Abstract
The aurora kinases facilitate transit from G2 through cytokinesis and, thus, are targets in cancer therapy. Multiple myeloma (MM) is a malignancy characterized by genetic instability, suggesting a disruption of checkpoints that arrest cells at G2M when injury to the mitotic machinery occurs. Since deficient checkpoints would prevent cell cycle arrest and may render cells susceptible to apoptosis in mitosis and since aurora kinases are intermediaries in checkpoint pathways, we tested antimyeloma effects of 2 agents that inhibit aurora kinases. Both inhibited growth of MM lines and primary myeloma samples at nanomolar concentrations while having less of an effect on proliferating lymphocytes and hematopoietic cells. MM cells were not protected by IL-6 or activating mutations of Ras. Antimyeloma effects included induction of tetraploidy followed by apoptosis. Apoptosis correlated with inhibition of aurora activity as shown by reduction of histone 3B phosphorylation. Ectopic expression of aurora A protected MM cells against aurora inhibitors but had no effect on apoptosis induced by bortezomib. As expression of RHAMM in MM contributes to genetic instability, we tested effects of RHAMM. RHAMM overexpression enhanced sensitivity to apoptosis and RHAMM silencing decreased sensitivity. These results suggest potential for aurora kinase inhibitors in MM especially in patients in whom RHAMM is overexpressed.
Introduction
The aurora kinases regulate cell cycle transit from G2 through to cytokinesis (reviewed in Andrews et al1 ). There are 3 mammalian aurora kinase genes, encoding aurora A, B, and C, which may have diverged from a single gene present in yeast. Although relatively little is known about aurora kinase C function, intense investigation has focused on aurora A and B as they appear to play a role in oncogenesis,2 with aurora A identified as a low-penetrance tumor-susceptibility gene in mice and humans.3 Thus, these kinases could be potential targets for novel small-molecule inhibitors
Aurora A is recruited into the centrosome early in G2 and has been implicated in the activation of CDK1/cyclin B on the centrosome.4 Activated aurora A, in turn, phosphorylates numerous centrosomal proteins and has a role in centrosome maturation and mitotic spindle formation. The aurora A gene is frequently amplified in cancer,5 amplification correlates with aneuploidy,5 and in vitro overexpression induces chromosome segregation anomalies associated with malignant transformation in vitro and in vivo.4,6
Aurora B is a chromosomal passenger protein that associates with centromeres during prometaphase and with the spindle midzone during anaphase and telophase. It is essential for chromosomal alignment on the spindle and cytokinesis. It resides in a complex with 2 other chromosome passenger proteins, INCENP and survivin, and recent work suggests these proteins work in concert for maintenance of the spindle assembly checkpoint.7 Aurora B is also highly expressed in multiple tumor types.8 Targeting aurora A and B with RNA interference,9 dominant-negative constructs,10 or small kinase inhibitors11,12 results in cell cycle slowing, induction of apoptosis,12 sensitization to chemotherapy,9 and suppression of tumor growth in a variety of xenograft models.12
Multiple myeloma is characterized by genetic instability with numeric chromosomal abnormalities.13 This suggests that, during the evolution of myeloma, disruption of cell cycle checkpoints has occurred that would arrest cells at the G2M transition or at mitosis when DNA damage or spindle abnormalities have occurred, allowing potential repair. Such deficient checkpoints may render myeloma cells particularly susceptible to induction of apoptotic death in mitosis (so-called mitotic catastrophe14 ) when further assaults on the mitotic machinery can be induced. For these reasons, we investigated potential effects of 2 agents that are inhibitors of aurora kinases. Both were capable of inducing tetraploidy followed by myeloma cell death. This antitumor effect correlated with inhibited phosphorylation of histone 3B, a known substrate of auroras, and was specifically prevented by ectopic expression of auroras. These results suggest that aurora kinases are potential targets for future antimyeloma therapy.
Materials and methods
Approval for these studies was obtained from the Greater Los Angeles Veterans Administration Healthcare System institutional review board (IRB). Informed consent was provided according to the Declaration of Helsinki.
Cell lines, primary cells, and reagents
The parental and activated N-ras–transfected ANBL-6 cell lines were gifts from Brian Van Ness, University of Minnesota.15 Primary patient myeloma bone marrow cells were isolated by positive selection for CD38 as previously described.16 Plasma cells were also isolated from a patient with plasma cell leukemia by density centrifugation of peripheral blood. The purity was more than 98% plasma cells. Peripheral blood lymphocytes (PBLs) and chronic lymphocytic leukemia (CLL) cells were also isolated by density centrifugation. The ZK inhibitor was a gift from Berlex (Richmond, CA). It was stored as a 10-mM stock solution diluted in DMSO and kept at −20°C. VX-680 was purchased from Kava Technology (San Diego, CA) and stored as a 1-mM solution in DMSO and also kept at −20°C. The antibody for detecting phosphorylated histone identified histone when phosphorylated at serine 10 and was purchased from Cell Signaling (catalog no. 9701; Beverly, MA).
Flow cytometric analyses for cell cycle distribution and apoptosis
Cell cycle distribution was determined by staining cells with propidium iodide and apoptosis identified by staining for expression of activated caspase 3 (BD Biosciences, San Jose, CA) as previously described.17
Stimulation of normal lymphohematopoietic cells
PBLs from healthy donors were isolated and stimulated with 2.5 μg/mL PHA for 72 hours in the presence of increasing concentrations of VX-680. To evaluate myeloid colony formation, frozen human bone marrow cells were purchased from Stem Cell Technologies (Vancouver, BC) and thawed. The colony-forming assay was performed in MethoCult medium as instructed by Stem Cell Technologies. Cells were plated in quadruplicate (at a density of 20 000 cells per 35-mm dish) in “Complete” methylcellulose medium with recombinant cytokines. VX-680 was added to the medium in increasing concentrations prior to addition of bone marrow cells. After a 14-day incubation, the number of erythroid–colony-forming units (CFU-Es), erythroid–burst-forming units (BFU-Es), granulocyte-macrophage–colony-forming units (CFU-GMs), and granulocyte-erythroid-monocyte-macrophage–colony-forming units (CFU-GEMMs) was determined using an inverted microscope and the criteria were defined by Stem Cell Technologies for each colony type. The averages were determined from the quadruplicate samples and inhibition induced by drug was calculated.
Immunoblot assays
Immunoblot assays were performed as previously described16,17 with the following exception: To assay expression of histone phosphorylation, cells were first washed × 1 in PBS, followed by cell lysis in lysis buffer (10 mM Hepes [pH 7.9], 1.5 mM MgCl2, 10 mM KCl). Sulfuric acid was added to 0.2 M and lysates were incubated on ice for 1 hour. The lysates were then centrifuged at 14 000 g for 15 minutes and the pellet was discarded. TCA at 15% was added to the supernatant, which was then kept overnight at 4°C. The supernatant was centrifuged and the pellet washed with acidic acetone × 1 and acetone × 1. The pellet was then resuspended in H2O and gel electrophoresis was performed.
Synchronization of cells
Cells were synchronized at the G1/S boundary by use of a double thymidine block as previously described.18 Briefly, cells were first blocked for 16 hours with 25 mM thymidine. The block was released by washing × 3 with PBS and culture in fresh media for 8 hours. Cells were then re-exposed to thymidine at 25 mM for an additional 16 hours, followed by release with 3 additional washings with PBS and fresh media.
Constructs and transfections
The aurora A construct was cloned into the pEGFP-C1 vector (Clontech, Palo Alto, CA) as previously described.18 Aurora A transfection of OPM-2 cells was accomplished by electroporation. Transfection of U266 cells with RHAMM was accomplished using a RHAMM-EGFP construct as described previously.19 The Amaxa Biosystems nucleofactor kit C (Gaithersburg, MD) was used to successfully transduce U266 cells. RHAMM silencing was accomplished by stable expression of an inhibitory short hairpin RHAMM RNA (shRNA). Briefly, the RHAMM shRNA was constructed in Plenti6/Block, a lentiviral vector (Invitrogen, Frederick, MD). The sequence was chosen from the software on the Invitrogen website. It was first cloned into the Block-it U6 RNAi vector. It was then recombined into the Plenti6/Block vector. The viral vector was then transfected into 3T3 cells to produce lentivirus expressing RHAMM shRNA. After centrifugation to remove cell debris, the virus-containing supernatant was harvested and frozen. The viral supernatant was diluted 1:1 to infect multiple myeloma (MM) cells in the presence of polybrene used at 1 nM. Twenty-four hours after infection, blasticidin was added at 2.5 μg/mL to select successfully transduced cells.
Cell survival assays
Survival assays were performed as previously described.17 After 48 to 72 hours, surviving cells were enumerated by trypan blue exclusion. Experimental groups were run in quadruplicate and the means of the replicates were used to calculate the percent inhibition of viable recovery compared with cell groups treated with vehicle alone. MTT assays were performed as previously described.16,17,20
Statistics
The t test was used to determine significance of differences between groups.
Results
Myeloma cells are sensitive to cytoreductive effects of aurora kinase inhibitors
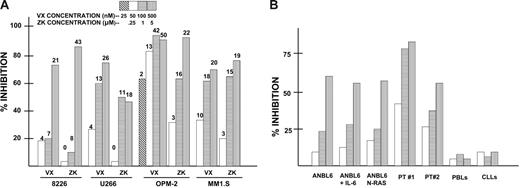
We tested 2 agents in short-term assays with myeloma cells. One, VX-680, is a well-known aurora kinase inhibitor.12 The other, ZK (Berlex), was initially identified in a small-molecule inhibitor screen that assayed inhibitory effects on PDK-1 stimulation of AKT activity but has been subsequently shown to also have aurora kinase inhibitory activity. Exposure of multiple myeloma cell lines for 72 hours to both drugs resulted in a dose-dependent inhibition of cell growth and induction of death (Figure 1A). Significant cytoreductive effects were also demonstrated after 48 hours of exposure (not shown). The data in Figure 1A represent mean percent inhibition of cell growth from 3 independent experiments (SDs were all < 5% of the means). The 3 VX-680 concentrations used were 50, 100, and 500 nM (OPM-2 cells also were challenged with a fourth concentration, 25 nM) and the ZK concentrations were 250 nM, 1 μM, and 5 μM. Thus, as shown in Figure 1A, on a molar basis, all myeloma cell lines tested were more sensitive to VX-680 compared with ZK. The 4 myeloma lines varied somewhat in their sensitivity. However, their relative sensitivities to both agents were comparable. For example, OPM-2 cells were the most sensitive to both agents (ID50 of 15 nM for VX-680 and 250 nM for ZK), and 8226 cells were least sensitive to both (ID50s of 200 nM and 3 μM, respectively). This inhibition of viable cell growth was accompanied by a loss of viability. The numbers at the tops of the bars in Figure 1A represent the percent of cells (mean of 3 independent experiments) that were nonviable above control groups (cell lines cultured for 72 hours in absence of inhibitors that had a baseline nonviability percent of 5%-15%). As shown, a significant loss of cell viability followed exposure to these agents, and this value roughly correlated with the degree of cell growth inhibition. Loss of viability was due to apoptosis (below).
Inhibitory effect of 2 aurora kinase inhibitors against growth of multiple myeloma cells. (A) MM cell lines were exposed to increasing concentrations (shown in key at top of figure) of either VX-680 or ZK inhibitors for 72 hours, after which MTT assays were performed as well as trypan blue exclusion. Bar data are percent inhibition of growth in MTT assays (vs vehicle alone; ie, no VX-680 or ZK), mean of 3 separate experiments where SDs were all less than 5% of the mean. Numbers at the tops of the bars represent percent of cells nonviable above control baseline nonviable percent (5%-15%, mean of 3 experiments). (B) Cells were cultured for 72 hours with increasing concentrations of VX-680 (at 50, 100, and 500 nM with symbols designated as in A), after which trypan blue exclusion was used to assess viable cell yield. Data are percent inhibition of viable cell yield (vs vehicle control), mean of 3 separate experiments. Cell targets are ANBL-6 MM cells, ANBL-6 cells first treated with 1000 U/mL recombinant IL-6 (ANBL-6 + IL-6), ANBL-6 cells stably transfected with an activated N-RAS allele, primary myeloma cells obtained from 2 MM patients (PT #1 and PT #2), peripheral blood lymphocytes (PBLs) from 3 healthy donors (data are means of 3 assays), and isolated B-cell CLL cells from 3 leukemic patients (data are means of 3 separate assays).
Inhibitory effect of 2 aurora kinase inhibitors against growth of multiple myeloma cells. (A) MM cell lines were exposed to increasing concentrations (shown in key at top of figure) of either VX-680 or ZK inhibitors for 72 hours, after which MTT assays were performed as well as trypan blue exclusion. Bar data are percent inhibition of growth in MTT assays (vs vehicle alone; ie, no VX-680 or ZK), mean of 3 separate experiments where SDs were all less than 5% of the mean. Numbers at the tops of the bars represent percent of cells nonviable above control baseline nonviable percent (5%-15%, mean of 3 experiments). (B) Cells were cultured for 72 hours with increasing concentrations of VX-680 (at 50, 100, and 500 nM with symbols designated as in A), after which trypan blue exclusion was used to assess viable cell yield. Data are percent inhibition of viable cell yield (vs vehicle control), mean of 3 separate experiments. Cell targets are ANBL-6 MM cells, ANBL-6 cells first treated with 1000 U/mL recombinant IL-6 (ANBL-6 + IL-6), ANBL-6 cells stably transfected with an activated N-RAS allele, primary myeloma cells obtained from 2 MM patients (PT #1 and PT #2), peripheral blood lymphocytes (PBLs) from 3 healthy donors (data are means of 3 assays), and isolated B-cell CLL cells from 3 leukemic patients (data are means of 3 separate assays).
Figure 1B presents results from 72-hour assays using only the VX-680 aurora inhibitor. As shown, the ANBL-6 MM cell line was also sensitive to VX-680's effects, and stimulation with the IL-6 MM growth and survival factor or stable transfection with an activated mutated N-ras allele, both maneuvers known to protect MM cells from other agents,21–23 failed to prevent the cytoreductive effects of VX-680. Figure 1B also shows that 2 freshly obtained primary myeloma specimens demonstrated significant sensitivity to the VX-680 aurora inhibitor. These MM cell preparations were isolated from 2 patients with very aggressive disease, one of which had plasma cell leukemia. In contrast, specimens of PBLs from 3 healthy donors and preparations of purified B-cell CLL lymphocytes from 3 leukemic patients were insensitive to effects of VX-680.
Since nonstimulated PBLs and CLL specimens are, for the most part, nonproliferative, they may not be the most relevant control nonmyeloma cells for comparison of aurora kinase inhibitory effects. Thus, we also tested proliferating lymphohematopoietic cells as targets. ZK's inhibitory effect on CD3/CD28-stimulated PBLs was minimal with an ID50 of between 5 to 10 μM. Thus, the sensitivity of CD3/CD28-stimulated proliferation of normal PBLs to ZK was comparable with that of 8226 and U266 MM cells but significantly less than the other MM lines tested (at least 10-fold less sensitive than the OPM-2 and MM1.S cell lines). The VX-680 inhibitor was also tested against marrow progenitors in colony-forming assays and PBLs stimulated by PHA. It depressed CFU-E, BFU-E, CFU-GM, and CFU-GEMM colony formation with an ID50 of approximately 200 nM for each colony type. Thus, OPM-2, U266, and MM1.S MM lines were significantly more sensitive than myeloid precursors to VX-680 (ID50s of 15 nM, 70 nM, and 60 nM, respectively), and ANBL-6 and 8226 cell lines were comparable. VX-680 also inhibited PHA-stimulated lymphocyte proliferation, although this required much higher concentrations than what was effective against MM cells (ID50 of 400 nM). Flow cytometric analysis for activated caspase 3 staining, however, did not reveal any increase in apoptosis induced by VX-680 in the inhibited lymphocyte cultures. As is shown below, the inhibitory effect of aurora kinase inhibitors on MM cells is associated with a significant induction of apoptosis.
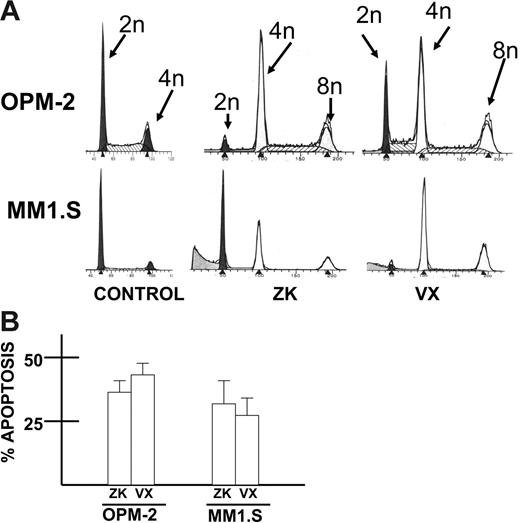
To ascertain effects on cell cycle distribution and apoptosis, OPM-2 or MM1.S cells were treated and stained with hypotonic propidium iodide (cell cycle analysis) or an antibody to activated caspase 3 (for apoptosis). As shown in Figure 2A, both agents induced tetraploidy in MM cells after 60 hours of treatment. Apoptosis was induced as well (Figure 2B) in OPM-2 and MM1.S cells with activated caspase 3 staining increasing to 32% to 47% with 100 nM VX-680 and 27% to 40% with 500 nM ZK (control cells [no inhibitors] showed 12% to 16% positive staining with the activated caspase 3 antibody).
Induction of tetraploidy and apoptosis in MM cells. (A) Cell cycle analysis (propidium iodide staining) was performed in OPM-2 and MM1.S MM cell lines after 48-hour exposure to vehicle alone (control), ZK (500 nM), or VX-680 (100 nM). Induction of tetraploidy is shown. (B) Similarly treated OPM-2 and MM1.S cells were assayed at 72 hours for apoptosis by flow cytometric analysis of activated caspase 3 expression. Results are mean percent apoptosis ± SD of 3 separate experiments (control cells treated with vehicle alone showed a mean of 16% positive staining with the caspase 3 antibody).
Induction of tetraploidy and apoptosis in MM cells. (A) Cell cycle analysis (propidium iodide staining) was performed in OPM-2 and MM1.S MM cell lines after 48-hour exposure to vehicle alone (control), ZK (500 nM), or VX-680 (100 nM). Induction of tetraploidy is shown. (B) Similarly treated OPM-2 and MM1.S cells were assayed at 72 hours for apoptosis by flow cytometric analysis of activated caspase 3 expression. Results are mean percent apoptosis ± SD of 3 separate experiments (control cells treated with vehicle alone showed a mean of 16% positive staining with the caspase 3 antibody).
Inhibition of MM cell growth is due to effects on aurora kinases
Histone 3B is a known substrate of aurora kinases.24 Thus, we next analyzed effects of VX-680 and ZK on phosphorylation of histone 3B. As shown in Figure 3A, although exposure of OPM-2 or MM1.S MM cell lines to increasing concentrations of ZK had no effect on expression of total histone 3B, the drug inhibited this aurora substrate's phosphorylation in both cell lines. Similar data were observed when OPM-2 cells (Figure 3B) or MM1.S cells (Figure 3C) were exposed to increasing concentrations of VX-680. At least for OPM-2 cells, VX-680 was more effective than ZK on a molar basis in its ability to inhibit histone phosphorylation (ID50 of approximately 50 nM compared with ID50 of > 500 nM for ZK), thus mirroring its more significant cytoreduction as shown in Figure 1.
Effect of inhibitors on histone 3B phosphorylation. (A) OPM-2 or MM1.S cells were exposed to increasing concentrations of the ZK inhibitor, after which immunoblot was performed for expression of total histone 3B or phosphorylated histone 3B (on serine 10). (B-C) Similar immunoblot experiments were performed on OPM-2 and MM1.S cells exposed to increasing concentrations of VX-680. The experiments depicted in panels A-C were repeated 3 times with identical results.
Effect of inhibitors on histone 3B phosphorylation. (A) OPM-2 or MM1.S cells were exposed to increasing concentrations of the ZK inhibitor, after which immunoblot was performed for expression of total histone 3B or phosphorylated histone 3B (on serine 10). (B-C) Similar immunoblot experiments were performed on OPM-2 and MM1.S cells exposed to increasing concentrations of VX-680. The experiments depicted in panels A-C were repeated 3 times with identical results.
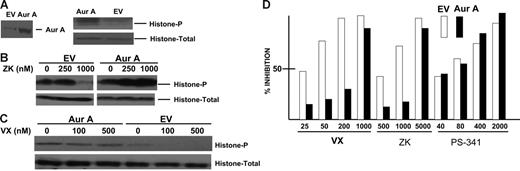
To assess whether our 2 agents were inducing antimyeloma effects specifically due to their inhibitory effects on aurora kinases, we attempted to stably transfect either aurora A or aurora B into OPM-2 MM cells. However, we were successful only with aurora A transfection. To confirm successful transfection of a functional aurora A construct, empty vector (EV) or aurora A–transfected cells were first synchronized at the G1/S boundary by use of a double thymidine block.18 Four hours after release of the block, lysates were immunoblotted for aurora A, histone, and phosphorylated histone. As shown in Figure 4A, the aurora A transgene was expressed and aurora A–transfected cells expressed significantly increased levels of phosphorylated histone relative to empty vector control cells. We then exposed these isogenic transfected cell lines to increasing concentrations of either ZK or VX-680 inhibitors. As shown in Figure 4B-C, both ZK and VX-680 inhibitors were able to significantly curtail histone phosphorylation in empty vector (EV) cells but not in aurora A transfectants. When the transfectants were tested in cell survival assays (Figure 4D), the data demonstrate that aurora A transfection significantly protected against both VX-680 and ZK. Although the highest doses of VX-680 (1000 nM) and ZK (5000 nM) were capable of an antitumor effect in aurora A–transfected cells, lower doses were considerably less effective and the ID50s for both were significantly greater in aurora A–transfected cells (500 nM for VX-680 compared with 30 nM in EV control cells and 3000 nM for ZK compared with 600 nM in EV control cells). Similar results were obtained when we assayed apoptosis. VX-680 used at 25, 50, 100, and 500 nM induced 1%, 13%, 42%, and 49% apoptosis, respectively, in empty vector control cells (means of 3 separate experiments where SDs were all < 5% of the mean). In contrast, minimal apoptosis was seen only at 500 nM VX-680 (15% ± 4%) when tested against aurora A–transfected cells. Exposure of the isogenic transfected MM cells to increasing concentrations of PS-341 (bortezomib), a commonly used antimyeloma agent, demonstrated comparable sensitivities (Figure 4D). Thus, the resistance afforded by stable transfection with aurora A is relatively specific for the antimyeloma action of aurora kinase inhibitors.
Ectopic aurora A expression inhibits antimyeloma effect of aurora inhibitors. OPM-2 cells were transfected with aurora A (Aur A) or empty vector (EV); cells were selected in vitro and then synchronized by double thymidine block. Four hours after release of the block, lysates were immunoblotted for aurora A expression as well as total histone 3B and phosphorylated histone (on serine 10) as shown in panel A. (B-C) Isogenic transfected cells were treated with increasing concentrations of either ZK (B) or VX-680 (C) and similarly synchronized and immunoblotted for total histone or phosphorylated histone. (D) Aurora A–transfected cells (▪) or empty vector control cells (EV, □) were exposed to increasing concentrations of VX-680, ZK, or bortezomib (PS-341) for 48 hours, after which percent inhibition of growth was determined in MTT assays. Results are means of 3 experiments. The percent inhibition induced by VX-680 and ZK was significantly less (P < .05) in aurora A–transfected cells treated with 25, 50, and 200 nM VX-680 and 500 and 1000 nM ZK.
Ectopic aurora A expression inhibits antimyeloma effect of aurora inhibitors. OPM-2 cells were transfected with aurora A (Aur A) or empty vector (EV); cells were selected in vitro and then synchronized by double thymidine block. Four hours after release of the block, lysates were immunoblotted for aurora A expression as well as total histone 3B and phosphorylated histone (on serine 10) as shown in panel A. (B-C) Isogenic transfected cells were treated with increasing concentrations of either ZK (B) or VX-680 (C) and similarly synchronized and immunoblotted for total histone or phosphorylated histone. (D) Aurora A–transfected cells (▪) or empty vector control cells (EV, □) were exposed to increasing concentrations of VX-680, ZK, or bortezomib (PS-341) for 48 hours, after which percent inhibition of growth was determined in MTT assays. Results are means of 3 experiments. The percent inhibition induced by VX-680 and ZK was significantly less (P < .05) in aurora A–transfected cells treated with 25, 50, and 200 nM VX-680 and 500 and 1000 nM ZK.
Effect of RHAMM transfection
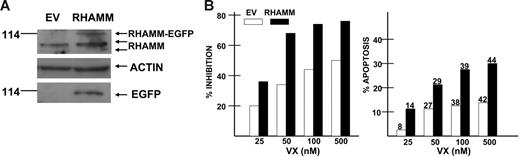
One possible cause of genetic instability in myeloma is overexpression of RHAMM, a centrosomal protein.19,25 RHAMM correlates with structural centrosomal and mitotic defects19 and, within the centrosome, RHAMM may interact with aurora A via common binding to the centrosomal protein TPX2.19 RHAMM overexpression may disrupt the mitotic checkpoints that normally arrest cells at the G2M transition or within mitosis when DNA damage has occurred. For these reasons, we tested the effect of RHAMM on sensitivity to VX-680 by stably expressing it in the U266 MM cell line. This MM cell line was transfected with full-length GFP-RHAMM19 by electroporation. After in vitro selection of transfected cells, ectopic RHAMM expression was demonstrated by immunoblot assay (Figure 5A top arrow). The immunoblot shows the 2 endogenous RHAMM proteins (Figure 5A second and third arrows from top) at approximately 85 to 95 kDa, one full length and one, more minimally expressed, that results from alternative splicing.25 For unexplained reasons, the endogenous RHAMM expression is approximately 1.5-fold higher in RHAMM-transfected cells. In addition, there is a larger RHAMM-immunoreactive protein in RHAMM-transfected cells that represents the ectopic RHAMM-GFP fusion product (Figure 5A top arrow). Reprobing the membrane for GFP protein demonstrates a single band at approximately 114 kDa, the location of the ectopically expressed RHAMM. By densitometry, the total amount of immunoreactive RHAMM was 2.5-fold higher in RHAMM-transfected cells 10 hours after release of the block and 2-fold higher 17 hours after release of the block (versus empty vector–transfected cells).
Effect of ectopic RHAMM expression. U266 MM cells were transfected with a RHAMM-EGFP construct (▪) or control empty vector (EV, □). (A) Western assay of transfected isogenic cells synchronized by double thymidine block and then immunoblotted for expression of RHAMM, actin, or EGFP. (B) The transfected cells were exposed to increasing concentrations of VX-680 for 72 hours and then assessed for percent inhibition of growth in MTT assays (left panel) and percent apoptosis (right panel, flow cytometric analysis of activated caspase 3 expression). Dark bars are RHAMM transfectants, and open bars are empty vector control cells. Results are means of 3 separate experiments where the SDs were all less than 5% of the mean. Percent of cells demonstrating tetraploidy are shown on top of bars of right panel. The percent inhibition of growth and percent induced apoptosis was significantly greater (P < .05) for RHAMM-transfected cells at all doses of VX-680.
Effect of ectopic RHAMM expression. U266 MM cells were transfected with a RHAMM-EGFP construct (▪) or control empty vector (EV, □). (A) Western assay of transfected isogenic cells synchronized by double thymidine block and then immunoblotted for expression of RHAMM, actin, or EGFP. (B) The transfected cells were exposed to increasing concentrations of VX-680 for 72 hours and then assessed for percent inhibition of growth in MTT assays (left panel) and percent apoptosis (right panel, flow cytometric analysis of activated caspase 3 expression). Dark bars are RHAMM transfectants, and open bars are empty vector control cells. Results are means of 3 separate experiments where the SDs were all less than 5% of the mean. Percent of cells demonstrating tetraploidy are shown on top of bars of right panel. The percent inhibition of growth and percent induced apoptosis was significantly greater (P < .05) for RHAMM-transfected cells at all doses of VX-680.
To ascertain a possible effect of this modest RHAMM overexpression on sensitivity to aurora inhibitors, the 2 transfected isogenic U266 MM cell lines were exposed to increasing concentrations of VX-680 inhibitor. As shown in Figure 5B, the RHAMM transfectants (darkened bars) were significantly more sensitive than EV control cells (open bars) to growth inhibition (left panel) induced by increasing concentrations of VX-680 as well as apoptosis induction (activated caspase 3 staining, right panel). The enhanced sensitivity is modest, approximately a 2-fold increase in sensitivity. Of interest, except for the lowest dose of VX-680 (25 nM), there was little difference in the ability of VX-680 to induce tetraploidy in these isogenic MM cells (numbers above the bars of right panel in Figure 5B are percent tetraploid cells). Further support for a role of RHAMM in regulating sensitivity to aurora inhibitors was found by silencing RHAMM expression. Inhibitory short hairpin RHAMM RNA was stably expressed in AF-10 MM cells by lentivirus infection. Control infections used a scrambled sequence. We chose AF-10 cells as they expressed higher amounts of endogenous RHAMM compared with other MM cell lines. After infection with shRNA-expressing lentivirus, AF-10 cells were selected by exposure to blasticidin in vitro. The top of Figure 6 demonstrates successful silencing of RHAMM in shRNA-expressing cells. When exposed to VX-680, the RHAMM-silenced MM cells were significantly less sensitive to cytoreduction (left panel, bottom of Figure 6) and apoptosis (right panel, bottom of Figure 6).
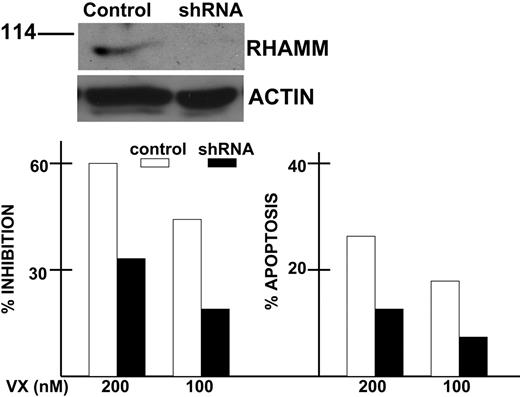
Effect of RHAMM silencing. AF-10 cells were infected with lentivirus expressing shRNA for RHAMM or a scrambled sequence (Control). After selection of transduced clones in blasticidin, the isogenic cell lines were first immunoblotted for expression of endogenous RHAMM (top). Immunoblot was performed 6 hours after synchronizing cells as described in Figure 5. Isogenic cell lines were then exposed to 0, 100, or 200 nM VX-680 for 72 hours, after which percent inhibition was calculated from growth assays and percent apoptosis was assayed by flow cytometric analysis of activated caspase 3 expression (bottom). Results are means of 3 experiments where SDs were less than 5% of the mean for all groups. The degree of percent inhibition and apoptosis in shRNA-expressing cells (▪) was significantly less (P < .05) than that of control cells (□).
Effect of RHAMM silencing. AF-10 cells were infected with lentivirus expressing shRNA for RHAMM or a scrambled sequence (Control). After selection of transduced clones in blasticidin, the isogenic cell lines were first immunoblotted for expression of endogenous RHAMM (top). Immunoblot was performed 6 hours after synchronizing cells as described in Figure 5. Isogenic cell lines were then exposed to 0, 100, or 200 nM VX-680 for 72 hours, after which percent inhibition was calculated from growth assays and percent apoptosis was assayed by flow cytometric analysis of activated caspase 3 expression (bottom). Results are means of 3 experiments where SDs were less than 5% of the mean for all groups. The degree of percent inhibition and apoptosis in shRNA-expressing cells (▪) was significantly less (P < .05) than that of control cells (□).
Discussion
This study suggests that therapeutically targeting aurora kinases has potential in multiple myeloma. A known aurora inhibitor, VX-680, was effective at nanomolar concentrations, inducing cytoreduction and apoptosis of MM cell lines and primary patient specimens. Mutant N-ras genes and the MM growth factor, IL-6, were unable to protect against VX-680, although they are known antiapoptotic factors against other antimyeloma drugs.21–23 A second independent agent, ZK, likewise was effective against MM cell lines and primary samples, although at slightly higher drug concentrations.
Our data strongly support that both VX-680 and ZK induce their adverse effects against myeloma cells by inhibiting aurora activity. First, the concentrations of both drugs that are effective against MM cells correlate with concentrations that inhibit aurora activity as determined by a decrease in histone phosphorylation. VX-680 is more effective than ZK in inhibiting histone phosphorylation in OPM-2 and MM1.S cells and is more effective in inducing OPM-2 and MM1.S cytoreduction. In addition, as shown in Figure 3B-C, OPM-2 cells are more sensitive to inhibition of histone phosphorylation than MM1.S cells and are more sensitive to the induced cytoreduction. Second, cell cycle analysis demonstrates an induction of tetraploidy that precedes the apoptotic response. This effect is comparable with the tetraploidy/apoptosis response in other cell models that used aurora kinase siRNA as interventions.9 Third, stable transfection with aurora A specifically protected myeloma cells against the effects of the agents while having no effect on myeloma apoptosis induced by PS-341. Since aurora A influences spindle checkpoint function,26 possibly through its ternary complex with microtubule-associated proteins, and is involved in stabilization of kinetochore-microtubule attachment of bipolar spindles,27,28 it is likely that ablation of aurora A function causes spindle checkpoint dysfunction leading to ploidy alterations and apoptosis. Although it is unknown whether additional inhibition of aurora B also plays a role in our results, it is clear that aurora A targeting can be effective therapy in this preclinical study.
The resistance of normal PBLs or CLL cells to aurora inhibitors is probably due to their exceedingly low proliferative rate. In contrast, experiments with normal lymphohematopoietic cells stimulated to proliferate with mitogens or specific growth factors demonstrated some degree of sensitivity to the inhibitors. The ID50s for inhibition of myeloid colony formation (approximately 200 nM) and PHA-stimulated lymphocyte proliferation (400 nM) were generally higher than that of the MM cell lines, suggesting a possible therapeutic window might exist in treated patients. An additional therapeutic window could result from the fact that the inhibitors readily induced apoptosis in MM cells while, at least for PHA-stimulated lymphocytes, no apoptosis was identified.
Our efforts to test an effect of RHAMM expression on the sensitivity of myeloma cells to aurora inhibitors were prompted by recent work indicating RHAMM overexpression in myeloma cells and its possible role in the genetic instability of myeloma clones.19 RHAMM localizes to the centrosome where it functions to maintain spindle integrity.29,30 Structural centrosomal abnormalities in primary patient myeloma cells correlate with RHAMM expression,19 and RHAMM overexpression in vitro results in mitotic defects. Thus, we wondered whether RHAMM expression in MM cells disrupted mitotic checkpoints that could render them more sensitive to aurora inhibitors. Stable transfection of U266 MM cells with full-length RHAMM resulted in only a very modest overexpression of RHAMM probably because higher expression results in multipolar spindles,19 which might not allow transfected cell outgrowth. A significant increase in sensitivity in RHAMM-transfected cells was seen in terms of decreased viable cell recovery and increased apoptosis. This enhanced sensitivity was not identified in the inhibitor-induced tetraploidy, suggesting it is the sensitivity to the subsequent apoptotic program that is altered by RHAMM rather than the initial molecular effects. In keeping with a role for RHAMM, when its expression was silenced by stable expression of shRNA in AF-10 MM cells, a significant decrease in sensitivity to VX-680 was seen.
In summary, our results suggest a therapeutic potential of aurora kinase inhibitors in patients with multiple myeloma. Deficient mitotic checkpoints, reflected in the disease's genetic instability, may render the malignancy particularly sensitive to these drugs. Furthermore, the enhanced sensitivity of RHAMM-overexpressing MM cells to VX-680 suggests that aurora kinase inhibitors may be especially effective in patients whose myeloma clones overexpress RHAMM.
Authorship
Contribution: Y.S. performed research; T.R. contributed RHAMM constructs and suggested experimental design; W.L. contributed reagents; C.A.M. generated the RHAMM construct; L.P. contributed constructs; T.R.D. performed colony-forming assays; S.S. generated and contributed aurora A construct and contributed to writing of the paper; M.L.P. designed colony-forming assays; R.F. contributed an aurora inhibitor and assisted in experimental design; A.L. designed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alan Lichtenstein, W111H, VA West LA Hospital, 11301 Wilshire Blvd, Los Angeles, CA, 90073; e-mail: alan.lichtenstein@med.va.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants CA96920, CA86915, CA107023, CA107023-02S1, CA89716, and CA111448 from NIH/NCI; and by research funds of the VA, the Alberta Heritage Foundation for Medical Research, the Canadian Institutes of Health, the Alberta Cancer Foundation, and the Lymphoma Foundation Canada.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal