions of the cells or whether it was due to cellular modifications caused by UV irradiation we conducted a second approach with HMEC-1 cells exposed to a combination of TNF-α (3 nM) and CPT (0.15 μM). The combination of the 2 agents was necessary because neither TNF-α nor CPT alone induced apoptosis in HMEC-1 cells. TNF-α/CPT-treated apoptotic HMEC-1 cells induced up-regulation of IL-8 transcript in the recipient cells after 3 hours of coincubation (> 2-fold increase, n = 5), too. The impact of TNF-α/CPT-treated HMEC-1 cells on MCP1 expression was much weaker (1.5-fold increase, n = 5; Figure 3).
Enhanced expression of IL-8 and MCP1 is independent of the method of apoptosis induction. Apoptosis was induced in HMEC-1 cells either by UV irradiation or by treatment with a combination of TNF-α (3 nM) and CPT (0.15 μM) for 24 hours. Healthy HMEC-1 cells were exposed to the apoptotic cells for 3 hours and mRNA level of IL-8 and MCP1 were determined by real-time qPCR. Data are given as mean ± SD of 5 independent experiments.
Enhanced expression of IL-8 and MCP1 is independent of the method of apoptosis induction. Apoptosis was induced in HMEC-1 cells either by UV irradiation or by treatment with a combination of TNF-α (3 nM) and CPT (0.15 μM) for 24 hours. Healthy HMEC-1 cells were exposed to the apoptotic cells for 3 hours and mRNA level of IL-8 and MCP1 were determined by real-time qPCR. Data are given as mean ± SD of 5 independent experiments.
Further experiments were conducted to rule out that contaminating RNA from irradiated cells was responsible for any of these effects. Total RNA was extracted from HMEC-1 cells 16 hours after exposure to UV light. Already low UV doses of 40 J/cm2 caused slight RNA degradation and UV doses in excess of 85 J/cm2 led to a total degradation of RNA (Figure S1). These results showed that all mRNA results reflected altered expression in healthy endothelium and not contamination from irradiated ECs.
To study the importance of cell-to-cell contact for the differences in cytokine synthesis, apoptotic or necrotic cells and healthy HMEC-1 cells were grown separately on opposite sides of a permeable membrane in transwell chambers. Untreated HMEC-1 cells from the upper chamber were removed and subjected to RNA extraction and qPCR after different periods of coincubation. In these experiments IL-8 and MCP1 mRNA amounts did not differ from controls, suggesting that any increase in cytokine synthesis after coincubation is mediated by direct cell-to-cell contact and not by soluble markers that may be released by the irradiated cells (data not shown).
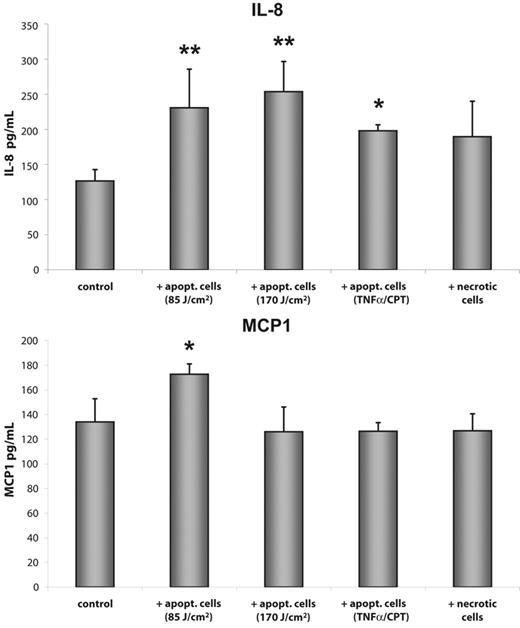
Next we measured the release of IL-8 and MCP1 protein into the supernatant of HMEC-1 cells after exposure for 3 hours to apoptotic or necrotic cells. In accordance with RNA results, apoptotic and necrotic cells induced a significant increase in IL-8 protein release from HMEC-1 cells. In contrast, only exposure of HMEC-1 cells to ECs that were exposed to UV light at a dose of 85 J/cm2 showed a significant increase in protein synthesis (Figure 4). We also measured the release of IL-8 and MCP1 from cells either exposed to UV light or treated with TNF-α/CPT. Neither condition caused any significant increase in IL-8 or MCP1 protein amount compared to untreated controls (data not shown).
Exposure to apoptotic ECs resulted in enhanced release of IL-8 and MCP1 protein. HMEC-1 cells were incubated with apoptotic or necrotic ECs for 3 hours and the release of IL-8 and MCP1 protein into the supernatant was measured by ELISA. Data are given as mean ± SD of 5 independent experiments (*P < .01; **P < .001).
Exposure to apoptotic ECs resulted in enhanced release of IL-8 and MCP1 protein. HMEC-1 cells were incubated with apoptotic or necrotic ECs for 3 hours and the release of IL-8 and MCP1 protein into the supernatant was measured by ELISA. Data are given as mean ± SD of 5 independent experiments (*P < .01; **P < .001).
Phagocytosis of apoptotic or necrotic cells by HMEC-1 cells and HUVECs
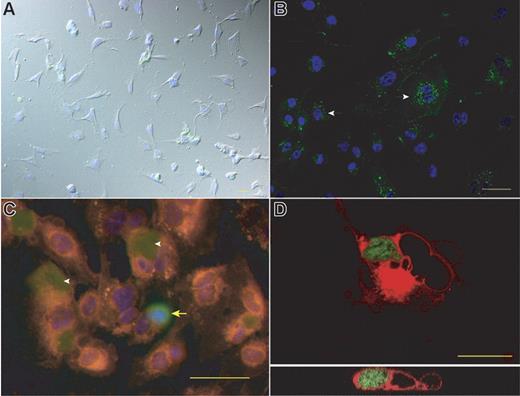
In the following experiments we tried to determine the fate of apoptotic or necrotic cells that were coincubated with HMEC-1 cells or HUVECs. Both cell types rapidly bound and engulfed apoptotic-cell corpses or necrotic-cell fragments. After 3 hours of exposure of necrotic cells to HMEC-1 fluorescent vesicles containing endothelial fragments were clearly visible inside the cell (Figure 5A). Prolonged coincubation led to the appearance of fluorescent dye in lysosomes, indicating lysosomal digestion of EC fragments (Figure 5B). Also apoptotic HMEC-1 cells were bound and engulfed by untreated HMEC-1 cells (Figure 5C). Confocal microscopy on HUVECs demonstrated that apoptotic HMEC-1 cells not only bound to the surface of HUVECs but were rather ingested (Figure 5D). Because both HUVECs and HMEC-1 cells were able to engulf damaged cells, phagocytosis of endothelial debris by healthy ECs seems to represent a general concept in clearance of dead cells.
Engulfment of apoptotic or necrotic ECs by HMEC-1 cells and HUVECs. (A) Exposure of HMEC-1 cells to necrotic HMEC-1 cells for 3 hours resulted in engulfment of labeled cell fragments. (B) After 6 hours of coincubation phagocytosed particles appear in the lysosomes around the nucleus (arrows). (C) CM-Dil labeled HMEC-1 cells were incubated with CMFDA-labeled apoptotic HMEC-1 cells. After 1 hour apoptotic cells were engulfed by HUVECs (white arrowheads). The yellow arrow indicates an apoptotic cell that binds to the surface of a HUVEC. (D) CM-Dil stained HUVECs were exposed for 2.5 hours to apoptotic HMEC-1 cells. The micrograph shows a confocal image of a cell engulfing an apoptotic (green) cell. A cross-section of the whole cell is shown in the micrograph at the bottom. Bars represent 50 μm (A-C) or 20 μm (D).
Engulfment of apoptotic or necrotic ECs by HMEC-1 cells and HUVECs. (A) Exposure of HMEC-1 cells to necrotic HMEC-1 cells for 3 hours resulted in engulfment of labeled cell fragments. (B) After 6 hours of coincubation phagocytosed particles appear in the lysosomes around the nucleus (arrows). (C) CM-Dil labeled HMEC-1 cells were incubated with CMFDA-labeled apoptotic HMEC-1 cells. After 1 hour apoptotic cells were engulfed by HUVECs (white arrowheads). The yellow arrow indicates an apoptotic cell that binds to the surface of a HUVEC. (D) CM-Dil stained HUVECs were exposed for 2.5 hours to apoptotic HMEC-1 cells. The micrograph shows a confocal image of a cell engulfing an apoptotic (green) cell. A cross-section of the whole cell is shown in the micrograph at the bottom. Bars represent 50 μm (A-C) or 20 μm (D).
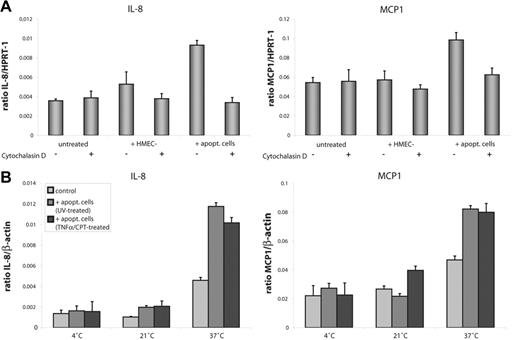
To further study the effect of engulfment of apoptotic cells on expression of cytokines, HMEC-1 cells were treated with cytochalasin D prior to exposure to damaged cells. Cytochalasin D inhibits polymerization of the cytoskeleton and prevents uptake, but not the binding, of damaged cells or cell fragments. Pretreatment of HMEC-1 cells with cytochalasin D (4 μM) did not have any effects on basal mRNA synthesis of HMEC-1 cells, but it reversed the enhanced synthesis of IL-8 and MCP1 transcript in HMEC-1 cells exposed to apoptotic cells (Figure 6A). We also studied whether different temperatures have any influence on cytokine expression. HMEC-1 cells were exposed to apoptotic cells for 3 hours at 4°C, 21°C, and 37°C. At low temperatures mRNA expression of IL-8 was reduced compared to 37°C, but there were no differences between controls and cells that bound apoptotic cells. At 21°C a slight increase in IL-8 expression was seen in HMEC-1 cells exposed to apoptotic cells. The same pattern was seen in MCP1 expression. These data implicate that the binding of apoptotic-cell fragments to the surface of the recipient cell is not sufficient and that functional internalization of apoptotic cells may be required for the induction of inflammatory gene synthesis (Figure 6B).
Engulfment of apoptotic cells by HMEC-1 cells is necessary for enhanced synthesis of IL-8 and MCP1 mRNA. (A) HMEC-1 cells were exposed to untreated or apoptotic cells for 3 hours and IL-8 and MCP1 expression was measured by real-time qPCR. Pretreatment with cytochalasin D (4 μM) reversed the enhanced synthesis of IL-8 and MCP1 to the level of the controls. (B) At temperatures where the internalization of bound cells is prevented, apoptotic cells failed to induce enhanced expression of IL-8 and MCP1 transcript. Data are given as mean ± SD of 3 independent experiments.
Engulfment of apoptotic cells by HMEC-1 cells is necessary for enhanced synthesis of IL-8 and MCP1 mRNA. (A) HMEC-1 cells were exposed to untreated or apoptotic cells for 3 hours and IL-8 and MCP1 expression was measured by real-time qPCR. Pretreatment with cytochalasin D (4 μM) reversed the enhanced synthesis of IL-8 and MCP1 to the level of the controls. (B) At temperatures where the internalization of bound cells is prevented, apoptotic cells failed to induce enhanced expression of IL-8 and MCP1 transcript. Data are given as mean ± SD of 3 independent experiments.
Exposure of apoptotic cells to HMEC-1 cells altered adhesion properties of leukocytes
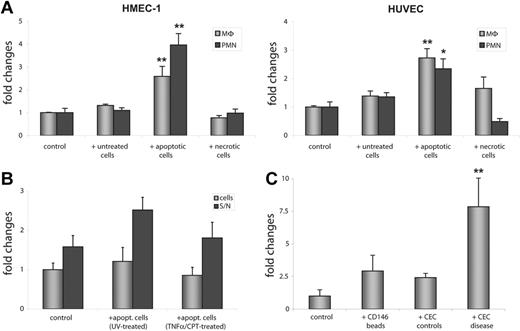
Adhesion of neutrophils (> 3.9-fold increase, P < .001) and macrophages (> 2.5-fold increase, P < .001) to HMEC-1 cells increased significantly after contact with apoptotic cells for 4 hours, whereas exposure of HMEC-1 cells to necrotic or untreated cells did not have any significant effect on neutrophil or macrophage adhesion. We repeated this assay with macrovascular HUVECs and could confirm this finding in that pre-exposure of HUVECs to apoptotic HMEC-1 cells but not to necrotic or control cells resulted in elevated binding of neutrophils (> 2-fold increase, P < .01) and macrophages (> 2.3-fold increase, P < .001, Figure 7A). Because we could not measure any significant changes in ICAM-1 expression of HMEC-1 cells exposed to damaged cells, we wondered whether the enhanced binding of neutrophils was dependent on the supernatant of the recipient cells or on the cells itself. Therefore, we transferred the supernatant of HMEC-1 cells that were exposed to apoptotic cells to untreated HMEC-1 cells and measured adhesion of neutrophils. Surprisingly, the numbers of bound neutrophils on the pretreated ECs was much lower compared to the number of neutrophils that adhere to HMEC-1 cells receiving the preconditioned supernatant. Moreover, the number of neutrophils that bound to the pretreated cells did not differ between controls and HMEC-1 cells that were exposed to apoptotic cells. In contrast, supernatant of HMEC-1 cells exposed to apoptotic cells caused an increase in neutrophil binding as compared to supernatant from controls (Figure 7B).
Apoptotic cells trigger enhanced binding of leukocytes to endothelial cells. (A) Exposure of HMEC-1 cells and HUVECs to apoptotic HMEC-1 cells for 4 hours increased adhesion of PMNs and MΦ, whereas exposure to necrotic cells failed to induce enhanced binding of leukocytes to HMEC-1 cells. HUVECs coincubated with necrotic HMEC-1 cells showed enhanced binding of MΦ but not of PMNs. (B) The supernatant of HMEC-1 cells that were exposed to apoptotic ECs is sufficient to promote increased binding of neutrophils to HMEC-1 cells, whereas the pretreated cells alone do not show enhanced binding of neutrophils. (C) Incubation of HMEC-1 cells with CECs isolated from patients with PR3-ANCA–associated vasculitis leads to a significant increase in adhesion of neutrophils. MΦ indicates primary blood mononuclear cells (monocytes/macrophages); PMN, polymorphonuclear cells (neutrophils). Data represent mean ± SEM of 5 (A,C) or 3 (B) independent experiments. Mann-Whitney U test was performed to compare differences in leukocyte adhesion (*P < .01; *P < .001).
Apoptotic cells trigger enhanced binding of leukocytes to endothelial cells. (A) Exposure of HMEC-1 cells and HUVECs to apoptotic HMEC-1 cells for 4 hours increased adhesion of PMNs and MΦ, whereas exposure to necrotic cells failed to induce enhanced binding of leukocytes to HMEC-1 cells. HUVECs coincubated with necrotic HMEC-1 cells showed enhanced binding of MΦ but not of PMNs. (B) The supernatant of HMEC-1 cells that were exposed to apoptotic ECs is sufficient to promote increased binding of neutrophils to HMEC-1 cells, whereas the pretreated cells alone do not show enhanced binding of neutrophils. (C) Incubation of HMEC-1 cells with CECs isolated from patients with PR3-ANCA–associated vasculitis leads to a significant increase in adhesion of neutrophils. MΦ indicates primary blood mononuclear cells (monocytes/macrophages); PMN, polymorphonuclear cells (neutrophils). Data represent mean ± SEM of 5 (A,C) or 3 (B) independent experiments. Mann-Whitney U test was performed to compare differences in leukocyte adhesion (*P < .01; *P < .001).
CECs from patients increased adhesion of neutrophils to HMEC-1 cells
To test whether the data we obtained from the in vitro experiments may reflect the conditions in vivo, we incubated HMEC-1 cells with CECs isolated from patients with ANCA-associated vasculitis or from healthy controls. Adhesion of healthy neutrophils significantly increased (> 7.5-fold increase; P < .001, n = 4) under conditions where HMEC-1 cells were exposed to CECs from patients, whereas CECs from healthy subjects (n = 4) or incubation with CD146-coated Dynabeads (n = 4) induced a slight and insignificant rise in the number of bound neutrophils (Figure 7C).
Discussion
Since their first description, CECs have been used as markers of vascular damage across an ever-widening variety of diseases.12 The clinical use of this marker has been demonstrated in a broad range of vascular disorders and technical consensus is currently discussed to permit a more widespread use.33 However, the accumulation of CECs in the blood not only serves as an easily accessible diagnostic marker but also represents a high-risk factor for the onset of inflammation in the vasculature.
Up to now, we could only speculate about the phenotype of the CECs. It is conceivable that these circulating cells undergo anoikis, a specific type of apoptosis that is caused by detachment of the cell from its supportive matrix.34 Apoptosis is a naturally occurring process during normal growth or development and is necessary to remove excess cells from tissue by neighboring cells or macrophages. The complete removal of apoptotic cells is important because only effective clearance of apoptotic cells prevents proinflammatory responses.2,35 In vasculitis, however, markedly elevated numbers of CECs seem to exhaust the existing clearance mechanisms. Few data, if any, shed light on the impact of apoptotic or necrotic endothelial corpses on other cell subsets. In this study, we provide, for the first time, proof of such interactions between apoptotic and necrotic ECs and healthy endothelium in vitro. We observed an inflammatory phenotype with elevated level of proinflammatory IL-8 and MCP1 transcripts after exposure to apoptotic cells. These results differ from previous findings where engulfment of apoptotic cells by phagocytes suppresses the onset of inflammation and immune responses through release of anti-inflammatory cytokines.36-39 Similarly, nonprofessional phagocytes like epithelial cells release a set of growth and survival factors and promote EC proliferation when exposed to apoptotic cells.40 Others, however, have described that engulfment of apoptotic cells by phagocytes results in secretion of proinflammatory cytokines.41,42 Khan and colleagues studied the influence of oxidized lipoproteins on the release of proinflammatory cytokines in macrophages (MΦ) that were exposed to apoptotic cells. Under normal conditions engulfment of apoptotic cells by MΦ suppressed the release of proinflammatory cytokines. In contrast, pretreatment with oxidized lipoprotein prevented this suppression.43 Our work lends further support to the hypothesis that apoptotic cells can induce proinflammatory reactions under certain conditions. Ongoing apoptosis is a key feature of multicellular organisms and a sustained proinflammatory reaction would be detrimental. We speculate that anti-inflammatory effects dominate when apoptosis occurs under normal conditions. However, in disorders where high amounts of apoptotic cells are present nonprofessional phagocytes may trigger proinflammatory signals, but these signals are overridden by anti-inflammatory effects in phagocytes. The biologic importance of an endothelial proinflammatory response after exposure to apoptotic cells certainly deserves further study.
It is tempting to speculate about the effects of IL-8 and MCP1 synthesis by ECs in our model, since both IL-8 and MCP1 are involved in inflammatory diseases. IL-8 plays a role in the activation of monocytes. Induction of IL-8 in healthy endothelium by apoptotic ECs may recruit monocytes to vascular lesions.44 MCP1 has been implicated in vasculogenesis, the development of atherosclerotic lesions, and in thrombosis.45,46 Both chemokines have previously been detected in ECs under various conditions.47,48 In our model, exposure of HMEC-1 cells to apoptotic cells markedly increased the mRNA expression of IL-8 and MCP1. We also demonstrated that direct contact of apoptotic cells to HMEC-1 cells is essential for the elevated release of IL-8 and MCP1. The proinflammatory response was accompanied by enhanced binding of neutrophils and macrophages to HMEC-1 cells and to primary macrovascular HUVECs. However, we were not able to detect any significant changes in expression of the adhesion molecules ICAM-1. This could be explained by the fact that the inflammatory responses in our in vitro model occurred in the first 3 hours and that transcriptional regulation of ICAM-1 may occur later on. Another possibility may be that the main factor that drives the increased binding of neutrophils is not the EC itself but its release of chemotactic factors. This hypothesis is supported by our findings that the preconditioned supernatants of HMEC-1 cells exposed to apoptotic cells triggered enhanced binding of neutrophils to untreated ECs.
Taken together, these results indicate that apoptotic ECs can be involved in leukocyte recruitment and adhesion to healthy endothelium. Surprisingly, exposure of HMEC-1 cells to necrotic HMEC-1 cells induced neither increased binding of leukocytes nor release of enhanced amounts of MCP1.
Recent data by Secchiero and colleagues suggest a role for TRAIL and its receptors in mediating cytokine-induced adherence of leukocytes to ECs by selective down-regulation of CCL8 and CXCL10 chemokines and promoting the survival/proliferation of endothelial cells.49,50 TRAIL is structurally related to the TNF family of cytokines and was described as a major factor for propagating proapoptotic signals.51,52 Other studies suggest a role for TRAIL and its receptors in mediating prosurvival signals, possibly via activation of the extracellular-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) and nuclear factor κB pathways.53 We were interested to note that prolonged exposure to apoptotic or necrotic ECs led to a marked decline in TRAIL mRNA. These data suggest that prolonged exposure provides an anti-inflammatory signal as a negative regulatory mechanism.
Previous studies have demonstrated that endothelial cells phagocytose latex beads, crystals, and bacteria but these cells have not been implicated in clearance of apoptotic cells.54-57 Here we show that HMEC-1 cells as well as HUVECs engulf necrotic and apoptotic endothelial material. Under physiologic conditions engulfment of apoptotic cells by healthy endothelium may be of minor importance because this task is performed by macrophages and granulocytes. The situation may be different in vascular diseases, such as ANCA-associated vasculitis or active atherosclerosis, where engulfment of apoptotic cells is delayed or the larger numbers of apoptotic cells may overwhelm the clearance by professional phagocytes. Under these conditions, engulfment of apoptotic ECs by healthy endothelium may represent a last resort to clear apoptotic material from peripheral blood. Finally, the proinflammatory response observed here could serve to recruit immunocompetent cells to sites of ongoing vascular damage. Our results may well have considerable importance in vascular diseases although this hypothesis needs to be confirmed by further studies.
It may be argued that our findings have limited relevance to the situation in vivo since we incubated healthy endothelium with much higher cell numbers of damaged ECs than were normally found in the blood of patients. It must be appreciated, however, that CECs have only been detected in peripheral blood and that cell numbers within vascular lesions may be much higher. Finally, CECs isolated from patients with vasculitis caused a much higher increase in neutrophil adhesion to cocultivated HMEC-1 cells than do apoptotic HMEC-1 cells, although they were applied in much lower concentrations. Therefore, the proinflammatory potential of CECs seems to be much greater than that of experimentally damaged HMEC-1 cells. In this regard, our findings also underscore the need for further analysis of CECs in terms of phenotype and expression of surface markers.
Unfortunately, CECs are few in number and it has been difficult to study these cells in detail.14 We have described mainly necrotic cells in systemic ANCA-associated vasculitis although the distinction between apoptosis and necrosis remains difficult in this setting. Moreover, it cannot be excluded that apoptotic circulating cells undergo secondary necrosis. The lack of markers that permit information as to the origin of CECs (microvascular versus macrovascular) is also troubling. In summary, we do not have any information about the original condition of the detached cells at the site of damage. Finally, different techniques used for the enrichment of CECs may yield different results. For instance, a recent study described a subpopulation of inflammatory circulating endothelial cells in patients with PR3-ANCA–associated vasculitis that were able to re-adhere to fibronectin-coated wells and to form a monolayer, demonstrating that this specific subpopulation of CECs seems to be neither of an apoptotic nor a necrotic phenotype.58
In conclusion, we demonstrate that apoptotic ECs induce IL-8 and MCP1 synthesis in healthy human microvascular ECs and lead to enhanced adherence of leukocytes. Direct cell-to-cell contact is mandatory for the induction of this response. Finally, we demonstrate engulfment of apoptotic and necrotic endothelial material by healthy human endothelium and that engulfment, rather than binding of the damaged cells, is a prerequisite for the inflammatory responses. These findings may provide an important mechanism by which inflammatory CECs gain pathogenetic importance locally or distant from sites of injury. Interactions of CECs with healthy endothelium warrant further studies and the importance of our findings in vivo remains to be elucidated.
Authorship
Contributions: T.K. designed and performed experiments and wrote the paper; A.W. analyzed data and wrote the paper; M.B. performed experiments; K.W. prepared and maintained HUVECs and HMEC-1 cells; J.K.P. performed fluorescence microscopy; U.E. designed and supervised FACS analysis; B.H. performed FACS analysis; H.H. designed research; and M.H. designed the study, supervised experiments, analyzed data, and contributed to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marion Haubitz, Division of Nephrology, Department of Medicine, Hannover Medical School, Carl-Neuberg-Strasse 1, 30625 Hannover, Germany; e-mail: haubitz.marion@mh-hannover.de.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG Wo 907/1-1; A.W. and M.H.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal