Abstract
Protein C is best known for its mild deficiency associated with venous thrombosis risk and severe deficiency associated with neonatal purpura fulminans. Activated protein C (APC) anticoagulant activity involves proteolytic inactivation of factors Va and VIIIa, and APC resistance is often caused by factor V Leiden. Less known is the clinical success of APC in reducing mortality in severe sepsis patients (PROWESS trial) that gave impetus to new directions for basic and preclinical research on APC. This review summarizes insights gleaned from recent in vitro and in vivo studies of the direct cytoprotective effects of APC that include beneficial alterations in gene expression profiles, anti-inflammatory actions, antiapoptotic activities, and stabilization of endothelial barriers. APC's cytoprotection requires its receptor, endothelial cell protein C receptor, and protease-activated receptor-1. Because of its pleiotropic activities, APC has potential roles in the treatment of complex disorders, including sepsis, thrombosis, and ischemic stroke. Although much about molecular mechanisms for APC's effects on cells remains unclear, it is clear that APC's structural features mediating anticoagulant actions and related bleeding risks are distinct from those mediating cytoprotective actions, suggesting the possibility of developing APC variants with an improved profile for the ratio of cytoprotective to anticoagulant actions.
Genetic deficiencies of protein C
Protein C circulates in plasma at 70 nM as the zymogen of the anticoagulant serine protease, activated protein C (APC), which averages 40 pM (∼ 2.3 ng/mL) in normal plasma.1 The physiologic importance of the protein C system is most clearly demonstrated by the massive, usually lethal, thrombotic complications occurring in infants with severe homozygous protein C deficiency and the significantly increased risk for venous thrombosis in heterozygous deficient adults.2,3 The most commonly identifiable hereditary risk factor for venous thrombosis among whites involves an APC-cleavage site mutation (Arg506Gln, factor V Leiden) that is the major target for factor Va inactivation by APC.4-6 Targeted deletion of the protein C gene in mice results in perinatal lethality.7,8 Nevertheless, in the absence of fetal protein C, embryogenesis and development occur, potentially due to maternal protein C in the fetus. Although protein C–null embryos develop at the expected Mendelian distribution until embryonic day 17.5, these embryos show extensive bleeding, coagulopathy, fibrin deposition, and liver necrosis.7,8 In moderately to severely deficient mice, protein C levels of 1% to 18% suffice for development and birth, although such mice are prone to early onset thrombosis and inflammation, indicating protein C's physiological role as an antithrombotic and anti-inflammatory protein.9
The anticoagulant protein C pathway
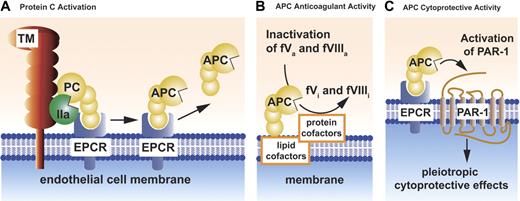
Cell surfaces localize and promote a variety of biochemical transformations involving protein C and various cellular protein receptors (Figure 1A-C). These biochemical reactions include protein C activation, expression of APC anticoagulant activity, and initiation of APC's cytoprotective actions (see “The cytoprotective protein C pathway”).
Schematic models of PC activation and APC activities. Protein C (PC) activation occurs on the endothelial cell membrane and requires thrombomodulin (TM) (A). Thrombin (IIa) bound to thrombomodulin activates protein C. Binding of protein C to its endothelial receptor, EPCR, provides for the most efficient activation of protein C. Protein C and APC have a similar affinity for EPCR. Dissociation of APC from EPCR allows expression of APC's anticoagulant activity (B), whereas retention of APC bound to EPCR allows APC to express multiple direct cellular activities (C). APC conveys its anticoagulant activity (B) when bound to cell membrane surfaces, various microparticles, or lipoproteins (eg, HDL). As an anticoagulant, APC cleaves the activated cofactors Va (fVa) and VIIIa (fVIIIa) to yield the inactivated cofactors fVi and fVIIIi. Inactivation of factors Va and VIIIa by APC is enhanced by a number of different protein cofactors (eg, protein S, factor V) and various lipids cofactors (eg, phosphatidylserine, cardiolipin, glucosylceramide, HDL). Beneficial activities of APC that involve direct effects of APC on cells require the cellular receptors EPCR and PAR-1 (C). These activities include APC-mediated alteration of gene expression, anti-inflammatory activities, antiapoptotic activities, and protection of endothelial barrier functions. Collectively, these activities are referred to as APC's cytoprotective activities.
Schematic models of PC activation and APC activities. Protein C (PC) activation occurs on the endothelial cell membrane and requires thrombomodulin (TM) (A). Thrombin (IIa) bound to thrombomodulin activates protein C. Binding of protein C to its endothelial receptor, EPCR, provides for the most efficient activation of protein C. Protein C and APC have a similar affinity for EPCR. Dissociation of APC from EPCR allows expression of APC's anticoagulant activity (B), whereas retention of APC bound to EPCR allows APC to express multiple direct cellular activities (C). APC conveys its anticoagulant activity (B) when bound to cell membrane surfaces, various microparticles, or lipoproteins (eg, HDL). As an anticoagulant, APC cleaves the activated cofactors Va (fVa) and VIIIa (fVIIIa) to yield the inactivated cofactors fVi and fVIIIi. Inactivation of factors Va and VIIIa by APC is enhanced by a number of different protein cofactors (eg, protein S, factor V) and various lipids cofactors (eg, phosphatidylserine, cardiolipin, glucosylceramide, HDL). Beneficial activities of APC that involve direct effects of APC on cells require the cellular receptors EPCR and PAR-1 (C). These activities include APC-mediated alteration of gene expression, anti-inflammatory activities, antiapoptotic activities, and protection of endothelial barrier functions. Collectively, these activities are referred to as APC's cytoprotective activities.
Physiological proteolytic activation of protein C by thrombin occurs on the surface of the endothelial cell and involves the 2 membrane receptors, thrombomodulin and endothelial protein C receptor (EPCR) (Figure 1A).10 Binding of thrombin to thrombomodulin on the endothelial surface shields thrombin's procoagulant exosite I and promotes its anticoagulant properties by activation of protein C by the thrombin-thrombomodulin complex. This reaction is augmented by localization of protein C on the endothelial surface by its binding to EPCR.11
The anticoagulant actions of APC are primarily based on the irreversible proteolytic inactivation of factors Va and VIIIa with contributions by various cofactors (Figure 1B). These anticoagulant APC cofactors comprise both proteins and lipids, including protein S, factor V, high-density lipoprotein, anionic phospholipids (eg, phosphatidylserine, cardiolipin), and glycosphingolipids (eg, glucosylceramide). The reader is referred to excellent previous reviews for discussions of APC's anticoagulant mechanisms that are beyond the scope of this review.10,12,13
The cytoprotective protein C pathway
In reactions mediated by EPCR10 and the effector receptor, protease-activated receptor-1 (PAR-114 ), APC remarkably acts directly on cells to exert multiple cytoprotective effects including (1) alteration of gene expression profiles; (2) anti-inflammatory activities; (3) antiapoptotic activity; and (4) protection of endothelial barrier function (Figure 1C).15-19 As clear from the schemes presented in Figure 1B-C, the pathways for anticoagulant and cytoprotective pathways differ not only in terms of biologic effects but also in terms of substrates and cofactors. APC's substrates for anticoagulant action are factors Va and VIIIa and for cytoprotective actions, PAR-1. We would like to emphasize making an explicit distinction between the protein C cytoprotective pathway and the protein C anticoagulant pathway because this distinction is useful for design and interpretation of preclinical and clinical results and of in vitro experiments. Moreover, this distinction may also be made based on genetic engineering of APC variants with selectively altered specificities for factor Va versus PAR-1. Such APC variants may facilitate experimental distinction of the relative importance of APC's direct effects on cells versus APC's anticoagulant activity (see “Roles for APC's anticoagulant activity versus APC's cytoprotective activities”).
The tissue distribution of EPCR and thrombomodulin is notably distinct and different, with higher levels of EPCR on the surface of large vessels versus smaller vessels, whereas thrombomodulin shows the opposite pattern.10,20-23 No studies have defined the tissue distribution or cellular localization of EPCR relative to thrombomodulin or PAR-1. It will be interesting to establish the extent to which thrombomodulin and EPCR colocalize in lipid membrane rafts in different tissues where protein C activation can be localized versus the colocalization of PAR-1 and EPCR. Such data may enable speculation about the tissue specificity for protein C activation versus expression of APC cytoprotective activities.
Mechanisms for APC beneficial effects
Both the anticoagulant and cytoprotective protein C pathways are important for APC's beneficial effects in various settings. As noted in “The anticoagulant protein C pathway,” the anticoagulant protein C pathway is centered on inactivation of factors Va and VIIIa by APC and involves protein and lipid cofactors (Figure 1B). Until recently, investigators often presumed that the systemic anticoagulant activity of APC mediated APC's beneficial anti-inflammatory effects because APC down-regulates generation of thrombin, which was recognized for its proinflammatory properties and because no other mechanism for APC's anti-inflammatory activity was apparent. However, both clinical trial data and basic lab findings have changed this viewpoint and stimulated research on the protein C cytoprotective pathway. In clinical trials, the ability of APC, but not of other anticoagulants such as antithrombin and tissue factor pathway inhibitor, to reduce mortality in severe sepsis patients in comparable large phase 3 trials implicates the less well-defined cytoprotective actions of APC as potentially very important for APC's pharmacologic success.24-26 In the laboratory, extensive in vivo and in vitro studies related to murine ischemic stroke models imply that APC's neuroprotective effects, at least in part, are independent of APC's anticoagulant activity,16 and recent studies show that APC variants with severely reduced anticoagulant activity prevent endotoxemia-induced death in mice (see “Roles for APC's anticoagulant activity versus APC's cytoprotective activities”).27 These various observations support the notion that APC's beneficial cytoprotective effects are beneficial in vivo and that they are, at least in part, independent of APC anticoagulant activity.27-29
The direct effects of APC on cells, that is, APC's cytoprotective effects, include (1) alteration of gene expression profiles; (2) anti-inflammatory activities; (3) antiapoptotic activity; and (4) endothelial barrier stabilization. Although potentially interrelated, each of these activities of APC is distinct and may or may not involve the same intracellular mechanisms with their particular APC dose-response characteristics, depending on a cell's receptor profile and on a particular cell's location, condition, or particular properties. Much remains to be clarified about these issues.
As summarized under the next 4 subheadings, EPCR is required for most if not all of APC's known cytoprotective actions, and a requirement for PAR-1 is also implicated for many of APC's effects on cells.
APC-mediated alteration of gene expression profiles
Transcriptional profiling of alterations in gene expression patterns caused by APC's treatment of cells revealed modulation of gene expression for the major pathways of inflammation and apoptosis.15,16,19,30-33 The effects of APC generally involved down-regulation of proinflammatory and proapoptotic pathways and up-regulation of anti-inflammatory and antiapoptotic pathways (Table 1), thus helping to explain the anti-inflammatory and antiapoptotic activities of APC. APC suppresses nuclear transcription factor κB (NFκB)–modulated genes by directly reducing NFκB expression and functional activity; this causes inhibition of cytokine signaling and inhibition of tumor necrosis factor-α (TNFα)–dependent induction of adhesion molecules. APC up-regulates antiapoptotic gene products related to Bcl-2 and suppresses proapoptotic p53 and Bax expression.
Up-regulation and down-regulation of gene expression by APC
| Gene* . | Official gene name . | Pathway affected . | Reference sequence . | Other protein name . | Sources . |
|---|---|---|---|---|---|
| Up-regulation | |||||
| BCL2A1 | BCL2-related protein A1 | Apoptosis | NM_004049 | A1 | Joyce et al,15 Riewald and Ruf30 |
| BMP2 | Bone morphogenetic protein 2 | Differentiation | NM_001200 | BMP-2 | Riewald et al,19 Riewald and Ruf30 |
| CCL2 | Chemokine (C-C motif) ligand 2 | Inflammation | NM_002982 | MCP-1 | Riewald et al,19 Franscini et al33 |
| CX3CL1† | Chemokine (C-X3-C motif) ligand 1 | Adhesion | NM_002996 | Fractalkine | Joyce et al,15 Franscini et al,33 Brueckmann et al34 |
| CXCL2 | Chemokine (C-X-C motif) ligand 2 | Inflammation | NM_002089 | MIP-2, GRO | Riewald et al,19 Riewald and Ruf,30 Franscini et al33 |
| DDX21 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 21 | Transcription | NM_004728 | RNA helicase II/Gu-α | Joyce et al15 |
| F3 | Coagulation factor III (thromboplastin, tissue factor) | Coagulation | NM_001993 | TF, CD142 | Riewald et al,19 Riewald and Ruf30 |
| HBEGF | Heparin-binding EGF-like growth factor | Proliferation | NM_001945 | HBEGF | Riewald et al19 |
| IL1RI† | Interleukin 1 receptor, type 1 | Inflammation | NM_000877 | IL-1R1, CD121a | Riewald and Ruf,30 Franscini et al33 |
| IL6 | Interleukin 6 (interferon, beta 2) | Inflammation | NM_000600 | IL-6 | Franscini et al,33 Hooper et al35 |
| IL8 | Interleukin 8 | Inflammation | NM_000584 | IL-8, CXCL-8 | Franscini et al,33 Hooper et al,35 Brueckmann et al36 |
| NFKB1† | Nuclear factor of kappa light polypeptide gene enhancer 1 | Transcription | NM_003998 | NF-κB | Joyce et al,15 Joyce and Grinnell,32 Franscini et al33 |
| NFKB2† | Nuclear factor of kappa light polypeptide gene enhancer 2 | Transcription | NM_002502 | NF-κB2 p100 | Joyce et al,15 Riewald and Ruf,30 Franscini et al33 |
| NOS3 | Nitric oxide synthase 3 (endothelial cell) | Inflammation | NM_000603 | eNOS | Joyce et al15 |
| NR4A1 | Nuclear receptor subfamily 4, group A, member 1 | Apoptosis | NM_173157 | TR3, Nur77, NA4A1 | Riewald et al,19 Riewald and Ruf,30 Franscini et al33 |
| NR4A2 | Nuclear receptor subfamily 4, group A, member 2 | Transcription | NM_006186 | NURR1, NA4A2 | Riewald et al,19 Riewald and Ruf30 |
| NR4A3 | Nuclear receptor subfamily 4, group A, member 3 | Transcription | NM_006981 | NOR-1, NA4A3 | Riewald et al,19 Riewald and Ruf30 |
| PCNA | Proliferating cell nuclear antigen | Cell cycle | NM_002592 | PCNA | Joyce et al15 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 | Inflammation | NM_000963 | COX-2 | Riewald et al,19 Riewald and Ruf,30 Brueckmann et al37 |
| SMAD3 | SMAD, mothers against DPP homolog 3 (Drosophila) | Apoptosis | NM_005902 | SMAD-3 | Joyce et al15 |
| TNFAIP3 | Tumor necrosis factor, alpha-induced protein 3 | Apoptosis | NM_006290 | A20 | Joyce et al,15 Riewald et al19 |
| Down-regulation | |||||
| B2M | Beta-2-microglobulin | Inflammation | NM_004048 | B2M | Joyce and Grinnell32 |
| BIRC5 | Baculoviral IAP repeat-containing 5 (survivin) | Apoptosis | NM_001168 | Survivin | Joyce and Grinnell,32 Franscini et al33 |
| CALR | Calreticulin | Inflammation | NM_004343 | Ro, cC1qR | Joyce et al,15 Joyce and Grinnell32 |
| CMKOR1 | Chemokine orphan receptor 1 | Cell signaling | NM_020311 | RDC1 | Joyce et al15 |
| CX3CL1† | Chemokine (C-X3-C motif) ligand 1 | Adhesion | NM_002996 | Fractalkine | Joyce et al,15 Franscini et al,33 Brueckmann et al34 |
| EFNA1 | Ephrin-A1 | Chemoattract | NM_004428 | Ephrin-A1 | Joyce et al15 |
| ICAM1 | Intercellular adhesion molecule 1 (CD54) | Adhesion | NM_000201 | ICAM-1, CD54 | Joyce et al,15 Franscini et al33 |
| IL1R1† | Interleukin 1 receptor, type I | Inflammation | NM_000877 | IL-1R1, CD121a | Riewald and Ruf,30 Franscini et al33 |
| LTB | Lymphotoxin beta (TNF superfamily, member 3) | Inflammation | NM_002341 | LT-β | Joyce and Grinnell32 |
| MMP10 | Matrix metallopeptidase 10 (stromelysin 2) | Proteolysis | NM_002425 | MMP-10 | Joyce et al15 |
| NFKB1† | Nuclear factor of kappa light polypeptide gene enhancer 1 | Transcription | NM_003998 | NF-κB | Joyce et al,15 Joyce and Grinnell,32 Franscini et al33 |
| NFKB2† | Nuclear factor of kappa light polypeptide gene enhancer 2 | Transcription | NM_002502 | NF-κB2 p100 | Joyce et al,15 Riewald and Ruf30 |
| SELE | Selectin E (endothelial adhesion molecule 1) | Adhesion | NM_000450 | E-selectin, CD62E | Joyce et al,15 Franscini et al33 |
| SOD2 | Superoxide dismutase 2, mitochondrial | Oxidation | NM_000636 | MnSOD | Joyce et al15 |
| THBS1‡ | Thrombospondin 1 | Adhesion | NM_003246 | TSP-1 | Riewald and Ruf30 |
| TP53‡ | Tumor protein p53 (Li-Fraumeni syndrome) | Apoptosis | NM_000546 | p53 | Cheng et al,16 Riewald and Ruf,30 Guo et al31 |
| VCAM1 | Vascular cell adhesion molecule 1 | Adhesion | NM_080682 | VCAM-1, CD106 | Joyce et al,15 Franscini et al33 |
| Gene* . | Official gene name . | Pathway affected . | Reference sequence . | Other protein name . | Sources . |
|---|---|---|---|---|---|
| Up-regulation | |||||
| BCL2A1 | BCL2-related protein A1 | Apoptosis | NM_004049 | A1 | Joyce et al,15 Riewald and Ruf30 |
| BMP2 | Bone morphogenetic protein 2 | Differentiation | NM_001200 | BMP-2 | Riewald et al,19 Riewald and Ruf30 |
| CCL2 | Chemokine (C-C motif) ligand 2 | Inflammation | NM_002982 | MCP-1 | Riewald et al,19 Franscini et al33 |
| CX3CL1† | Chemokine (C-X3-C motif) ligand 1 | Adhesion | NM_002996 | Fractalkine | Joyce et al,15 Franscini et al,33 Brueckmann et al34 |
| CXCL2 | Chemokine (C-X-C motif) ligand 2 | Inflammation | NM_002089 | MIP-2, GRO | Riewald et al,19 Riewald and Ruf,30 Franscini et al33 |
| DDX21 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 21 | Transcription | NM_004728 | RNA helicase II/Gu-α | Joyce et al15 |
| F3 | Coagulation factor III (thromboplastin, tissue factor) | Coagulation | NM_001993 | TF, CD142 | Riewald et al,19 Riewald and Ruf30 |
| HBEGF | Heparin-binding EGF-like growth factor | Proliferation | NM_001945 | HBEGF | Riewald et al19 |
| IL1RI† | Interleukin 1 receptor, type 1 | Inflammation | NM_000877 | IL-1R1, CD121a | Riewald and Ruf,30 Franscini et al33 |
| IL6 | Interleukin 6 (interferon, beta 2) | Inflammation | NM_000600 | IL-6 | Franscini et al,33 Hooper et al35 |
| IL8 | Interleukin 8 | Inflammation | NM_000584 | IL-8, CXCL-8 | Franscini et al,33 Hooper et al,35 Brueckmann et al36 |
| NFKB1† | Nuclear factor of kappa light polypeptide gene enhancer 1 | Transcription | NM_003998 | NF-κB | Joyce et al,15 Joyce and Grinnell,32 Franscini et al33 |
| NFKB2† | Nuclear factor of kappa light polypeptide gene enhancer 2 | Transcription | NM_002502 | NF-κB2 p100 | Joyce et al,15 Riewald and Ruf,30 Franscini et al33 |
| NOS3 | Nitric oxide synthase 3 (endothelial cell) | Inflammation | NM_000603 | eNOS | Joyce et al15 |
| NR4A1 | Nuclear receptor subfamily 4, group A, member 1 | Apoptosis | NM_173157 | TR3, Nur77, NA4A1 | Riewald et al,19 Riewald and Ruf,30 Franscini et al33 |
| NR4A2 | Nuclear receptor subfamily 4, group A, member 2 | Transcription | NM_006186 | NURR1, NA4A2 | Riewald et al,19 Riewald and Ruf30 |
| NR4A3 | Nuclear receptor subfamily 4, group A, member 3 | Transcription | NM_006981 | NOR-1, NA4A3 | Riewald et al,19 Riewald and Ruf30 |
| PCNA | Proliferating cell nuclear antigen | Cell cycle | NM_002592 | PCNA | Joyce et al15 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 | Inflammation | NM_000963 | COX-2 | Riewald et al,19 Riewald and Ruf,30 Brueckmann et al37 |
| SMAD3 | SMAD, mothers against DPP homolog 3 (Drosophila) | Apoptosis | NM_005902 | SMAD-3 | Joyce et al15 |
| TNFAIP3 | Tumor necrosis factor, alpha-induced protein 3 | Apoptosis | NM_006290 | A20 | Joyce et al,15 Riewald et al19 |
| Down-regulation | |||||
| B2M | Beta-2-microglobulin | Inflammation | NM_004048 | B2M | Joyce and Grinnell32 |
| BIRC5 | Baculoviral IAP repeat-containing 5 (survivin) | Apoptosis | NM_001168 | Survivin | Joyce and Grinnell,32 Franscini et al33 |
| CALR | Calreticulin | Inflammation | NM_004343 | Ro, cC1qR | Joyce et al,15 Joyce and Grinnell32 |
| CMKOR1 | Chemokine orphan receptor 1 | Cell signaling | NM_020311 | RDC1 | Joyce et al15 |
| CX3CL1† | Chemokine (C-X3-C motif) ligand 1 | Adhesion | NM_002996 | Fractalkine | Joyce et al,15 Franscini et al,33 Brueckmann et al34 |
| EFNA1 | Ephrin-A1 | Chemoattract | NM_004428 | Ephrin-A1 | Joyce et al15 |
| ICAM1 | Intercellular adhesion molecule 1 (CD54) | Adhesion | NM_000201 | ICAM-1, CD54 | Joyce et al,15 Franscini et al33 |
| IL1R1† | Interleukin 1 receptor, type I | Inflammation | NM_000877 | IL-1R1, CD121a | Riewald and Ruf,30 Franscini et al33 |
| LTB | Lymphotoxin beta (TNF superfamily, member 3) | Inflammation | NM_002341 | LT-β | Joyce and Grinnell32 |
| MMP10 | Matrix metallopeptidase 10 (stromelysin 2) | Proteolysis | NM_002425 | MMP-10 | Joyce et al15 |
| NFKB1† | Nuclear factor of kappa light polypeptide gene enhancer 1 | Transcription | NM_003998 | NF-κB | Joyce et al,15 Joyce and Grinnell,32 Franscini et al33 |
| NFKB2† | Nuclear factor of kappa light polypeptide gene enhancer 2 | Transcription | NM_002502 | NF-κB2 p100 | Joyce et al,15 Riewald and Ruf30 |
| SELE | Selectin E (endothelial adhesion molecule 1) | Adhesion | NM_000450 | E-selectin, CD62E | Joyce et al,15 Franscini et al33 |
| SOD2 | Superoxide dismutase 2, mitochondrial | Oxidation | NM_000636 | MnSOD | Joyce et al15 |
| THBS1‡ | Thrombospondin 1 | Adhesion | NM_003246 | TSP-1 | Riewald and Ruf30 |
| TP53‡ | Tumor protein p53 (Li-Fraumeni syndrome) | Apoptosis | NM_000546 | p53 | Cheng et al,16 Riewald and Ruf,30 Guo et al31 |
| VCAM1 | Vascular cell adhesion molecule 1 | Adhesion | NM_080682 | VCAM-1, CD106 | Joyce et al,15 Franscini et al33 |
eNOS indicates endothelial nitric oxide synthase.
Selected genes whose expression is modulated by APC. Genes were selected from available microarray data. Gene selection criteria are based on at least 2-fold up-regulation or down-regulation by APC and independent confirmation of the gene array results. The list is not a complete list of all potentially important changes in gene expression. Nomenclature according to HUGO gene nomenclature committee.
These genes are down-regulated by APC in TNFα-stimulated human umbilical vein endothelial cells (HUVECs) but up-regulated by APC when human coronary artery endothelial cells (HCAECs) are stimulated with a mixture of interleukin-1β, TNFα, and interferon-γ.15,33
Modulation of the expression of thrombospondin-1 and p53 by APC in the microarray analysis was less than 2-fold. Nevertheless, APC-induced changes in these genes are shown to be functionally significant and are herein included (see “APC-mediated alteration of gene expression profiles”).
The effects of APC on gene expression profiles are at least in part due to the inhibitory effect of APC on transcription factor activity. For instance, APC suppressed inflammation-activated transcription factors of the activator protein-1 (AP-1) family c-Fos and FosB, which directly induce intercellular adhesion molecule-1 (ICAM-1) and monocyte chemoattractant protein-1 (MCP-1) in endothelial cells, as well as c-Rel, a member of the NFκB family.15
The molecular mechanisms for APC's effects on gene expression remain to be elucidated, but much of APC's modulation of gene expression profiles is mediated by EPCR-dependent PAR-1 activation. Curiously, APC-induced alterations of gene expression that required PAR-1 were not remarkably different from those induced by thrombin when the effects of these 2 proteases on unperturbed endothelial cells were studied in vitro. However, after endothelial cells were stimulated by TNFα, some of the effects on gene expression were different for APC compared with thrombin.30 Up-regulation of p53 mRNA by thrombin was most notably reduced by APC.16 Similar results were observed for expression of thrombospondin-1 that was down-regulated by APC but not by thrombin.30 APC, but not thrombin, down-regulates both p53 mRNA and protein by APC.16,30
Thus, for stressed endothelial cells, APC and thrombin clearly do not coincide in their transcriptome alteration effects, although they both can activate the same receptor, PAR-1. The basis for how 2 protease agonists can activate the same G-protein–coupled receptor, PAR-1, with different outcomes has not been clarified. Evidently, APC alters the transcriptome via mechanisms that are, at least in part, distinct from those used by thrombin.
APC anti-inflammatory activity
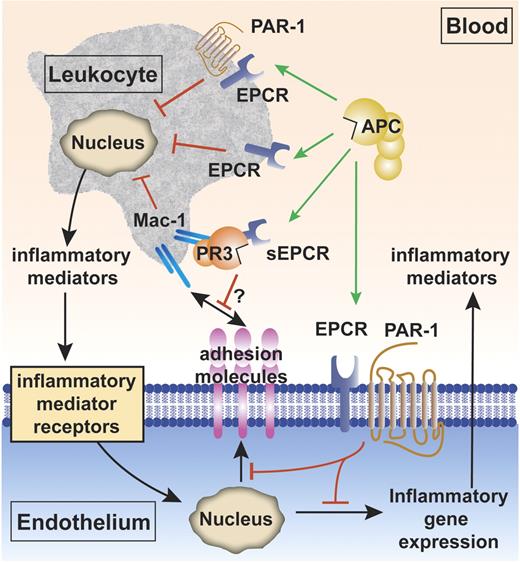
The anti-inflammatory vascular effects of APC can be divided into its effects on endothelial cells and its effects on leukocytes. APC's effects on endothelial cells include inhibition of inflammatory mediator release and down-regulation of vascular adhesion molecules, thereby reducing leukocyte adhesion and infiltration of tissues and limiting damage to underlying tissue (Figure 2). In addition, APC protects and maintains endothelial barrier function and reduces the chemotactic potential of several potent chemotactic agents.43-48
The cytoprotective protein C pathway for APC anti-inflammatory activity. Anti-inflammatory effects of APC include APC's effects on vascular endothelial cells and APC's effects on leukocytes. Inhibition of inflammatory gene expression on endothelial cells by APC is EPCR and PAR-1 dependent (green arrow). APC also down-regulates expression of vascular adhesion molecules (such as ICAM-1, VCAM-1, and E-selectin) on the endothelial surface in the presence of inflammatory mediators (red block), thereby limiting leukocyte adhesion and infiltration. APC reduces proinflammatory mediator release (such as TNFα and IL-1β) from leukocytes (red block). The mechanisms by which APC acts on leukocytes are incompletely resolved and could involve the receptors EPCR and PAR-1, EPCR alone, or others (green arrows).38-41 The complex of soluble EPCR (sEPCR) and proteinase 3 (PR3) binds to the integrin complex CD11b/CD18 (αMβ2; Mac-1; CR3) on activated neutrophils. Although speculative at present (indicated by the question mark), binding of (A)PC to sEPCR is retained when sEPCR is bound to proteinase 3, suggesting that sEPCR might mediate APC cellular signals and/or activation of protein C on leukocytes.42
The cytoprotective protein C pathway for APC anti-inflammatory activity. Anti-inflammatory effects of APC include APC's effects on vascular endothelial cells and APC's effects on leukocytes. Inhibition of inflammatory gene expression on endothelial cells by APC is EPCR and PAR-1 dependent (green arrow). APC also down-regulates expression of vascular adhesion molecules (such as ICAM-1, VCAM-1, and E-selectin) on the endothelial surface in the presence of inflammatory mediators (red block), thereby limiting leukocyte adhesion and infiltration. APC reduces proinflammatory mediator release (such as TNFα and IL-1β) from leukocytes (red block). The mechanisms by which APC acts on leukocytes are incompletely resolved and could involve the receptors EPCR and PAR-1, EPCR alone, or others (green arrows).38-41 The complex of soluble EPCR (sEPCR) and proteinase 3 (PR3) binds to the integrin complex CD11b/CD18 (αMβ2; Mac-1; CR3) on activated neutrophils. Although speculative at present (indicated by the question mark), binding of (A)PC to sEPCR is retained when sEPCR is bound to proteinase 3, suggesting that sEPCR might mediate APC cellular signals and/or activation of protein C on leukocytes.42
APC inhibits inflammatory mediator release by leukocytes as well as endothelial cells. APC diminishes cytokine release from leukocytes and thereby may attenuate initiation of systemic inflammatory responses. This APC action might reduce the so-called cytokine storm that is associated with sepsis. For example, APC inhibits lipopolysaccharide (LPS)–induced production of proinflammatory mediators by monocytes.49-52
Mechanisms for APC's anti-inflammatory effects on leukocytes are not completely clear; however, evidence indicates an important role for EPCR38-41 that is on the surface of monocytes, CD56+ natural killer cells, neutrophils, and eosinophils, but not T cells and B cells.48,53-55 Of interest, soluble EPCR lacking the transmembrane helix of native EPCR interacts with the integrin CD11b/CD18 (Mac-1) (αMβ2) (CR3) on leukocytes, suggesting that binding of soluble EPCR to CD11b/CD18 might interfere with leukocyte adhesion (Figure 2).42 Proteinase-3 (PR3), a serine protease with elastase-like properties stored in granules of neutrophils, also binds both CD11b/CD18 and soluble EPCR. PR3 expression on the plasma membrane of neutrophils is increased upon neutrophil stimulation. Although one may speculate that PR3 can mediate binding of soluble EPCR to CD11b/CD18, proof for a functional role of the EPCR:PR3:CD11b/CD18 complex remains elusive. But since the soluble EPCR:PR3 complex binds protein C and APC, it is possible that this complex may mediate APC cellular signals and/or activation of protein C on leukocytes.42
Animal injury studies are consistent with the hypothesis that APC inhibits leukocyte cytokine release, chemotaxis, and migration in vivo. In both rats and humans, APC inhibits endotoxin-induced pulmonary injury and inflammation, at least in part through inhibition of leukocyte accumulation and chemotaxis.44,45 In a rat compression-induced spinal cord injury model, APC reduces TNFα levels, neutrophil accumulation, and motor disturbances.56 It should be noted, however, that neutrophil accumulation is enhanced by fibrin. Thus, the anticoagulant action of APC, which decreases thrombin generation, may indirectly reduce neutrophil accumulation in tissues by reducing fibrin deposition.
APC prolongs the lifespan of in vivo circulating monocytes by inhibition of spontaneous monocyte apoptosis (normal half-life ∼ 24 hours).32,38 Inhibition of monocyte apoptosis by APC requires the receptors EPCR and PAR-1, consistent with results for inhibition of endothelial cell apoptosis by APC.38 Whether a lengthened survival of monocytes is beneficial during sepsis is controversial as the consequential prolonged proinflammatory mediator release may worsen tissue damage and augment the inflammatory response. On the other hand, immediate host responses to invading microorganisms and phagocytosis-mediated clearance of invading pathogens would benefit from a decreased monocyte turnover. Selective inhibition of proinflammatory mediator release in monocytes by APC while leaving the antimicrobial phagocytic capacity of monocytes intact suggests that APC antiapoptotic activity in monocytes is likely to contribute an overall anti-inflammatory effect.38
APC antiapoptotic activity
Apoptosis is a tightly regulated process to execute an efficient cell death program.57-59 Apoptotic signals coming from inside the cell activate the intrinsic pathway in response to cellular stress (eg, hypoxia), and release of cytochrome c from mitochondria and subsequent procaspase-3 activation are key steps for the intrinsic pathway. Key regulators, such as the tumor-suppressor protein, p53, and the Bcl-2 family of proteins, determine the mitochondria's roles by balancing apoptotic versus survival signals. Activation of the extrinsic apoptotic pathway, which occurs via activation of initiator caspases, such as procaspase-8, is dependent on membrane-bound death receptors and sensors of the extracellular environment. The extrinsic apoptotic pathway may also use elements of the intrinsic pathway via activation of the proapoptotic members of the Bcl-2 family or may directly activate the common pathway by activation of procaspase-3. Whether activated via the intrinsic or the extrinsic pathway, caspase-3 cleaves specific cellular substrates, leading to the morphologic and biochemical features characteristic of apoptotic cell death.57-59
APC manifests antiapoptotic activity both in vitro and in vivo, and this activity requires the enzymatic active site of APC and its receptors, EPCR and PAR-1 (Figure 3).15,16,18,60 In murine injury models, APC inhibits apoptosis while providing neuroprotection. Reduction of apoptosis is associated with improved survival in sepsis, suggesting a potential important role for APC's antiapoptotic activity in reducing mortality in sepsis.61
The cytoprotective protein C pathway for APC antiapoptotic activity. APC antiapoptotic activity requires the APC receptors EPCR and PAR-1. APC antiapoptotic activity is at least partially dependent on modulation of gene expression (green arrow). APC down-regulates proapoptotic p53 and Bax protein (red blocks) and maintains protective antiapoptotic Bcl-2 protein levels (green arrow), thereby beneficially affecting the Bax/Bcl-2 ratio. APC inhibits activation (red block) of both initiator caspases (eg, tPA-induced caspase-8 activation60 ) as well as activation of effector caspases (eg, staurosporine or hypoxia/hypoglycemia-induced caspase-3 activation16,18 ). Further work is needed to clarify exactly how APC exerts antiapoptotic activity and what the relative contributions of APC's effects on gene expression, of APC-specific signaling, and of APC-specific proteolysis involving particular receptors or effectors might be for APC's antiapoptotic actions. Green arrows indicate stimulation by APC, red blocks indicate inhibition by APC, and black arrows/blocks denote stimulation/inhibition of (downstream) reactions.
The cytoprotective protein C pathway for APC antiapoptotic activity. APC antiapoptotic activity requires the APC receptors EPCR and PAR-1. APC antiapoptotic activity is at least partially dependent on modulation of gene expression (green arrow). APC down-regulates proapoptotic p53 and Bax protein (red blocks) and maintains protective antiapoptotic Bcl-2 protein levels (green arrow), thereby beneficially affecting the Bax/Bcl-2 ratio. APC inhibits activation (red block) of both initiator caspases (eg, tPA-induced caspase-8 activation60 ) as well as activation of effector caspases (eg, staurosporine or hypoxia/hypoglycemia-induced caspase-3 activation16,18 ). Further work is needed to clarify exactly how APC exerts antiapoptotic activity and what the relative contributions of APC's effects on gene expression, of APC-specific signaling, and of APC-specific proteolysis involving particular receptors or effectors might be for APC's antiapoptotic actions. Green arrows indicate stimulation by APC, red blocks indicate inhibition by APC, and black arrows/blocks denote stimulation/inhibition of (downstream) reactions.
APC reduces many characteristic apoptotic features, including DNA degradation, caspase-3 activation, and phosphatidylserine translocation to the outer cell membrane.15,16,18,60 At this time, specific intracellular targets for APC that mediate inhibition of apoptosis have not been identified. However, in stressed human brain endothelial cells (BECs), APC reduces the amounts of p53 protein and mRNA induced by hypoxia (Figure 3).16 Curiously, only a small number of gene products, including p53 and thrombospondin-1, show PAR-1–dependent down-regulation by APC in contrast to their up-regulation by thrombin.30 During hypoxic stress of brain endothelial cells, APC reduces up-regulation of proapoptotic Bax and maintains levels of protective Bcl-2 protein, thereby blunting stimulation of the intrinsic apoptotic pathway.16
The antiapoptotic effects of APC are not limited to the intrinsic apoptotic pathway as APC counteracts the neurotoxicity of tissue plasminogen activator (tPA), which exerts proapoptotic activity via the extrinsic pathway.60 In the setting of tPA-induced apoptosis of brain cells, APC inhibits caspase-8 activation, showing APC's antiapoptotic effects when either the extrinsic pathway or the intrinsic pathway is activated in the brain. Thus, the antiapoptotic effects of APC have been shown to be broadly cytoprotective both in vitro and in vivo.
Further work is needed to clarify exactly how APC exerts antiapoptotic activity and whether this activity is limited to APC's effects on gene expression or whether other additional effects, such as APC-specific signaling or APC-specific proteolysis involving particular receptors or effectors, might play direct roles.
APC-mediated endothelial barrier stabilization
Breakdown of the endothelial barrier is a key factor in the pathogenesis of inflammation. The consequences of increased endothelial permeability involve swelling, hypotension, and promotion of inflammation, and these processes can contribute to the physiopathology of sepsis, acute lung injury, and organ failure (see reviews62-64 ). APC induces potent barrier protective effects via EPCR-dependent PAR-1 activation, induction of sphingosine kinase-1 (SphK-1), and up-regulation of sphingosine-1-phosphate (S1P) formation via sphingosine kinase (Figure 4).43,46 S1P is a biologically active sphingolipid that signals via the S1P receptor-1 (S1P1), a G-protein–coupled receptor belonging to the endothelial differentiation gene (Edg) family. Activation of the S1P1 by S1P reduces endothelial permeability and stabilizes the cellular cytoskeleton via stabilization of cytoskeletal elements that are dependent on modulation of Rho family GTPases and mitogen-activated protein kinases (MAPKs) (see reviews62,64,65 ).
The cytoprotective protein C pathway for APC-mediated endothelial barrier protection. Protection of endothelial barrier function by APC requires EPCR and PAR-1.43,46 Activation of PAR-1 by APC stimulates sphingosine kinase-1 (SphK-1) to form sphingosine-1-phosphate (S1P) from sphingosine.43 S1P either released from activated platelets or formed intracellularly by SphK-1 activates the sphingosine-1-phosphate receptor 1 (S1P1) to promote increased endothelial barrier protection. A direct interaction of EPCR with S1P1 was postulated, but a mechanistic contribution to barrier protective effects remains unclear (indicated by question mark).46 In addition to barrier protective effects, S1P also promotes cell survival. In contrast, ceramide and sphingosine contribute to signals inducing cell death. The dynamic equilibrium between the formation and degradation of ceramide, sphingosine, and S1P is a sphingolipid rheostat for the cell, and the setting of this rheostat balances death-initiating and death-preventing signaling. It is currently unclear whether the S1P cell survival signals contribute to APC antiapoptotic activity or other cytoprotective activities.
The cytoprotective protein C pathway for APC-mediated endothelial barrier protection. Protection of endothelial barrier function by APC requires EPCR and PAR-1.43,46 Activation of PAR-1 by APC stimulates sphingosine kinase-1 (SphK-1) to form sphingosine-1-phosphate (S1P) from sphingosine.43 S1P either released from activated platelets or formed intracellularly by SphK-1 activates the sphingosine-1-phosphate receptor 1 (S1P1) to promote increased endothelial barrier protection. A direct interaction of EPCR with S1P1 was postulated, but a mechanistic contribution to barrier protective effects remains unclear (indicated by question mark).46 In addition to barrier protective effects, S1P also promotes cell survival. In contrast, ceramide and sphingosine contribute to signals inducing cell death. The dynamic equilibrium between the formation and degradation of ceramide, sphingosine, and S1P is a sphingolipid rheostat for the cell, and the setting of this rheostat balances death-initiating and death-preventing signaling. It is currently unclear whether the S1P cell survival signals contribute to APC antiapoptotic activity or other cytoprotective activities.
APC-enhanced endothelial barrier protection requires PAR-1. Curiously, robust signaling of PAR-1 by thrombin leads to endothelial barrier destabilization, whereas PAR-1 activation by APC leads to barrier stabilization.43 Differences in barrier effects mediated by thrombin and APC are dependent on the level of S1P induction; S1P concentration-dependent selectivity for S1P receptor–mediated second messenger Rac (protective) and Rho (destabilizing) signaling; and recruitment of S1P receptors to caveolin-rich microdomains.65 In vitro studies suggest an apparent functional interaction of EPCR with S1P1 associated with endothelial barrier stabilization.46 These protective effects of APC appear to be more effectively achieved by endogenously generated APC than by exogenously added APC (Figure 1C).66 This finding highlights a novel potential mechanistic link between the protein C cytoprotective pathway and pathways for S1P-mediated barrier modulation (Figure 4).43,46,66
S1P not only promotes endothelial barrier stabilization but also mediates antiapoptotic signals. The sphingolipids, ceramide and sphingosine, are in dynamic metabolic equilibrium with S1P. The ratio of ceramide to sphingosine, sometimes referred to as a sphingolipid rheostat, appears to mediate proapoptotic signaling. Whether APC-induced S1P up-regulation and concomitant ceramide and sphingosine down-regulation contribute to APC antiapoptotic activity is currently unclear. Glucosylceramide, a major metabolic product of ceramide, enhances APC anticoagulant activity,67,68 suggesting that interactions between the sphingolipid regulatory pathway and the protein C pathways might be both more complicated and more significant than previously appreciated.
APC and protease-activated receptors
Protease-activated receptors (PARs) are G-protein–coupled receptors and are activated by proteolytic cleavage of an extracellular N-terminal tail that forms an intramolecular tethered ligand that triggers intracellular signaling.69 In reactions requiring EPCR, APC can activate PAR-1 on endothelial cells and both PAR-1 and PAR-2 on transfected cells, although to date only the activation of PAR-1 has been shown to alter cellular functional activity.16-19 In the presence of EPCR, APC induces PAR-1–dependent MAPK phosphorylation, increases intracellular calcium fluxes, and modulates PAR-1–specific gene products in endothelial cells.17,19,30 Dependent on EPCR and PAR-1, APC inhibits hypoxia/hypoglycemia or staurosporine-induced apoptosis in various endothelial cells types.15,16,18 This indicates that in the presence of EPCR, APC can induce biologically relevant intracellular signaling transduction through PAR-1 on endothelial cells (Figure 1C).
A number of the remarkable in vivo anti-inflammatory and neuroprotective effects of APC require PAR-1 and EPCR. Using murine APC and mice with targeted gene deletions of PARs or mice that were severely deficient in EPCR, it was shown that PAR-1 and EPCR are required for pharmacological beneficial effects of APC in vivo in mouse models for ischemic stroke.16,31,60,70 On murine neurons, PAR-3 seems to play a supporting role in APC-mediated cytoprotective effects in addition to PAR-1.31 Thrombin activates human platelets via PAR-1 and PAR-4, whereas it activates mouse platelets via PAR-3 and PAR-4.71,72 Curiously, murine PAR-3 is not directly activated by thrombin to generate cellular signaling, but rather it is hypothesized to serve as a cofactor for thrombin's proteolytic activation of murine PAR-4.72 These species differences might complicate comparison of results for the mechanistic roles of specific PARs based on studies using murine injury models compared with human cells or with results observed for humans. Nonetheless, it is clear that the beneficial pharmacologic effects of APC in various injury models require PAR-1 where the mechanism has been carefully assessed.
PAR-1 signaling for good or bad: APC versus thrombin
The observation that the beneficial pharmacologic effects of APC require PAR-1 may seem somewhat surprising as PAR-1, the archetype thrombin receptor, is often described as mediating proinflammatory signals.73 Curiously, a gene expression profiling study of TNFα-perturbed endothelial cells demonstrated some specific PAR-1–dependent effects of APC that were opposite those of PAR-1–dependent effects of thrombin.30 Strikingly, both p53 and thrombospondin-1 expression were down-regulated by APC but up-regulated by thrombin, showing that this same receptor on the same cell type can mediate opposite biologic effects by 2 different plasma serine proteases.30 One may wonder whether the cytoprotective effects of APC might also be achieved by other PAR-1 agonists or whether they are specific to APC's interactions with PAR-1. A PAR-1 agonist peptide showed partial reduction of hypoxia-induced apoptosis of human brain endothelial cells and of N-methyl-D-aspartate (NMDA)–induced apoptosis of murine cortical neurons, but it failed to inhibit staurosporine-induced apoptosis of transformed endothelial cells.16,18 Furthermore, thrombin did not protect against staurosporine-induced endothelial cell apoptosis.18 These various observations illustrate that the molecular mechanisms for the effects caused by PAR-1 activation on cells are incompletely resolved; although the requirement for PAR-1 in the pharmacologic actions of APC has been proven, a role for PAR-1 in the physiologic actions of APC is a subject of current debate.74-76
In vivo beneficial effects of APC
Beneficial effects of APC have been demonstrated in severe sepsis trials in humans and a variety of animal injury model systems (Table 2). As discussed in the following subsections, current and potential therapeutic applications for APC's beneficial effects include, among others, severe sepsis, lung injury and inflammation, ischemic stroke, and angiogenesis and wound healing.
In vivo APC beneficial effects
| Model, host . | Trigger . | Read out . | APC effect . | Sources . |
|---|---|---|---|---|
| Sepsis | ||||
| Human | Sepsis (high risk of death) | Death | Inhibits | Bernard et al,24 Bernard et al77 |
| Human | Sepsis (low risk of death) | Death | No effect | Abraham et al78 |
| Human | LPS | Thrombosis/inflammation | No effect | Kalil et al79 |
| Baboon* | E coli | Death | Inhibits | Taylor et al80 |
| Hamster | LPS | Inflammation | Inhibits | Hoffmann et al81 |
| Rat | LPS | Inflammation | Inhibits | Iba et al82 |
| Rat | LPS | Arterial blood pressure | Increases | Isobe et al83 |
| Rat | LPS | Cardiovascular dysfunction/inflammation | Inhibits | Favory et al84 |
| Pulmonary inflammation and injury | ||||
| Human | LPS | Thrombosis | Inhibits | Van der Poll et al85 |
| Human | LPS | Inflammation | Inhibits | Nick et al45 |
| Rat | LPS | Inflammation | Inhibits | Murakami et al44 |
| Rat | Acid aspiration | Inflammation | Inhibits | Jian et al86 |
| Rat | Smoke | Inflammation | Inhibits | Wong et al87 |
| Mouse* | Bleomycin | Fibrosis/inflammation | Inhibits | Shimizu et al,40 Yasui et al88 |
| Mouse* | Ovalbumin | Inflammation | Inhibits | Yuda et al41 |
| Stroke | ||||
| Rabbit | I/R | Neurologic injury | Inhibits | Yamauchi et al89 |
| Rat | I/R | Neurologic injury/thrombosis/inflammation | Inhibits | Zlokovic et al90 |
| Mouse*† | I/R | Neurologic injury/thrombosis/inflammation | Inhibits | Cheng et al,16 Cheng et al,70 Zlokovic et al,90 Fernández et al,91 Shibata et al92 |
| Mouse†‡ | NMDA | Apoptosis/neurologic injury | Inhibits | Guo et al,31 Liu et al60 |
| Wound healing/angiogenesis | ||||
| Rat | Biopsy | Wound closure | Promotes | Xue et al,93 Jackson et al94 |
| Mouse | None | Capillary formation | Promotes | Uchiba et al95 |
| Chick | None | Capillary formation | Promotes | Xue et al93 |
| Miscellaneous | ||||
| Intestinal | ||||
| Rat | I/R | Inflammation | Inhibits | Schoots et al96 |
| Acute pancreatitis | ||||
| Rat | Taurocholate | Inflammation | Inhibits | Yamenel et al97 |
| Gastric injury | ||||
| Rat | Stress | Thrombosis/inflammation | Inhibits | Isobe et al98 |
| Spinal cord injury | ||||
| Rat | Compression | Motor disturbance/inflammation | Inhibits | Taoka et al56 |
| Islet transplantation | ||||
| Mouse | Transplantation | Apoptosis/inflammation | Inhibits | Contreras et al99 |
| Model, host . | Trigger . | Read out . | APC effect . | Sources . |
|---|---|---|---|---|
| Sepsis | ||||
| Human | Sepsis (high risk of death) | Death | Inhibits | Bernard et al,24 Bernard et al77 |
| Human | Sepsis (low risk of death) | Death | No effect | Abraham et al78 |
| Human | LPS | Thrombosis/inflammation | No effect | Kalil et al79 |
| Baboon* | E coli | Death | Inhibits | Taylor et al80 |
| Hamster | LPS | Inflammation | Inhibits | Hoffmann et al81 |
| Rat | LPS | Inflammation | Inhibits | Iba et al82 |
| Rat | LPS | Arterial blood pressure | Increases | Isobe et al83 |
| Rat | LPS | Cardiovascular dysfunction/inflammation | Inhibits | Favory et al84 |
| Pulmonary inflammation and injury | ||||
| Human | LPS | Thrombosis | Inhibits | Van der Poll et al85 |
| Human | LPS | Inflammation | Inhibits | Nick et al45 |
| Rat | LPS | Inflammation | Inhibits | Murakami et al44 |
| Rat | Acid aspiration | Inflammation | Inhibits | Jian et al86 |
| Rat | Smoke | Inflammation | Inhibits | Wong et al87 |
| Mouse* | Bleomycin | Fibrosis/inflammation | Inhibits | Shimizu et al,40 Yasui et al88 |
| Mouse* | Ovalbumin | Inflammation | Inhibits | Yuda et al41 |
| Stroke | ||||
| Rabbit | I/R | Neurologic injury | Inhibits | Yamauchi et al89 |
| Rat | I/R | Neurologic injury/thrombosis/inflammation | Inhibits | Zlokovic et al90 |
| Mouse*† | I/R | Neurologic injury/thrombosis/inflammation | Inhibits | Cheng et al,16 Cheng et al,70 Zlokovic et al,90 Fernández et al,91 Shibata et al92 |
| Mouse†‡ | NMDA | Apoptosis/neurologic injury | Inhibits | Guo et al,31 Liu et al60 |
| Wound healing/angiogenesis | ||||
| Rat | Biopsy | Wound closure | Promotes | Xue et al,93 Jackson et al94 |
| Mouse | None | Capillary formation | Promotes | Uchiba et al95 |
| Chick | None | Capillary formation | Promotes | Xue et al93 |
| Miscellaneous | ||||
| Intestinal | ||||
| Rat | I/R | Inflammation | Inhibits | Schoots et al96 |
| Acute pancreatitis | ||||
| Rat | Taurocholate | Inflammation | Inhibits | Yamenel et al97 |
| Gastric injury | ||||
| Rat | Stress | Thrombosis/inflammation | Inhibits | Isobe et al98 |
| Spinal cord injury | ||||
| Rat | Compression | Motor disturbance/inflammation | Inhibits | Taoka et al56 |
| Islet transplantation | ||||
| Mouse | Transplantation | Apoptosis/inflammation | Inhibits | Contreras et al99 |
LPS indicates lipopolysaccharide; I/R, ischemia/reperfusion; and NMDA, N-methyl-D-aspartate.
EPCR required.
PAR-1 required.
PAR-3 required.
APC in severe sepsis
The landmark Protein C Evaluation in Severe Sepsis (PROWESS) study24 was the first positive study in recent years of clinical sepsis trials that demonstrated a significant reduction of 28-day all-cause mortality in patients with severe sepsis. In this randomized, double-blind, placebo-controlled multicenter trial, drotrecogin alpha-activated (recombinant APC) reduced the risk of death by 6.1% (relative risk reduction, 19.4% [95% confident interval, 6.6% to 30.5%]) when given for 4 days by infusion at 24 μg/kg per hour (plasma APC levels averaging ∼ 50 ng/mL). These results were especially remarkable since 2 other potent anticoagulant proteins, tissue factor pathway inhibitor (TFPI) and antithrombin III (ATIII), failed to reduce mortality in similar phase 3 clinical trials (OPTIMIST and KyberSept).25,26 This reasonably implies that APC anticoagulant activity alone does not explain APC's success in reducing mortality. Thus, this implies that APC's anti-inflammatory and antiapoptotic activities are relevant for reduction of mortality.
The PROWESS trial results for severe sepsis patients were reported to be confirmed in the ENHANCE US trial.77 Nevertheless, the absence of an effect of APC on mortality in patients with sepsis with a low risk of death in the ADDRESS trial and in a pediatric trial clearly indicates that the currently used APC therapeutic regimen has its limitations.78 A low but significant increased risk of serious bleeding is associated with the PROWESS regimen for APC infusion (24 μg/kg per hour for 96 hours). It is unclear whether APC administration at higher doses and/or for shorter periods might have less risk of serious bleeding and equivalent or greater efficacy. Almost none of the protocols for APC's beneficial effects in animal injury models used a protocol like that of the PROWESS trial, and serious bleeding was not a notable finding in any of the reported animal injury studies. This suggests that APC dosage regimens that have not yet been tested in humans, for example, bolus or bolus plus brief infusions (0.5 to 4 hours) at APC doses higher than 24 μg/kg per hour, might have less side effects and equivalent or greater efficacy. Moreover, as discussed in “Roles for APC's anticoagulant activity versus APC's cytoprotective activities,” genetically engineered variants of APC with reduced anticoagulant activity but with normal cytoprotective activity might provide superior properties in clinical situations, such as severe sepsis; such APC variants with attenuated anticoagulant activity might permit higher doses given by bolus or short infusions at different times with less risk of serious bleeding.
APC in lung injury and inflammation
APC's beneficial actions against inflammatory injury were shown in models of lung injury. Clinical studies show that reduced plasma protein C levels and impaired APC generation in the intra-alveolar space and bronchoalveolar lavage fluid in patients with lung injury and airway inflammation were associated with increased collagen deposition in the lung and worse clinical outcome.100,101 In a mouse bleomycin-induced lung injury model, APC inhibited development of lung fibrosis and, in a mouse ovalbumin-induced bronchial asthma model, APC prevented the development of an allergic inflammation.41,88 Furthermore, in the lungs of bleomycin-challenged mice, APC inhibited platelet-derived growth factor effects that include potent mitogenic action, chemoattractant activity, and induction of matrix-related gene expression (eg, collagen and fibronectin).40 When APC is given at pharmacologic doses, part of APC's protective effects on lung injury and inflammation is mediated through APC-dependent inhibition of leukocyte accumulation and chemotaxis.44,45 These studies may provide novel promising directions for treatments of lung inflammation, fibrosis, and asthma. Of interest, airway epithelial cells express protein C, thrombomodulin, and EPCR, and these cells support generation of APC in the presence of thrombin.102 Thus, it is reasonable that the endogenous protein C system, as well as pharmacologically administered APC, can help protect the lung against injury.
APC in ischemic stroke
Based on preclinical animal studies and observational human studies, a very promising untested area for potential APC human therapy involves ischemic stroke.103 The prospective epidemiologic Atherosclerosis Risk in Communities (ARIC) study reported that plasma protein C appeared protective against ischemic stroke (OR = 0.65 [0.4-1.0]).104 In murine models of focal cerebral ischemia, APC provided remarkable anti-inflammatory and neuroprotective effects.16,91,92 The neuroprotective action of APC was apparent in both rat and murine stroke models even when APC was given 6 hours after onset of brain ischemia.90
tPA, one of the few available treatment options in ischemic stroke, effectively promotes plasmin-dependent lysis of thrombotic occlusions, but this drug has notable side effects. Not only is tPA directly neurotoxic to neurovascular cells as it induces apoptosis in brain endothelial cells and neurons, but tPA also aggravates the risk for hemorrhagic transformation due to up-regulation and activation of pro–matrix metalloprotease 9 (pro-MMP9), especially when tPA is administered after 3 hours of onset of stroke. A potential breakthrough for ischemic stroke treatment is also implicit in recent in vivo studies of combined APC and tPA given for neuroprotection during murine ischemic stroke and for prevention of excitotoxic injury following administration of NMDA.60,70 In both rat and murine stroke models, the cytoprotective activities of APC effectively counteract tPA's neurotoxic effects on endothelial cells, the blood-brain barrier, and neurons, thus improving the overall beneficial activity of tPA.60,70 APC prevents tPA-induced bleeding by specifically counteracting tPA's up-regulation of pro-MMP9 expression in stressed human brain endothelium in vitro and in hypoxic murine brain in vivo.70 In summary, APC appears promising for treatment of ischemic stroke in the absence as well as in the presence of tPA.
APC in angiogenesis, wound healing, and inflammation
APC can induce endothelial cell proliferation and angiogenesis in vitro and in vivo, which is dependent on EPCR and PAR-1.95 APC can stimulate keratinocyte proliferation and migration and APC can contribute to extracellular matrix degradation by activation of the gelatinase, matrix metalloproteinase-2 (MMP-2).105,106 Thus, beneficial effects of APC in regenerative processes (eg, angiogenesis and wound healing) have been demonstrated. Furthermore, APC may induce wound repair and promote endothelial cell migration and proliferation via up-regulation of endothelial cytokines, as was shown for interleukin-6 (IL-6) and IL-8, or chemokines such as MCP-1.19,35,49,107 In certain conditions, APC can up-regulate MCP-1 expression at both the RNA and protein levels by suppression of nitric oxide (NO) synthesis and stabilization of mRNA.36,107 However, a clear application for pharmacologic APC in the setting of angiogenesis or wound healing is less obvious than in the settings of severe sepsis or ischemic stroke (see “APC in severe sepsis” and “APC in ischemic stroke”). The intimate relationships between inflammation and host defense and between inflammation and wound healing are complex, and further work is needed to clarify the physiologic or pharmacologic roles for APC in these processes.
Roles for APC's anticoagulant activity versus APC's cytoprotective activities
Given the widely observed beneficial effects of APC in humans and in various animal injury models (Table 2) and given the multiplicity of APC activities (Figure 1), one wonders, what are the mechanistic secrets to APC's successes? For example, is the anticoagulant activity of APC essential both for success in the PROWESS trial and also for the increased risk for serious bleeding in severe sepsis patients, especially during the 4-day period of APC infusion.24,108 Alternatively, given the failure of other anticoagulants (eg, antithrombin and tissue factor pathway inhibitor) in severe sepsis trials, is one or more of the direct actions of APC on cells critical to reducing mortality in patients? And in various kinds of injury, what activities of APC are critical and which are dispensable? To help address these questions about APC's mechanisms of action and, ultimately, to obtain safer yet effective APC variants, we have undertaken a series of in vitro and in vivo studies of engineered APC variants.29
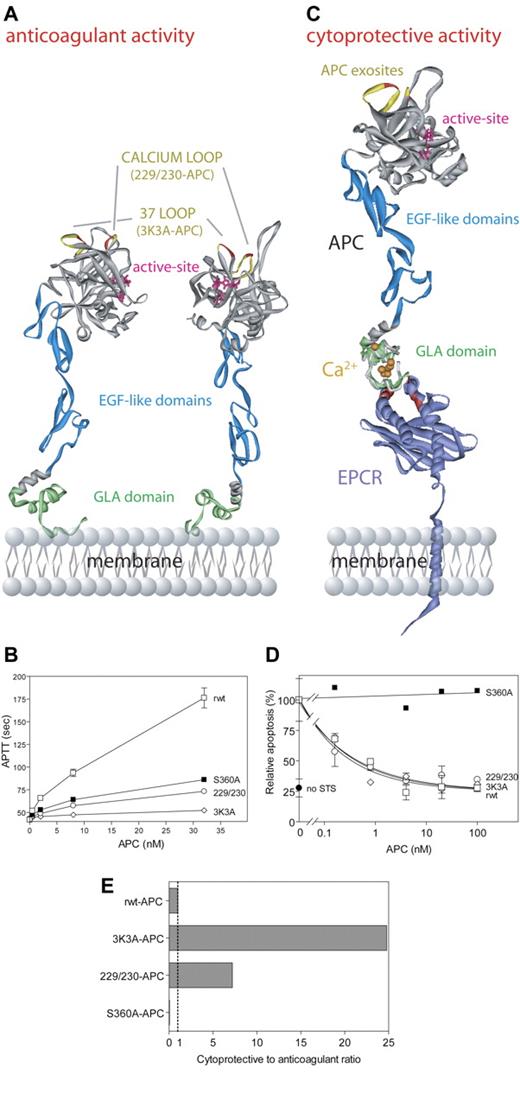
Our first goal was to construct potentially safer APC variants with reduced risk of bleeding due to reduced anticoagulant activity that retained normal direct actions on cells, so we altered factor Va binding exosites in APC without affecting exosites that recognize PAR-1. The anticoagulant action of APC critically involves a cleavage site at Arg506 in factor Va, and this cleavage depends on certain positively charged residues in surface loops on APC's protease domain, including loop 37 (protein C residues 190-193, equivalent to chymotrypsin [CHT] residues 36-39), the Ca++-binding loop (residues 225-235, CHT residues 70-80), and the autolysis loop (residues 301-316, CHT residues 142-153) (Figure 5A).112,114-119 Two APC variants were made with Ala mutations in 2 surface loops and involved Ala replacements of Arg229 and Arg230 and of Lys191, Lys192, and Lys193 (Figures 5A,C). Each APC variant had little anticoagulant activity (4% to 14% compared with wild-type APC) (Figure 5B), whereas each APC variant retained normal antiapoptotic activity, requiring PAR-1 and EPCR (Figure 5D).29 When the ratio of cytoprotective to anticoagulant activities of these APC variants was compared with wild-type APC (normalized to a ratio of 1:1), the 2 APC variants designated 3K3A-APC and 229/230-APC exhibited 25-times and 7-times greater antiapoptotic activity relative to that of wild-type APC (Figure 5E). Thus, genetic engineering of APC can reduce anticoagulant activity while preserving APC's direct actions on cells that are EPCR-dependent and that require PAR-1.
APC cytoprotective activities mediated by the cytoprotective protein C pathway are independent of APC anticoagulant activity. APC anticoagulant activity requires binding of the APC Gla-domain (green) (A) to negatively charged phospholipids on cell and/or platelet membranes or microparticles, whereas APC cytoprotective activity requires binding of the APC Gla-domain (green) to EPCR109 (purple) (C). EPCR residues important for protein C/APC binding, as determined by alanine mutagenesis, are indicated in red (C).110 Interactions of APC with its macromolecular substrates (factor Va, factor VIIIa, and PAR-1) are dictated by exosite interactions of APC protease domain surface loops (A,C). Important exosite loops in APC for interactions with factor Va are the autolysis loop, the 37-loop (yellow), and the calcium-binding loop (yellow). In particular, residues Lys191, Lys192, and Lys193 (red; A,C) in the 37-loop and residues Arg229 and Arg230 (red; A,C) in the calcium-binding loop contribute significantly to interactions with factor Va, and mutation of these residues to Ala severely compromises APC anticoagulant activity (B). Exosite specificity is illustrated by the fact that both the 37-loop residues (red/yellow) and the calcium-binding loop residues (red/yellow) (A,C) are required for normal cleavage of factor Va at Arg506 and thus for normal anticoagulant activity (B), while they are not involved in proteolytic activation of PAR-1 that generates antiapoptotic, cytoprotective activity (D). Based on the exosite specificity, a molecular engineering approach of APC surface loops was used to design APC variants with reduced risk of bleeding due to reduced anticoagulant activity but with full cytoprotective activity.29 Two such APC variants, 3K3A-APC and 229/230-APC, displayed increased ratios of cytoprotective (antiapoptotic) to anticoagulant activities (7- to 25-fold) compared with the reference ratio of 1.0 for recombinant wild-type APC (rwt-APC) (E). The ratio for a catalytically inactive mutant, S360A-APC (active site residue Ser360 mutated to Ala), is 0.0 (E). Data (B,D,E) were from Mosnier et al.29 Deep View Swiss PDB viewer (http://www.expasy.org/spdbv/) was used for the structural alignment. (A) The model of full-length APC111 is based on the serine protease domain structure of APC (Protein Data Bank entry 1AUT112 ). (C) The Gla-domain of the model of full-length APC was aligned with the protein C Gla-domain peptide crystallized in complex with soluble EPCR (1LQV).111,113 The model of full-length EPCR (kindly provided by Dr B. Dahlbäck, http://www.klkemi.mas.lu.se/dahlback/index.php) was aligned with the crystal structure of soluble EPCR to obtain a model representation of APC bound to EPCR on the endothelial cell membrane. (B, D) Error bars indicate SEM.
APC cytoprotective activities mediated by the cytoprotective protein C pathway are independent of APC anticoagulant activity. APC anticoagulant activity requires binding of the APC Gla-domain (green) (A) to negatively charged phospholipids on cell and/or platelet membranes or microparticles, whereas APC cytoprotective activity requires binding of the APC Gla-domain (green) to EPCR109 (purple) (C). EPCR residues important for protein C/APC binding, as determined by alanine mutagenesis, are indicated in red (C).110 Interactions of APC with its macromolecular substrates (factor Va, factor VIIIa, and PAR-1) are dictated by exosite interactions of APC protease domain surface loops (A,C). Important exosite loops in APC for interactions with factor Va are the autolysis loop, the 37-loop (yellow), and the calcium-binding loop (yellow). In particular, residues Lys191, Lys192, and Lys193 (red; A,C) in the 37-loop and residues Arg229 and Arg230 (red; A,C) in the calcium-binding loop contribute significantly to interactions with factor Va, and mutation of these residues to Ala severely compromises APC anticoagulant activity (B). Exosite specificity is illustrated by the fact that both the 37-loop residues (red/yellow) and the calcium-binding loop residues (red/yellow) (A,C) are required for normal cleavage of factor Va at Arg506 and thus for normal anticoagulant activity (B), while they are not involved in proteolytic activation of PAR-1 that generates antiapoptotic, cytoprotective activity (D). Based on the exosite specificity, a molecular engineering approach of APC surface loops was used to design APC variants with reduced risk of bleeding due to reduced anticoagulant activity but with full cytoprotective activity.29 Two such APC variants, 3K3A-APC and 229/230-APC, displayed increased ratios of cytoprotective (antiapoptotic) to anticoagulant activities (7- to 25-fold) compared with the reference ratio of 1.0 for recombinant wild-type APC (rwt-APC) (E). The ratio for a catalytically inactive mutant, S360A-APC (active site residue Ser360 mutated to Ala), is 0.0 (E). Data (B,D,E) were from Mosnier et al.29 Deep View Swiss PDB viewer (http://www.expasy.org/spdbv/) was used for the structural alignment. (A) The model of full-length APC111 is based on the serine protease domain structure of APC (Protein Data Bank entry 1AUT112 ). (C) The Gla-domain of the model of full-length APC was aligned with the protein C Gla-domain peptide crystallized in complex with soluble EPCR (1LQV).111,113 The model of full-length EPCR (kindly provided by Dr B. Dahlbäck, http://www.klkemi.mas.lu.se/dahlback/index.php) was aligned with the crystal structure of soluble EPCR to obtain a model representation of APC bound to EPCR on the endothelial cell membrane. (B, D) Error bars indicate SEM.
The availability of APC variants such as 3K3A-APC and 229/230-APC with reduced anticoagulant activity but normal cytoprotective activities for preclinical and clinical studies can help clarify whether the anticoagulant activity or the cytoprotective actions of APC or both are crucial for its various beneficial properties. In preliminary studies, the recombinant murine 3K3A-APC variant appeared as active as wild-type murine APC in preventing death from endotoxemia in mice,27,28 and this variant was also similar to wild-type APC in its potency to provide neuroprotection in murine ischemic stroke models (B.V.Z., L.O.M., J.H.G., unpublished results, March 2005). These data suggest a primary role for APC's in vivo beneficial activities in these injury models. If these preliminary findings for the mechanism of APC's beneficial actions in sepsis and stroke are confirmed to be of primary importance in additional studies, then one might use such APC variants for therapy and minimize risk of serious bleeding. Moreover, safer APC variants with less bleeding risk may allow studies of higher APC doses for shorter times to evaluate the possibility that higher doses of APC may stabilize stressed cells at risk for excessive inflammation or apoptosis.
Conclusions and future direction
Inflammation, apoptosis, and thrombosis are intimately connected in the tangled web of host defense. Many inflammatory and thrombotic feedback loops cooperate to exacerbate pathogenic reactions. Both basic, preclinical and clinical studies have provided an expanding body of research on the cytoprotective protein C pathway that is distinct from the anticoagulant protein C pathway. Although much remains to be characterized about the multiple activities of APC, the pharmacologic use of this fascinating enzyme holds remarkable promise. Therapeutic APC variants may be engineered with decreased anticoagulant activity but normal cytoprotective activity, and such APC variants hold the promise of reducing risks for serious bleeding while retaining this agent's beneficial cytoprotective properties.
Authorship
Conflict-of-interest disclosure: B.V.Z. is scientific founder of Socratech LLC, a startup biotech company with intellectual property involving activated protein C. The Scripps Research Institute has patents or patent applications related to activated protein C. The other authors declare no competing financial interests.
Correspondence: John H. Griffin, Department of Molecular and Experimental Medicine (MEM-180), The Scripps Research Institute, 10550 North Torrey Pines Rd, La Jolla, CA; e-mail: jgriffin@scripps.edu.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) grants (HL21544, HL31950, HL52246, and HL87618) and by a Basic Research Scholar Award from the American Society of Hematology (L.O.M.).
We gratefully acknowledge members of the Griffin and Zlokovic laboratories for their helpful discussions and ongoing research contributions to the field of the cytoprotective protein C pathway. We apologize to our respected colleagues whose important publications we were not able to cite or discuss due to space limitations.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal