It has become apparent that we should eradicate leukemic stem cells in order to successfully treat acute leukemias. Román-Gómez and colleagues in this issue of Blood demonstrate the potential of targeting the WNT/β-catenin signaling pathway in ALL in this respect.
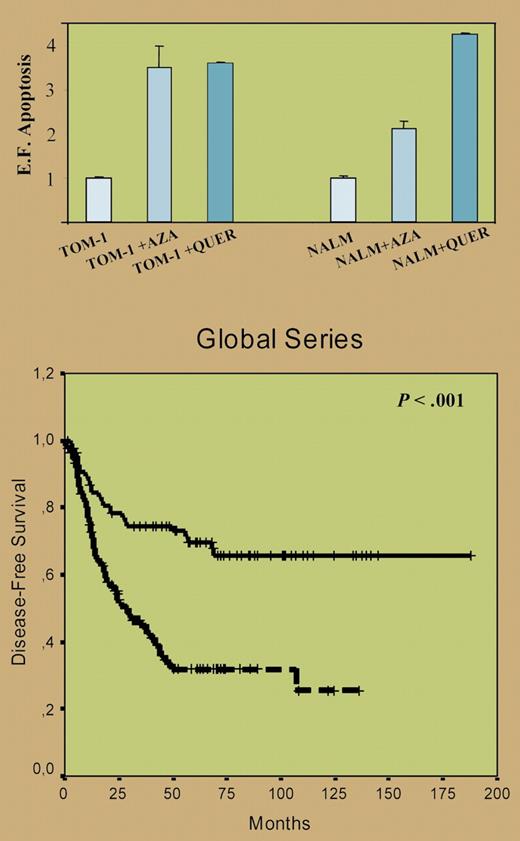
For the first time, and in a large group of 261 newly diagnosed acute lymphoblastic leukemia (ALL) patients, Román-Gómez and colleagues provide strong evidence for a relation between hypermethylation of natural WNT inhibitors and activation of the WNT signaling pathway in ALL. Moreover, the authors demonstrated that treatment in vitro with the WNT inhibitor quercetin or the demethylating agent 5-aza-2′-deoxycytidine inactivated the WNT pathway and resulted in apoptosis of the ALL cells (top panel of figure). Finally, hypermethylation of at least 1 of the 7 natural WNT inhibitors that were studied provided an independent adverse prognostic factor for disease-free and overall survival in both children and adults with ALL (bottom panel of figure).
(Top) Inhibition of the Wnt signaling by 5-aza-2′deoxycytidine and quercetin induces apoptosis of ALL cells. Apoptosis of ALL cells was significantly higher after treatment with quercetin (P < .05). (Bottom) Kaplan-Meier survivor function for patients with ALL. DFS curves for all the patients enrolled in this study, with an adverse impact of hypermethylated WNT inhibitors. See the complete figures in the article beginning on page 3462.
(Top) Inhibition of the Wnt signaling by 5-aza-2′deoxycytidine and quercetin induces apoptosis of ALL cells. Apoptosis of ALL cells was significantly higher after treatment with quercetin (P < .05). (Bottom) Kaplan-Meier survivor function for patients with ALL. DFS curves for all the patients enrolled in this study, with an adverse impact of hypermethylated WNT inhibitors. See the complete figures in the article beginning on page 3462.
Several unanswered questions remain, partly because of some limitations of this study. Intriguing is why there seems no biologic and/or clinical significance of either 1 or up to 7 WNT inhibitor genes being hypermethylated. Is each of these genes so crucial that low expression by promoter hypermethylation already results in activation of the WNT signaling pathway? Will it be technically feasible to study these methylation patterns in minimal residual disease (MRD) or even leukemic stem cells? Without a correlation with white blood cell count, how should we explain the worse prognosis of ALL patients with hypermethylation of WNT inhibitors (ie, an activated WNT pathway), a worse prognosis that was mainly caused by an increased relapse rate. Does an activated WNT pathway correlate with increased cellular drug resistance, thereby resulting in higher levels of MRD (the complete remission [CR] rate was not affected by hypermethylation) and subsequently a higher relapse rate? Do ALL patients with an activated WNT pathway have a higher leukemic stem cell burden, which was demonstrated to predict a worse outcome in adult acute myeloid leukemia (AML) by van Rhenen et al?1 Or is the (largely unexplained) therapeutic effect of maintenance treatment correlated with the WNT/β-catenin signaling pathway? And finally, can the prognostic significance of hypermethylation of natural WNT inhibitors be overcome by allogeneic stem cell transplantation?
This study by Román-Gómez et al has important clinical implications. It provides a new and strong prognostic factor, which might be used for risk-group stratification. Other groups still have to reproduce this in the context of their own treatment protocol, with rigorous validation of the techniques being used. Moreover, it indicates a new therapeutic strategy, interfering with the WNT signaling pathway. According to this study, about two thirds of ALL patients might benefit from treatment with a WNT inhibitor or with a demethylating agent. Of note, this susceptibility seems similar in newly diagnosed and relapsed ALL according to the unchanged pattern of hypermethylation in paired cases (albeit in a low number of patients). Studying the in vitro and in vivo antileukemic effect of a combination of such agents, possibly with the addition of an HDAC inhibitor, would be of interest. Methylation status or expression of WNT inhibitors might also provide biologic markers to determine biologically effective drug doses. The important role of WNT signaling has been demonstrated in leukemic stem cells,2,3 stem cells that now have been found in ALL as well.4,5 Therefore, clinical studies on the benefit of targeting the WNT signaling pathway might be performed after eradication of the bulk of the leukemic cells and after minimizing the amount of MRD, targeting the remaining MRD and especially the leukemic stem cells.
We are entering an exciting era of new therapeutic strategies in acute leukemias, and the paper by Román-Gómez and colleagues in this issue of Blood describes WNT signaling pathway interference as one of them.
The author declares no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal